On the other hand, the first oral
l-C supplementation studies failed to obtain improvements on moderate-intensity exercise performance (50–79%
V˙O
2 max)
[12], measured as changes in heart rate responses during different cycle tests at 50% V˙O
2 max after supplementation with 1 g/day for 14 or 28 days
[18][17]. Likewise, Soop et al. did not show differences in substrate oxidation or blood lactate concentration during prolonged moderate intensity cycling exercise
[18][17]. These results may have been because the duration and/or dose was insufficient to increase the muscle carnitine pool and fatty-acid oxidation during prolonged exercise
[19,20][18][19]. However, Stephens et al. demonstrated with muscle biopsies that an insulin stimulus, by using insulin intravenous infusions
[21,22][20][21] or CHO intake
[9[9][10],
10], increased carnitine of muscle content after oral L-C supplementation. In this line, Wall et al. presented positive results regarding muscle glycogen saving during moderate-intensity exercise, which produced an increase in working capacity and a decrease in the perceived exertion scale in subsequent tests of greater intensity after a chronic supplementation of 4 g/day of oral
l-C supplementation together with 80 g/day CHO (24 weeks)
[23][22]. On the other hand, in studies with acute oral
l-C supplementation protocols (2–3 g of oral
l-C 2–3 h before exercise) together with CHO intake, no changes were reported in the use of substrates; hence, the increase in muscle carnitine pool was seemingly not enough to produce improvements in this type of exercise performance
[20,24][19][23]. These results could indicate that, in addition to the content of the muscle carnitine pool, there could be other mechanisms via which
l-C could be effective on sports performance.
Therefore, although oral
l-C or glycine-propionyl
l-Carnitine (GPL-C) or
l-Carnitine
l-tartrate (
l-CLT) supplementation could be effective in both high- and moderate-intensity sports performance, the current scientific evidence is controversial. In this sense, to the best of author’s knowledge, there is no clear information regarding the different effects involved in high- and moderate-intensity performance, which are needed to understand whether oral
l-C supplementation can be effective. For that reason, the main aim of the present systematic review was to determine the effects of oral
l-C, glycine-propionyl
l-Carnitine (GPL-C), or
l-Carnitine
l-tartrate (
l-CLT) supplementation on high- and moderate-intensity exercise performance, as well as to identify the effective doses and ideal timing of their intake.
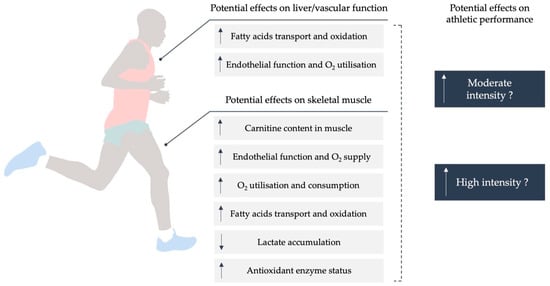
The amino acid carnitine is well known for its role in the transport of long-chain fatty acids into the mitochondrial matrix, where they are oxidized
[8]. Furthermore, by generating acylcarnitines,
l-C defends the cell from acyl-CoA accretion
[2]. Carnitine is mostly obtained from animal-based foods, with endogenous production in the liver and kidney providing a minor contribution. Due to the muscle’s inability to generate carnitine, it is dependent on uptake from the bloodstream
[36][24]. Mitochondrial fatty-acid oxidation is a significant source of energy for muscle metabolism, especially during exercise
[37][25]. However, the availability of free
l-C in the mitochondria appears to be limited during this process, especially during high-intensity exercise
[8]. Therefore, fatty-acid oxidation decreases significantly as exercise intensity rises from moderate to high. Given the importance of fatty acids in muscle bioenergetics and the limiting influence of free carnitine on fatty-acid oxidation during endurance exercise,
l-C supplementation has been proposed as a means of improving exercise performance
[2]. The function of
l-C supplementation on muscular performance has not yet been conclusively established. Different experimental outcomes were obtained due to differences in exercise (high and low–moderate) intensity, amount of
l-C provided, method, and timing of administration relative to the exercise (acute and chronic). In this review, the role of
l-C in muscle energetics was discussed, as well as the primary reasons for contradicting findings on the supplementation of
l-C.
2. Effect of Oral l-Carnitine Supplementation on Exercise Performance Based on the Exercise Intensity
2.1. Effects of l-C Supplementation on High-Intensity Exercise Performance (≥80% V˙O2 max)
When exercise intensity is greater than 80% V˙O
2 max, the use of glycogen begins to predominate over fatty acids as an energy source. It represents a key fuel during long-duration (>1 h) and high-intensity exercise
[8]. In this sense, during high-intensity exercise, muscle carnitine loading by
l-C supplementation could result in a better matching of glycolysis, pyruvate dehydrogenase complex (PDC), and mitochondrial flux, thereby reducing muscle anaerobic energy generation
[23][22]. In this sense, carnitine’s principal physiological roles during high-intensity exercise goes from acetyl group buffering (i.e., generating acetylcarnitine) to maintaining a pool of free co-enzyme A, which is required for mitochondrial flow to proceed (including the pyruvate dehydrogenase complex reaction)
[38][26]. However, there is still a rise in the acetyl-coenzyme A (acetyl-CoA)/CoASH ratio during this type of activity, presumably due to the considerable depletion of the free carnitine pool (to <6 mmol/kg dry muscle) induced by acetylcarnitine synthesis
[39][27].
As a result, an increase in skeletal muscle total carnitine content may provide more effective buffering of acetyl-CoA production during high-intensity exercise, counteracting the increase in the acetyl-CoA/CoASH ratio and enhancing PDC flow and mitochondrial ATP production
[23][22]. This would diminish the contribution of glycolysis and PCr hydrolysis to ATP synthesis, especially during the rest-to-exercise transition, when inertia in mitochondrial ATP production is known to exist at the level of PDC activation and flux
[40][28]. Furthermore, increasing PDC flow during high-intensity exercise is likely to lower muscle lactate generation, potentially improving exercise performance by reducing muscular acidosis
[41][29]. These metabolic actions could have significant implications for anaerobic ATP during high-intensity exercise. In this way, improved muscle strength (number of repetitions and third set lifting volume)
[33][30] and anaerobic performance by 30 s Wingate test (mean and peak power)
[32[31][30],
33], as well as decreased perceived exertion effort and an increase in work production
[16[16][22],
23], has been reported. Moreover, decreasing post-exercise blood lactate levels after graded exercise test on the treadmill were revealed
[16]. These data suggest that both chronic and acute
l-C supplementation could result in improvements in high-intensity performance
[16,23,32,33][16][22][31][30].
2.1.1. Chronic l-C Supplementation Protocol for High-Intensity Exercise Performance (≥80% V˙O2 max)
According to the results of included studies, 2 to 2.72 g/day of
l-C for 9 to 24 weeks could be beneficial in improving athletic performance during high-intensity exercise. Wall et al.
[23][22] revealed that supplementation with 4 g of
l-CLT (2.72 g of
l-C) with 160 g of CHO distributed two times per day for 24 weeks led to a 35% improvement in work capacity in an “all out” test. This test was performed for 30 min at 50% V˙O
2 max, followed immediately by 30 min at 80% V˙O
2 max [23][22]. Authors indicated that this advantage in the “all out” test was because the group supplemented with carnitine had 71% more muscle glycogen compared to the control, particularly due to savings in the 30 min at 50% V˙O
2 maxtest
[23][22]. On the other hand, Koozehchian et al.
[33][30] described improvements in peak power and absolute mean power derived from a lower accumulation of blood lactate concentration during a 30 s Wingate test after 2 g/day of
l-C supplementation for 9 weeks. In addition, these authors observed improvements in the number of repetitions and lifting volume in the third set of leg press exercises with the same previous protocol
[33][30]. These improvements were attributed to the fact that the increase in training volume was greater in the supplemented group, since the training program intensity was moderate. Therefore, chronic
l-C supplementation could result in a greater fatty-acid oxidation rate, thereby preserving muscle glycogen stores
[23][22]. To support this idea, these studies found that increasing muscle carnitine content saves muscle glycogen during low-intensity exercise (consistent with an increase in muscle lipid utilization), but that increasing muscle carnitine content reduces muscle anaerobic ATP production during high-intensity exercise by better matching glycolytic, pyruvate dehydrogenase complex, and mitochondrial flux
[23][22]. Moreover, this effect could be due to decreased post-exercise lactate levels and attenuated exercise-induced oxidative stress markers
[33,42][30][32].
On the contrary, other studies with chronic supplementation protocols using lower or similar
l-C doses (1 to 3 g/day) for periods between 4 and 24 weeks did not obtain improvements in this type of performance
[15,30,34][15][33][34]. Smith et al.
[34] did not show significant differences in power, total work, or percentage of fatigue after a test of anaerobic power (30 s on cycle ergometer) when the athletes were supplemented with both 1 g/day of PL and 3 g/day of PL for 8 weeks. These authors attributed these results to inadequate testing methods or to the high degree of variability in participant response. In the same line, Broad et al.
[30][33] did not present improvements in 20 km time trial duration after 3 g/day of
l-CLT (2 g/day
l-C) for 4 weeks. In this case, the high content of prior muscle carnitine could have led to the participants not oxidizing more fatty acids during the test. In addition, the relatively short time of supplementation did not allow for improvements in these deposits. For this reason, the investigators considered that future studies could analyze carnitine administration along with CHO-rich feedings to induce hyperinsulinemia in order to increase skeletal muscle carnitine content
[34].
Lastly, HIIT emerged several decades ago in response to the need for training techniques for athletic events of high intensity and often also of long duration
[43][35]. It is well known that HIIT exercise increases glycolytic and mitochondrial ATP and can induce morphological changes such as a conversion of fiber type morphology or increased fiber type area in fast-twitch fibers
[44][36]. In this sense, after 24 weeks of 2 g/day
l-CLT (1.36
l-C) with 80 g/day of CHO supplementation during a HIIT training program, Shannon et al.
[15] did not find significant improvements derived from less blood lactate accumulation, such as better V˙O
2 max, power, and work performed. However, it is likely that the duration of exercises in this study (two sets of 3 min) was not enough to allow a plateau of acetyl accumulation, which is normally observed within 10 min at 75–90% VO
2; thus, the ability of
l-C to buffer acetyl groups would be less critical when it comes to finding improvements
[15]. The authors explained that increased reliance on nonmitochondrial ATP resynthesis during a second bout of intense exercise is accompanied by increased carnitine acetylation. However, muscle carnitine during 24 weeks of HIIT did not alter this effect, nor did it enhance muscle metabolic adaptation or performance gain compared with HIIT alone
[15]. Other studies also found no significant differences in performance parameters during high-intensity tests after chronic supplementation of 4 g of
l-C with CHO for 14 and 7 days
[14], confirming that the supplementation period was insufficient, the exercise intensity was perhaps too great, and the glycolytic flux exceeded the capacity of the carnitine pool to effectively maintain the acetyl CoA/CoA ratio.
2.1.2. Acute l-C Supplementation Protocol for High-Intensity Exercise Performance (≥80% V˙O2 max)
Doses from 3 to 4 g of
l-C or GPL-C supplementation ingested between 60 and 90 min before exercise have been shown to improve different performance parameters. Lactate threshold values achieved at higher speeds and lower levels of perceived exertion scales during incremental tests until exhaustion were presented in
[16]. Equally, an increase in peak and average power of the last 10 s sprints in Wingate tests on a cycle ergometer was identified
[32][31]. These effects could be explained by more effective use of the aerobic energy system with the buffering capacity of acylcarnitines, resulting in a delay in the arrival of the lactate threshold and, therefore, consequent fatigue
[8]. To provoke the insulin stimulus that increased the muscle carnitine content, Orer and Guzel prescribed 3 or 4 g/day of
l-C supplementation in combination with CHO consumed in orange juice
[16]. On the other hand, the type of supplementation used by Jacobs et al.
[32][31] was a combination of the amino acid glycine with the molecule propionyl
l-Carnitine in order to increase levels of nitric oxide, although the physiological mechanisms remain to be elucidated to the best of the author’s knowledge. It was in 2007 when the ability of glycine to increase the plasma concentration of nitrites and nitrates was reported. This phenomenon could have the potential effect of improving blood flow to skeletal muscle during exercise
[45][37]. Therefore, the significant improvements in Jacobs et al.’s study were attributed to the vasodilator effects of nitric oxide that caused an improvement in the exchange of nutrients and metabolic products in muscle tissue
[32][31]; hence, this kind of GPL-C supplementation could be an alternative to the use of CHO to increase muscle carnitine content.
However, before discovering the necessity to carry out an insulin stimulus in order to increase muscle carnitine reserves, some studies
[9,21,22][9][20][21] found lower accumulation of blood lactate concentration and an increase in V˙O
2 and work capacity after oral
l-C supplementation without insulin stimulus, particularly in high-intensity exercises
[46,47][38][39]. In addition, another study found a decrease in blood lactate accumulation and improvements in power after intravenous supplementation
[42][32]. An initial limitation may be the lack of methodology given that they did not present muscle biopsies, highlighting whether it was possible to increase the content of muscle carnitine. For that reason, the concluded improvement in parameters with
l-C supplementation is unfounded
[35][40].
On the contrary, other acute supplementation protocols with similar or lower doses (between 3 and 4 g) ingested earlier (between 2 and 3 h before testing) or during the same test did not obtain improvements in high-intensity exercise performance parameters
[20,24][19][23]. There was a lack of improvement in power or time to exhaustion during a test at 85% V˙O
2 on a cycle ergometer or in the speed achieved at 4 mmol/lactate in tests of submaximal performance after a marathon race
[24][23]. These results were attributed to the fact that the intake of the supplementation period with CHO was not sufficient to increase the carnitine reserves in muscle and modify the substrate used du
[20,24][19][23]. Furthermore, in the case of Burrus et al.
[24][23], despite meeting the inclusion criteria (45 mL/kg/min of V˙O
2 peak), participants had great variability in the number of days and the duration of the training sessions. Therefore, it may be that the duration to exhaustion was not affected by the differences in participants’ aerobic practice
[24][23]. For this reason, it is necessary to carry out more research to clarify these contradictions and identify the appropriate way to supplement with
l-C acutely.
The variability of the results after both acute and chronic supplementation may be derived from differences in methodological approaches, individual differences among participants, type of stimulus, and lack of muscle biopsy data on muscle carnitine content
[30][33]. Even so, from the studies included in this review, it can be concluded that supplementation with 3 to 4 g of
l-C or GPL-C ingested 60–90 min before exercise, as well as doses 2 to 2.72 g with CHO over periods of 9 to 24 weeks, could be beneficial to improve performance in incremental tests, 10 s sprints, Wingate tests, and all out tests. These effects could be better if supplementation with
l-C includes CHO.
2.2. Effects of l-C Supplementation on Moderate-Intensity Exercise Performance (50–79% V˙O2 max)
Great cardiovascular capacity and metabolic adaptation are required in moderate-intensity exercise performance to supply the O
2 needs during exercise
[48,49][41][42]. Although a genetic predisposition is important to perform this type of discipline, training and nutrition protocols are essential to improve performance in moderate-intensity exercise events
[50][43]. From a metabolic point of view, during exercise at 50% V˙O
2 maxintensities or below, fatty-acid oxidation is the most predominant source of energy, whereas, during exercise of moderate intensity (between 50 and 70% V˙O
2 max), the energy source is provided by both fatty acids and glucose, with a gradual increase in the intensity
[51][44].
In this sense, the main role of carnitine during the exercise of low intensity, when the PDC activation and the flow are minimum, very likely involves the translocation of mitochondrial fatty acids
[23][22]. Although it has been suggested that free carnitine only limits fat oxidation at exercise intensities greater than 70% V˙O
2 max, it has also been observed that an acute increase of 15% in the muscle carnitine content reduced glycolytic flow mediated by insulin and PDC activation compared to a control group
[22][21]. In addition, this effect was also followed by an increase in muscle glycogen and long-chain CoA, which indicates an increase in muscle fatty-acid oxidation and CHO storage mediated by carnitine
[22][21]. Therefore, free carnitine availability can be a limiting factor in mitochondrial fatty-acid translocation both at rest and during low-intensity exercise, and an increase in skeletal muscle total carnitine content would increase the fatty-acid oxidation while reducing PDC activation and glycogen utilization during low–moderate-intensity exercise
[23][22]. In this sense, the increase in muscle carnitine increased fat oxidation during low–moderate-intensity exercise could save glycogen reserves and, therefore, decrease the fatigue at intensities below blood lactate threshold
[13]. Lastly, another study looked at the effect of supplementation on aerobic capacity, fat oxidation, and
V˙
O
2 max, i.e., physical performance
[42][32]. Regarding the dose, differences were observed among acute and chronic supplementation protocols, although this review did not find significant improvements in moderate-intensity exercise performance parameters.
2.2.1. Chronic l-C Supplementation Protocol for Moderate-Intensity Exercise Performance (50–79% V˙O2 max)
More than 95% of the body’s carnitine pool is confined to skeletal muscle, where it fulfils two major metabolic roles. For any given tissue the normal carnitine content is that which is necessary for an optimal rate of long-chain fatty-acid oxidation
[52][45]. Firstly, in mitochondrial fatty-acid translocation and transport, carnitine is a substrate for carnitine palmitoyl-transferase 1 (CPT1)
[53][46]. For that reason, oral carnitine feeding has been advocated as a possible ergogenic aid, with the main premise that it increases muscle carnitine content. In consequence, it would increase muscle fat transport and oxidation, as well as delay muscle glycogen depletion
[23,53][22][46]. In three of the studies included in this systematic review, supplementation doses of 1 to 3 g of
l-C or GPL-C over a period of 4, 8, or 24 weeks did not observe significant differences in moderate-intensity exercise performance parameters
[23,30,34][22][33][34]. In the same line, a lack of improvements in a scale of perceived exertion,
V˙
O
2 peak, and total time in tests of gradual exercise on a treadmill and 90 min on a cycle ergometer at 65% V˙O
2 maxwas observed two 2 of the studies
[30,34][33][34]. One potential reason could the inability to increase muscle carnitine concentrations, although, in the case of Broad et al.
[30][33], no biopsies were performed to report this statement. Moreover, this suggests that improvements in moderate-intensity exercise parameters could have been found if the studies were carried out using less trained participants and if lower exercise intensities were evaluated. Therefore, variability in the type of population analyzed in the studies represents another limitation in order to find conclusive results.
On the other hand, Wall et al.
[23][22] supplemented
l-C together with the intake of CHO and found no improvements in a scale of perceived exertion during moderate-intensity exercise, but a more integrating variable is needed to argument the changes, since the perception was not enough despite the placebo effect. However, as mentioned previously, the saving of muscle glycogen and the increase in fat oxidation revealed by muscle biopsies during this test allowed an improvement in the work capacity of later tests at greater intensity
[23][22]. Unlike Wall et al.
[23][22], previous studies used higher doses of
l-C (5 to 6 g) for shorter periods (5, 7 and 14 days) and without taking CHO, but they did not report changes in the substrate utilization during moderate-intensity exercises
[18][17] or in the muscle carnitine content by biopsies
[54][47].
On the contrary, the observation that
l-C does not affect muscle carnitine content without prior insulin stimulation seems to be in contrast with two previous studies that used supplementation of 2 g of
l-C together with CHO intake for 4 weeks in moderate- to low-intensity tests
[54,55][47][48]. While muscle biopsies performed in one of them reported increases in the pyruvate dehydrogenase activity and VO
2 max of the participants
[54][47], in the other one, no biopsies were carried out; however, a lower respiratory quotient was reported, suggesting that performance improvements derived from increased fat oxidation and probable muscle glycogen sparing could be expected
[55][48]. However, in this study, there were no changes in parameters such as O
2. Furthermore, other measurements such as oxygen uptake, heart rate, blood glycerol, and resting plasma free fatty-acid concentrations would have been necessary to report the improvement in different performance parameters
[55][48]. For this reason, more studies are needed to be able to affirm with conviction the improvements in low–moderate-intensity performance derived from chronic supplementation protocols.
2.2.2. Acute Supplementation Protocol for Moderate-Intensity Exercise Performance (50–79% V˙O2 max)
Along with the intake of CHO, doses of 3 g of
l-C 3 h before exercise at 65% V˙O
2 max on a cycle ergometer and doses of 2 g taken 2 h before a marathon race and 20 km after start did not find significant improvements in the power achieved during 40 min on the cycle ergometer or in total time in a marathon
[20,24][19][23]. In another study with
l-C and
l-tartrate, they used the same supplementation protocol as Burrus et al.
[24][23] (3 g of
l-C 3 h before), and no changes were found in the substrate used during a 60 min test at 60% V˙O
2 max[56][49]. The lack of improvements in these types of studies concluded that the supplementation period was not sufficient to increase the
l-C reserves in the muscle and, therefore, the substrate utilization was not modified
[20,24,56][19][23][49]. More research is necessary to explain the results and provide greater clarity and scientific evidence about the acute supplementation protocol of
l-C in moderate-intensity exercise performance and related outcomes.
Related to moderate-intensity exercise performance, different methodological approaches presented contradictory results after both acute and chronic supplementation protocols. Although more studies are needed to confirm the real effect of supplementation on moderate-intensity exercise performance parameters, this systematic review concluded that, while
l-C supplementation with CHO and 2.72 g of
l-C for a prolonged period of 24 weeks could increase muscle carnitine content, fat oxidation, and glycogen sparing during low-intensity testing, it seemingly had no effect on moderate-intensity exercise performance.