Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lorenzo Saggiante | + 827 word(s) | 827 | 2021-11-22 07:42:28 | | | |
| 2 | Camila Xu | Meta information modification | 827 | 2021-12-15 02:25:00 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Saggiante, L. Image-Guided Locoregional Therapies (LRTs). Encyclopedia. Available online: https://encyclopedia.pub/entry/17064 (accessed on 07 February 2026).
Saggiante L. Image-Guided Locoregional Therapies (LRTs). Encyclopedia. Available at: https://encyclopedia.pub/entry/17064. Accessed February 07, 2026.
Saggiante, Lorenzo. "Image-Guided Locoregional Therapies (LRTs)" Encyclopedia, https://encyclopedia.pub/entry/17064 (accessed February 07, 2026).
Saggiante, L. (2021, December 13). Image-Guided Locoregional Therapies (LRTs). In Encyclopedia. https://encyclopedia.pub/entry/17064
Saggiante, Lorenzo. "Image-Guided Locoregional Therapies (LRTs)." Encyclopedia. Web. 13 December, 2021.
Copy Citation
Image-guided locoregional therapies (LRTs) are a crucial asset in the treatment of hepatocellular carcinoma (HCC), which has proven to be characterized by an impaired antitumor immune status. LRTs not only directly destroy tumor cells but also have an immunomodulating role, altering the tumor microenvironment with potential systemic effects.
locoregional therapies
Immunomodulation
liver
Hepatocellular carcinoma
Interventional radiology
Immunotherapy
1. Image-guided locoregional therapies (LRTs) and Immunomodulation
Image-guided locoregional therapies (LRTs) have the potential of shaping tumor immunity by altering the composition of the HCC microenvironment; in particular, they lead to the release from dying tumor cells of cryptic TAAs and tumor neoantigens that become accessible to the immune system, acting as novel targets for antigen-presenting cells (APCs), mainly dendritic cells (DCs).
The innate immune system, including neutrophils, macrophages, and natural killer (NK) cells, is the first to respond, followed by the more strong and sustained acquired response [1][2], characterized by significant intratumoral immune infiltrates [3][4][5][6][7][8]. In order to stimulate the acquired immune system, cancer cells must die thorough immunogenic cell death (ICD), which happens with necrosis and is characterized by the release from dying cells, along with antigens and preserved intracellular organelles, of damage-associated molecular patterns (DAMPs), such as DNA, RNA, heat shock proteins (HSPs), adenosine triphosphate (ATP), protein high-mobility group box 1 (HMGB1), calreticulin, and uric acid, which allow recruitment and activation of DCs (through the NF-κβ pathway activation). Activated DCs, after reaching regional lymph nodes, present antigens along with costimulatory signals to T cells that are therefore stimulated [9][10][11]. On the contrary, in non-ICD, which occurs with apoptosis, the dying tumor cells do not release DAMPs; without phagocytizing DAMPs, DCs cannot be activated and therefore do not present costimulatory signals to T cells [11][12], with the subsequent T-cell anergy and deletion leading to immune tolerance [13][14][15]. The antitumor response triggered by ICD takes place both locally and distantly from the primary tumor; this phenomenon, whereby a locally applied therapy elicits a distant antitumor response, is known as the abscopal effect [16].
It is not yet clear which ablative technique has the highest potential for releasing cryptic tumor antigens and creating the best immunostimulatory microenvironment.
2. Pro-Oncogenic Effects of LRTs
All locoregional and surgical approaches might also have a stimulatory effect of oncogenesis, as described by several studies, by means of tumor cell spreading, microenvironmental changes, and both angiogenetic and proliferative triggers.
In fact, LRTs, both thermal and non-thermal, applied to the liver as well as to other organs, have been correlated to a more aggressive tumor subtype, tumor growth [17][18][19], and to a higher incidence of tumor progression [20][21]. This can be attributed to local and systemic inflammation (via an increase in IL-6, IL-8, and HSP) and to the upregulation of pro-oncogenic growth factors such as hypoxia-inducible factor-1α (HIF-1α), VEGF, hepatocyte growth factor (HGF), hepatocyte growth factor receptor (HGF-R), matrix metalloproteinases (MMPs), cluster of differentiation 147 (CD147), and mammalian target of rapamycin (mTOR) [17][22][23][24][25][26][27][28]. Moreover, local hepatic thermal ablation has been linked, in some cases, to a higher rate of distant intrahepatic tumor development and to overall worse outcomes [18][21][28][29]. This may be particularly true for sublethal–incomplete treatments [30][31][32].
3. LRTs: Classification
The main types of LRTs available today are reported in Figure 1.
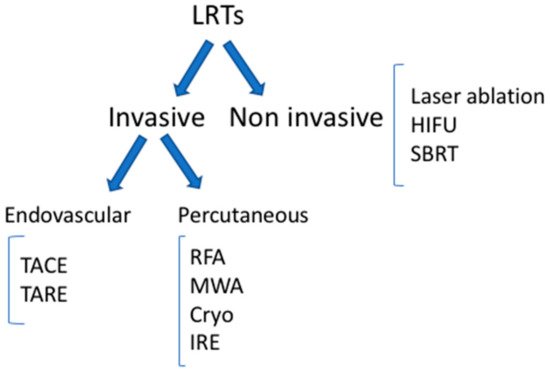
Figure 1. Locoregional therapies. TACE: trans-arterial chemoembolization; TARE: trans-arterial radioembolization; RFA: radiofrequency ablation; MWA: microwave ablation; IRE: irreversible electroporation; HIFU: high-intensity focal ultrasound; SBRT: stereotactic body radiation therapy.
In the following section, we briefly describe the technical properties and methods of functioning for each LRT, together with the existing evidence regarding its effect on the immune system. In Table 1, we summarize the effect of each LRT on the immune system.
Table 1. Summarized immunological effect of LRTs.
| RFA | Cryoablation | MWA | TACE | TARE | HIFU | Laser | SBRT | IRE | |
|---|---|---|---|---|---|---|---|---|---|
| Increased | DAMPs (RNA, DNA, HSPs, HMGB1) Inflammatory cytokines (IL-1β, IL-6, IL-8, TNF-α, IFN- γ) Tumor specific antibodies CD4+ T, CD8+ T, tumor-specific T, cytotoxic T, central memory lymphocytes, infiltrating CD45RO+ |
Inflammatory cytokines (IL-1, IL-6, TNF-α) NF-κβCD4+ T, CD8+ T, NK cells |
DAMPs (HSP-70) Inflammatory cytokines (IL-1, IL-6, IL-12) CD3+ T, CD56+ NK, CD8+ T cells) |
Circulating GPC3-specific cytotoxic T lymphocytes (CTL) IL-6 CD4+ cells CD4+/CD8+ ratio NK cells |
Inflammatory cytokines (TNF-α, IL-1, IL-6, IL-8) CD8+ T cells CD56+ NK, CD8+ CD56+ NKT, CD4+ T cells APCs oxidative stress markers (malondyaldehide) endothelial injury markers (vW factor, PAI-1) liver regeneration factors (FGF-19, HGF) |
DAMPs (HSPs, HMGB1) Inflammatory cytokines (IFN-γ, IL-2) CD4+, CD8+, CD3+, NK cells, B cells CD4+/CD8+ ratio DC Neutrophil |
DAMPs (HSPs) Inflammatory cytokines (IL-6) Macrophages CD8 T cells |
DAMPs (HMGB1)MHC I molecules Inflammatory cytokines IFN-α, IFN-β Lymphocyte infiltration in tumor tissue Specific CD8 T activation Peripheral NK, and CD3+CD56+NKT-like cells Treg |
Neutrophil and macrophage infiltration Inflammatory cytokines MIF Macrophage inflammatory protein-1b (MIP-1b)/chemokine ligand 4 (CCL4), TNF-α, and IL-17 |
| Decreased | TGF-ß, IL-10 Tregs |
IL-4, IL-10 | Treg CD8+ cells |
Immunosuppressive cytokines (VEGF, TGF-β1, TGF-β2 IL-4, and IL-10) |
DAMPs: danger-associated molecular patterns; HSPs: heat shock proteins; HMGB: high-mobility group box; GPC3: glypican-3; APCs: antigen--presenting cells; vW: von Willebrand; PAI-1: plasminogen activator inhibitor-1; FGF-19: fibroblast growth factor-19; HGF: hepatocyte growth factor; DCs: dendritic cells; MIF: macrophage migration inhibitory factor; MIP-1b: macrophage inflammatory protein-1b; MHC: major histocompatibility complex.
References
- Li, L.; Wang, W.; Pan, H.; Ma, G.; Shi, X.; Xie, H.; Liu, X.; Ding, Q.; Zhou, W.; Wang, S. Microwave Ablation Combined with OK-432 Induces Th1-Type Response and Specific Antitumor Immunity in a Murine Model of Breast Cancer. J. Transl. Med. 2017, 15, 23.
- Huang, K.W.; Jayant, K.; Lee, P.-H.; Yang, P.; Hsiao, C.-Y.; Habib, N.; Sodergren, M.H. Positive Immuno-Modulation Following Radiofrequency Assisted Liver Resection in Hepatocellular Carcinoma. J. Clin. Med. 2019, 8, 385.
- Nikfarjam, M.; Muralidharan, V.; Christophi, C. Mechanisms of Focal Heat Destruction of Liver Tumors. J. Surg. Res. 2005, 127, 208–223.
- Haen, S.P.; Pereira, P.L.; Salih, H.R.; Rammensee, H.-G.; Gouttefangeas, C. More Than Just Tumor Destruction: Immunomodulation by Thermal Ablation of Cancer. Clin. Dev. Immunol. 2011, 2011, 1–19.
- Dromi, S.A.; Walsh, M.P.; Herby, S.; Traughber, B.; Xie, J.; Sharma, K.V.; Sekhar, K.P.; Luk, A.; Liewehr, D.J.; Dreher, M.R.; et al. Radiofrequency Ablation Induces Antigen-Presenting Cell Infiltration and Amplification of Weak Tumor-Induced Immunity. Radiology 2009, 251, 58–66.
- Zerbini, A.; Pilli, M.; Laccabue, D.; Pelosi, G.; Molinari, A.; Negri, E.; Cerioni, S.; Fagnoni, F.; Soliani, P.; Ferrari, C.; et al. Radiofrequency Thermal Ablation for Hepatocellular Carcinoma Stimulates Autologous NK-Cell Response. Gastroenterology 2010, 138, 1931–1942.
- Dong, B.; Zhang, J.; Liang, P.; Yu, X.; Su, L.; Yu, D.; Ji, X.; Yu, G.; Yin, Z. Influencing Factors of Local Immunocyte Infiltration in Hepatocellular Carcinoma Tissues Pre- and Post-Percutaneous Microwave Coagulation Therapy. Zhonghua Yi Xue Za Zhi 2002, 82, 393–397.
- Kuang, M.; Liu, S.Q.; Saijo, K.; Uchimura, E.; Huang, L.; Leong, K.W.; Lu, M.D.; Huang, J.F.; Ohno, T. Microwave Tumour Coagulation plus in Situ Treatment with Cytokine-Microparticles: Induction of Potent Anti-Residual Tumour Immunity. Int. J. Hyperth. 2005, 21, 247–257.
- Kroemer, G.; Galluzzi, L.; Kepp, O.; Zitvogel, L. Immunogenic Cell Death in Cancer Therapy. Annu. Rev. Immunol. 2013, 31, 51–72.
- Sabel, M.S. Cryo-Immunology: A Review of the Literature and Proposed Mechanisms for Stimulatory versus Suppressive Immune Responses. Cryobiology 2009, 58, 1–11.
- Basu, S.; Binder, R.J.; Suto, R.; Anderson, K.M.; Srivastava, P.K. Necrotic but Not Apoptotic Cell Death Releases Heat Shock Proteins, Which Deliver a Partial Maturation Signal to Dendritic Cells and Activate the NF-ΚB Pathway. Int. Immunol. 2000, 12, 1539–1546.
- Bottero, V.; Withoff, S.; Verma, I.M. NF-ΚB and the Regulation of Hematopoiesis. Cell Death Differ. 2006, 13, 785–797.
- Chu, K.F.; Dupuy, D.E. Thermal Ablation of Tumours: Biological Mechanisms and Advances in Therapy. Nat. Rev. Cancer 2014, 14, 199–208.
- den Brok, M.H.M.G.M.; Sutmuller, R.P.M.; Nierkens, S.; Bennink, E.J.; Frielink, C.; Toonen, L.W.J.; Boerman, O.C.; Figdor, C.G.; Ruers, T.J.M.; Adema, G.J. Efficient Loading of Dendritic Cells Following Cryo and Radiofrequency Ablation in Combination with Immune Modulation Induces Anti-Tumour Immunity. Br. J. Cancer 2006, 95, 896–905.
- Ferguson, T.A.; Choi, J.; Green, D.R. Armed Response: How Dying Cells Influence T-Cell Functions. Immunol. Rev. 2011, 241, 77–88.
- Ng, J.; Dai, T. Radiation Therapy and the Abscopal Effect: A Concept Comes of Age. Ann. Transl. Med. 2016, 4, 118.
- Ahmed, M.; Kumar, G.; Moussa, M.; Wang, Y.; Rozenblum, N.; Galun, E.; Goldberg, S.N. Hepatic Radiofrequency Ablation–Induced Stimulation of Distant Tumor Growth Is Suppressed by c-Met Inhibition. Radiology 2016, 279, 103–117.
- Rozenblum, N.; Zeira, E.; Scaiewicz, V.; Bulvik, B.; Gourevitch, S.; Yotvat, H.; Galun, E.; Goldberg, S.N. Oncogenesis: An “Off-Target” Effect of Radiofrequency Ablation. Radiology 2015, 276, 426–432.
- Tanis, E.; Nordlinger, B.; Mauer, M.; Sorbye, H.; van Coevorden, F.; Gruenberger, T.; Schlag, P.M.; Punt, C.J.A.; Ledermann, J.; Ruers, T.J.M. Local Recurrence Rates after Radiofrequency Ablation or Resection of Colorectal Liver Metastases. Analysis of the European Organisation for Research and Treatment of Cancer #40004 and #40983. Eur. J. Cancer 2014, 50, 912–919.
- Erinjeri, J.P.; Thomas, C.T.; Samoilia, A.; Fleisher, M.; Gonen, M.; Sofocleous, C.T.; Thornton, R.H.; Siegelbaum, R.H.; Covey, A.M.; Brody, L.A.; et al. Image-Guided Thermal Ablation of Tumors Increases the Plasma Level of Interleukin-6 and Interleukin-10. J. Vasc. Interv. Radiol. 2013, 24, 1105–1112.
- Hinz, S.; Tepel, J.; Röder, C.; Kalthoff, H.; Becker, T. Profile of Serum Factors and Disseminated Tumor Cells before and after Radiofrequency Ablation Compared to Resection of Colorectal Liver Metastases—A Pilot Study. Anticancer Res. 2015, 35, 2961–2967.
- Gou, X.; Tang, X.; Kong, D.K.; He, X.; Gao, X.; Guo, N.; Hu, Z.; Zhao, Z.; Chen, Y. CD147 Is Increased in HCC Cells under Starvation and Reduces Cell Death through Upregulating P-MTOR in Vitro. Apoptosis 2016, 21, 110–119.
- Chao, Y.; Wu, C.-Y.; Kuo, C.-Y.; Wang, J.P.; Luo, J.-C.; Kao, C.-H.; Lee, R.-C.; Lee, W.-P.; Li, C.-P. Cytokines Are Associated with Postembolization Fever and Survival in Hepatocellular Carcinoma Patients Receiving Transcatheter Arterial Chemoembolization. Hepatol. Int. 2013, 7, 883–892.
- He, X.; Guo, X.; Zhang, H.; Kong, X.; Yang, F.; Zheng, C. Mechanism of Action and Efficacy of LY2109761, a TGF-β Receptor Inhibitor, Targeting Tumor Microenvironment in Liver Cancer after TACE. Oncotarget 2018, 9, 1130–1142.
- Zhao, Z.-W.; Fan, X.-X.; Song, J.-J.; Xu, M.; Chen, M.-J.; Tu, J.-F.; Wu, F.-Z.; Zhang, D.-K.; Liu, L.; Chen, L.; et al. ShRNA Knock-down of CXCR7 Inhibits Tumour Invasion and Metastasis in Hepatocellular Carcinoma after Transcatheter Arterial Chemoembolization. J. Cell. Mol. Med. 2017, 21, 1989–1999.
- Ahmed, M.; Kumar, G.; Navarro, G.; Wang, Y.; Gourevitch, S.; Moussa, M.H.; Rozenblum, N.; Levchenko, T.; Galun, E.; Torchilin, V.P.; et al. Systemic SiRNA Nanoparticle-Based Drugs Combined with Radiofrequency Ablation for Cancer Therapy. PLoS ONE 2015, 10, e0128910.
- Nikfarjam, M.; Muralidharan, V.; Christophi, C. Altered Growth Patterns of Colorectal Liver Metastases after Thermal Ablation. Surgery 2006, 139, 73–81.
- Nijkamp, M.W.; van der Bilt, J.D.W.; de Bruijn, M.T.; Molenaar, I.Q.; Voest, E.E.; van Diest, P.J.; Kranenburg, O.; Borel Rinkes, I.H.M. Accelerated Perinecrotic Outgrowth of Colorectal Liver Metastases Following Radiofrequency Ablation Is a Hypoxia-Driven Phenomenon. Ann. Surg. 2009, 249, 814–823.
- Kang, T.W.; Kim, J.M.; Rhim, H.; Lee, M.W.; Kim, Y.; Lim, H.K.; Choi, D.; Song, K.D.; Kwon, C.H.D.; Joh, J.-W.; et al. Small Hepatocellular Carcinoma: Radiofrequency Ablation versus Nonanatomic Resection—Propensity Score Analyses of Long-Term Outcomes. Radiology 2015, 275, 908–919.
- Shi, L.; Wang, J.; Ding, N.; Zhang, Y.; Zhu, Y.; Dong, S.; Wang, X.; Peng, C.; Zhou, C.; Zhou, L.; et al. Inflammation Induced by Incomplete Radiofrequency Ablation Accelerates Tumor Progression and Hinders PD-1 Immunotherapy. Nat. Commun. 2019, 10, 5421.
- Ruzzenente, A.; de Manzoni, G.; Molfetta, M.; Pachera, S.; Genco, B.; Donataccio, M.; Guglielmi, A. Rapid Progression of Hepatocellular Carcinoma after Radiofrequency Ablation. World J. Gastroenterol. 2004, 10, 1137.
- Zhao, Z.; Wu, J.; Liu, X.; Liang, M.; Zhou, X.; Ouyang, S.; Yao, J.; Wang, J.; Luo, B. Insufficient Radiofrequency Ablation Promotes Proliferation of Residual Hepatocellular Carcinoma via Autophagy. Cancer Lett. 2018, 421, 73–81.
More
Information
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
664
Revisions:
2 times
(View History)
Update Date:
15 Dec 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




