
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Juan Mielgo-Ayuso | + 4061 word(s) | 4061 | 2021-12-06 02:41:05 | | | |
| 2 | Bruce Ren | Meta information modification | 4061 | 2021-12-15 02:46:07 | | |
Video Upload Options
l-Carnitine (l-C) and any of its forms (glycine-propionyl l-Carnitine (GPL-C) or l-Carnitine l-tartrate (l-CLT)) has been frequently recommended as a supplement to improve sports performance due to, among others, its role in fat metabolism and in maintaining the mitochondrial acetyl-CoA/CoA ratio.
1. Introduction
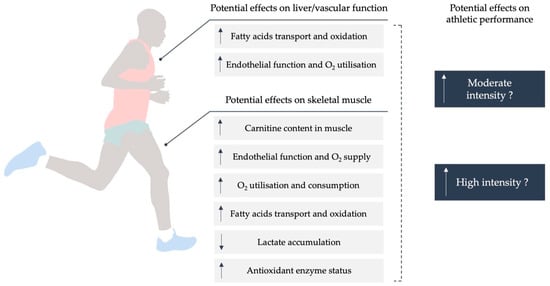
Regarding high-intensity exercise performance (≥80% V˙O2 max) [12], the role of carnitine in maintaining the acetyl-CoA/CoA ratio is especially important in maximum and supramaximal exercises, such as sprints competitions or mid-distance races between 50 and 400 m in swimming [13]. In fact, previous studies have reported that long-term oral l-C supplementation (2 g/day for 4 weeks) produced certain improvements on sports performance determinants such as V˙O2 max (mL/min/kg) and maximal work output (KJ) in both elite and amateur athletes
2. Effect of Oral l-Carnitine Supplementation on Exercise Performance Based on the Exercise Intensity
2.1. Effects of l-C Supplementation on High-Intensity Exercise Performance (≥80% V˙O2 max)
2.1.1. Chronic l-C Supplementation Protocol for High-Intensity Exercise Performance (≥80% V˙O2 max)
2.1.2. Acute l-C Supplementation Protocol for High-Intensity Exercise Performance (≥80% V˙O2 max)
2.2. Effects of l-C Supplementation on Moderate-Intensity Exercise Performance (50–79% V˙O2 max)
2.2.1. Chronic l-C Supplementation Protocol for Moderate-Intensity Exercise Performance (50–79% V˙O2 max)
2.2.2. Acute Supplementation Protocol for Moderate-Intensity Exercise Performance (50–79% V˙O2 max)
References
- Garthe, I.; Maughan, R.J. Athletes and supplements: Prevalence and perspectives. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 126–138.
- Karlic, H.; Lohninger, A. Supplementation of L-carnitine in athletes: Does it make sense? Nutrition 2004, 20, 709–715.
- Longo, N.; Frigeni, M.; Pasquali, M. Carnitine transport and fatty acid oxidation. Biochim. Biophys. Acta 2016, 1863, 2422.
- Kraemer, W.J.; Volek, J.S.; Dunn-Lewis, C. L-carnitine supplementation: Influence upon physiological function. Curr. Sports Med. Rep. 2008, 7, 218–223.
- Pekala, J.; Patkowska-Sokola, B.; Bodkowski, R.; Jamroz, D.; Nowakowski, P.; Lochynski, S.; Librowski, T. l-Carnitine—Metabolic Functions and Meaning in Humans Life. Curr. Drug Metab. 2011, 12, 667–678.
- Fielding, R.; Riede, L.; Lugo, J.P.; Bellamine, A. l-Carnitine Supplementation in Recovery after Exercise. Nutrients 2018, 10, 349.
- Mohammadi, H.; Djalali, M.; Daneshpazhooh, M.; Honarvar, N.M.; Chams-Davatchi, C.; Sepandar, F.; Fakhri, Z.; Yaghubi, E.; Zarei, M.; Javanbakht, M.H. Effects of L-carnitine supplementation on biomarkers of oxidative stress, antioxidant capacity and lipid profile, in patients with pemphigus vulgaris: A randomized, double-blind, placebo-controlled trial. Eur. J. Clin. Nutr. 2018, 72, 99–104.
- Gnoni, A.; Longo, S.; Gnoni, G.V.; Giudetti, A.M. Carnitine in human muscle bioenergetics: Can carnitine supplementation improve physical exercise? Molecules 2020, 25, 182.
- Stephens, F.B.; Evans, C.E.; Constantin-Teodosiu, D.; Greenhaff, P.L. Carbohydrate ingestion augments L-carnitine retention in humans. J. Appl. Physiol. 2007, 102, 1065–1070.
- Stephens, F.B.; Wall, B.T.; Marimuthu, K.; Shannon, C.E.; Constantin-Teodosiu, D.; Macdonald, I.A.; Greenhaff, P.L. Skeletal muscle carnitine loading increases energy expenditure, modulates fuel metabolism gene networks and prevents body fat accumulation in humans. J. Physiol. 2013, 591, 4655–4666.
- Oliveira, C.; Sousa, M. The effects of L-carnitine supplementation in athletic performance. Sci. Sport. 2019, 34, 63–72.
- Trinity, J.D.; Lee, J.F.; Pahnke, M.D.; Beck, K.C.; Coyle, E.F. Attenuated relationship between cardiac output and oxygen uptake during high-intensity exercise. Acta Physiol. 2012, 204, 362–370.
- Stephens, F.B.; Constantin-teodosiu, D.; Greenhaff, P.L. New insights concerning the role of carnitine in the regulation of fuel metabolism in skeletal muscle. J. Physiol. 2007, 581, 431–444.
- Trappe, S.W.; Costill, D.L.; Goodpaste, B.; Vukovich, M.D.; Fink, W.J. The effects of L-carnitine supplementation on performance during interval swimming. Int. J. Sports Med. 1994, 15, 181–185.
- Shannon, C.E.; Ghasemi, R.; Greenhaff, P.L.; Stephens, F.B. Increasing skeletal muscle carnitine availability does not alter the adaptations to high-intensity interval training. Scand. J. Med. Sci. Sport. 2018, 28, 107–115.
- Orer, G.E.; Guzel, N.A. The effects of acute l-carnitine supplementation on endurance performance of athletes. J. Strength Cond. Res. 2014, 28, 514–519.
- Soop, M.; Bjorkman, O.; Cederblad, G.; Hagenfeldt, L.; Wahren, J. Influence of carnitine supplementation on muscle substrate and carnitine metabolism during exercise. J. Appl. Physiol. 1988, 64, 2394–2399.
- Marconi, C.; Sassi, G.; Carpinelli, A.; Cerretelli, P. Effects of l-carnitine loading on the aerobic and anaerobic performance of endurance athletes. Eur. J. Appl. Physiol. Occup. Physiol. 1985, 54, 131–135.
- Colombani, P. Effects of L-carnitine supplementation on physical performance and energy metabolism of endurance-trained athletes: A double-blind crossover field study. Eur. J. Appl. Physiol. Occup. Physiol. 1996, 73, 434–439.
- Stephens, F.B.; Constantin-Teodosiu, D.; Laithwaite, D.; Simpson, E.J.; Greenhaff, P.L. Insulin stimulates L-carnitine accumulation in human skeletal muscle. FASEB J. 2005, 20, 377–379.
- Stephens, F.B.; Constantin-Teodosiu, D.; Laithwaite, D.; Simpson, E.J.; Greenhaff, P.L. An acute increase in skeletal muscle carnitine content alters fuel metabolism in resting human skeletal muscle. J. Clin. Endocrinol. Metab. 2006, 91, 5013–5018.
- Wall, B.T.; Stephens, F.B.; Constantin-Teodosiu, D.; Marimuthu, K.; Macdonald, I.A.; Greenhaff, P.L. Chronic oral ingestion of l-carnitine and carbohydrate increases muscle carnitine content and alters muscle fuel metabolism during exercise in humans. J. Physiol. 2011, 589, 963–973.
- Burrus, B.M.; Moscicki, B.M.; Matthews, T.D.; Paolone, V.J. The Effect of Acute L-carnitine and Carbohydrate Intake on Cycling Performance. Int. J. Exerc. Sci. 2018, 11, 404–416.
- Reuter, S.; Evans, A. Carnitine and acylcarnitines: Pharmacokinetic, pharmacological and clinical aspects. Clin. Pharmacokinet. 2012, 51, 553–572.
- Westerblad, H.; Bruton, J.; Katz, A. Skeletal muscle: Energy metabolism, fiber types, fatigue and adaptability. Exp. Cell Res. 2010, 316, 3093–3099.
- Harris, R.C.; Foster, C.V.L.; Hultman, E. Acetylcarnitine formation during intense muscular contraction in humans. J. Appl. Physiol. 1987, 63, 440–442.
- Van Loon, L.J.C.; Greenhaff, P.L.; Constantin-Teodosiu, D.; Saris, W.H.M.; Wagenmakers, A.J.M. The effects of increasing exercise intensity on muscle fuel utilisation in humans. J. Physiol. 2001, 536, 295.
- Kasper, J.D.; Meyer, R.A.; Beard, D.A.; Wiseman, R.W. Effects of altered pyruvate dehydrogenase activity on contracting skeletal muscle bioenergetics. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 316, R76–R86.
- Wan, J.J.; Qin, Z.; Wang, P.Y.; Sun, Y.; Liu, X. Muscle fatigue: General understanding and treatment. Exp. Mol. Med. 2017, 49, e384.
- Koozehchian, M.S.; Daneshfar, A.; Fallah, E.; Agha-Alinejad, H.; Samadi, M.; Kaviani, M.; Kaveh, B.M.; Jung, Y.P.; Sablouei, M.H. Effects of nine weeks l-Carnitine supplementation on exercise performance, anaerobic power, and exercise-induced oxidative stress in resistance-trained males. J. Exerc. Nutr. Biochem. 2018, 22, 7–19.
- Jacobs, P.L.; Goldstein, E.R.; Blackburn, W.; Orem, I.; Hughes, J.J. Glycine propionyl-L-carnitine produces enhanced anaerobic work capacity with reduced lactate accumulation in resistance trained males. J. Int. Soc. Sports Nutr. 2009, 6, 1–11.
- Drăgan, G.I.; Vasiliu, A.; Georgescu, E.; Dumas, I. Studies concerning chronic and acute effects of L-carnitine on some biological parameters in elite athletes. Physiologie 1987, 24, 23–28.
- Broad, E.M.; Maughan, R.J.; Galloway, S.D.R. Effects of four weeks l-Carnitine L-tartrate ingestion on substrate utilization during prolonged exercise. Int. J. Sport Nutr. Exerc. Metab. 2005, 15, 665–679.
- Smith, W.A.; Fry, A.C.; Tschume, L.C.; Bloomer, R.J. Effect of glycine propionyl-L-carnitine on aerobic and anaerobic exercise performance. Int. J. Sport Nutr. Exerc. Metab. 2008, 18, 19–36.
- Torma, F.; Gombos, Z.; Jokai, M.; Takeda, M.; Mimura, T.; Radak, Z. High intensity interval training and molecular adaptive response of skeletal muscle. Sport. Med. Health Sci. 2019, 1, 24–32.
- Ross, A.; Leveritt, M. Long-Term Metabolic and Skeletal Muscle Adaptations to Short-Sprint Training and Tapering. Sport. Med. 2001, 31, 1063–1082.
- Bloomer, R.J.; Smith, W.A.; Fisher-Wellman, K.H. Glycine propionyl-L-carnitine increases plasma nitrate/nitrite in resistance trained men. J. Int. Soc. Sports Nutr. 2007, 4, 22.
- Siliprandi, N.; Di Lisa, F.; Pieralisi, G.; Ripari, P.; Maccari, F.; Menabo, R.; Giamberardino, M.A.; Vecchiat, L. Metabolic changes induced by maximal exercise in human subjects following L-carnitine administration. BBA Gen. Subj. 1990, 1034, 17–21.
- Vecchiet, L.; Di Lisa, F.; Pieralisi, G.; Ripari, P.; Menab, R.; Giamberardino, M.A.; Siliprandi, N. Influence of L-carnitine administration on maximal physical exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1990, 61, 486–490.
- Barnett, C.; Costill, D.L.; Vukovich, M.D.; Cole, K.J.; Goodpaster, B.H.; Trappe, S.W.; Fink, W.J. Effect of l-carnitine supplementation on muscle and blood carnitine content and lactate accumulation during high-intensity sprint cycling. Int. J. Sport Nutr. 1994, 4, 280–288.
- Joyner, M.J.; Coyle, E.F. Endurance exercise performance: The physiology of champions. J. Physiol. 2008, 586, 35–44.
- San-Millán, I.; Brooks, G.A. Assessment of Metabolic Flexibility by Means of Measuring Blood Lactate, Fat, and Carbohydrate Oxidation Responses to Exercise in Professional Endurance Athletes and Less-Fit Individuals. Sport. Med. 2018, 48, 467–479.
- Moran, C.N.; Pitsiladis, Y.P. Tour de France Champions born or made: Where do we take the genetics of performance? J. Sports Sci. 2017, 35, 1411–1419.
- Gormley, S.; Swain, D.; High, R.; Spina, R.; Dowling, E.; Kotipalli, U.; Gandrakota, R. Effect of intensity of aerobic training on VO2max. Med. Sci. Sports Exerc. 2008, 40, 1336–1343.
- Duran, M.; Loof, N.; Ketting, D.; Dorland, L. Secondary carnitine deficiency. J. Clin. Chem. Clin. Biochem. 1990, 28, 359–363.
- Fritz, I.B.; Yue, K.T. Long-chain carnitine acyltransferase and the role of acylcarnitine derivatives in the catalytic increase of fatty acid oxidation induced by carnitine. J. Lipid Res. 1963, 4, 279–288.
- Arenas, J.; Huertas, R.; Campos, Y.; Díaz, A.E.; Villalón, J.M.; Vilas, E. Effects of l-carnitine on the pyruvate dehydrogenase complex and carnitine palmitoyl transferase activities in muscle of endurance athletes. FEBS Lett. 1994, 341, 91–93.
- Gorostiaga, E.M.; Maurer, A.C.; Eclache, J.P. Decrease in respiratory quotient during exercise following L-carnitine supplementation. Int. J. Sports Med. 1989, 10, 169–174.
- Abramowicz, W.N.; Galloway, S.D.R. Effects of acute versus chronic l-Carnitine L-tartrate supplementation on metabolic responses to steady state exercise in males and females. Int. J. Sport Nutr. Exerc. Metab. 2005, 15, 386–400.




