Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Image-guided locoregional therapies (LRTs) are a crucial asset in the treatment of hepatocellular carcinoma (HCC), which has proven to be characterized by an impaired antitumor immune status. LRTs not only directly destroy tumor cells but also have an immunomodulating role, altering the tumor microenvironment with potential systemic effects.
- locoregional therapies
- Immunomodulation
- liver
- Hepatocellular carcinoma
- Interventional radiology
- Immunotherapy
1. Image-guided locoregional therapies (LRTs) and Immunomodulation
Image-guided locoregional therapies (LRTs) have the potential of shaping tumor immunity by altering the composition of the HCC microenvironment; in particular, they lead to the release from dying tumor cells of cryptic TAAs and tumor neoantigens that become accessible to the immune system, acting as novel targets for antigen-presenting cells (APCs), mainly dendritic cells (DCs).
The innate immune system, including neutrophils, macrophages, and natural killer (NK) cells, is the first to respond, followed by the more strong and sustained acquired response [33,34], characterized by significant intratumoral immune infiltrates [35,36,37,38,39,40]. In order to stimulate the acquired immune system, cancer cells must die thorough immunogenic cell death (ICD), which happens with necrosis and is characterized by the release from dying cells, along with antigens and preserved intracellular organelles, of damage-associated molecular patterns (DAMPs), such as DNA, RNA, heat shock proteins (HSPs), adenosine triphosphate (ATP), protein high-mobility group box 1 (HMGB1), calreticulin, and uric acid, which allow recruitment and activation of DCs (through the NF-κβ pathway activation). Activated DCs, after reaching regional lymph nodes, present antigens along with costimulatory signals to T cells that are therefore stimulated [41,42,43]. On the contrary, in non-ICD, which occurs with apoptosis, the dying tumor cells do not release DAMPs; without phagocytizing DAMPs, DCs cannot be activated and therefore do not present costimulatory signals to T cells [43,44], with the subsequent T-cell anergy and deletion leading to immune tolerance [45,46,47]. The antitumor response triggered by ICD takes place both locally and distantly from the primary tumor; this phenomenon, whereby a locally applied therapy elicits a distant antitumor response, is known as the abscopal effect [48].
It is not yet clear which ablative technique has the highest potential for releasing cryptic tumor antigens and creating the best immunostimulatory microenvironment.
2. Pro-Oncogenic Effects of LRTs
All locoregional and surgical approaches might also have a stimulatory effect of oncogenesis, as described by several studies, by means of tumor cell spreading, microenvironmental changes, and both angiogenetic and proliferative triggers.
In fact, LRTs, both thermal and non-thermal, applied to the liver as well as to other organs, have been correlated to a more aggressive tumor subtype, tumor growth [49,50,51], and to a higher incidence of tumor progression [52,53]. This can be attributed to local and systemic inflammation (via an increase in IL-6, IL-8, and HSP) and to the upregulation of pro-oncogenic growth factors such as hypoxia-inducible factor-1α (HIF-1α), VEGF, hepatocyte growth factor (HGF), hepatocyte growth factor receptor (HGF-R), matrix metalloproteinases (MMPs), cluster of differentiation 147 (CD147), and mammalian target of rapamycin (mTOR) [49,54,55,56,57,58,59,60]. Moreover, local hepatic thermal ablation has been linked, in some cases, to a higher rate of distant intrahepatic tumor development and to overall worse outcomes [50,53,60,61]. This may be particularly true for sublethal–incomplete treatments [62,63,64].
3. LRTs: Classification
The main types of LRTs available today are reported in Figure 1.
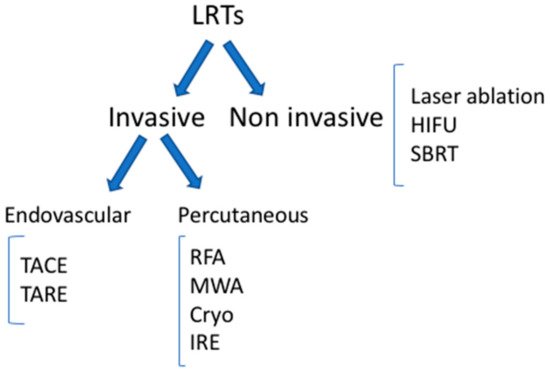
Figure 1. Locoregional therapies. TACE: trans-arterial chemoembolization; TARE: trans-arterial radioembolization; RFA: radiofrequency ablation; MWA: microwave ablation; IRE: irreversible electroporation; HIFU: high-intensity focal ultrasound; SBRT: stereotactic body radiation therapy.
In the following section, we briefly describe the technical properties and methods of functioning for each LRT, together with the existing evidence regarding its effect on the immune system. In Table 1, we summarize the effect of each LRT on the immune system.
Table 1. Summarized immunological effect of LRTs.
| RFA | Cryoablation | MWA | TACE | TARE | HIFU | Laser | SBRT | IRE | |
|---|---|---|---|---|---|---|---|---|---|
| Increased | DAMPs (RNA, DNA, HSPs, HMGB1) Inflammatory cytokines (IL-1β, IL-6, IL-8, TNF-α, IFN- γ) Tumor specific antibodies CD4+ T, CD8+ T, tumor-specific T, cytotoxic T, central memory lymphocytes, infiltrating CD45RO+ |
Inflammatory cytokines (IL-1, IL-6, TNF-α) NF-κβCD4+ T, CD8+ T, NK cells |
DAMPs (HSP-70) Inflammatory cytokines (IL-1, IL-6, IL-12) CD3+ T, CD56+ NK, CD8+ T cells) |
Circulating GPC3-specific cytotoxic T lymphocytes (CTL) IL-6 CD4+ cells CD4+/CD8+ ratio NK cells |
Inflammatory cytokines (TNF-α, IL-1, IL-6, IL-8) CD8+ T cells CD56+ NK, CD8+ CD56+ NKT, CD4+ T cells APCs oxidative stress markers (malondyaldehide) endothelial injury markers (vW factor, PAI-1) liver regeneration factors (FGF-19, HGF) |
DAMPs (HSPs, HMGB1) Inflammatory cytokines (IFN-γ, IL-2) CD4+, CD8+, CD3+, NK cells, B cells CD4+/CD8+ ratio DC Neutrophil |
DAMPs (HSPs) Inflammatory cytokines (IL-6) Macrophages CD8 T cells |
DAMPs (HMGB1)MHC I molecules Inflammatory cytokines IFN-α, IFN-β Lymphocyte infiltration in tumor tissue Specific CD8 T activation Peripheral NK, and CD3+CD56+NKT-like cells Treg |
Neutrophil and macrophage infiltration Inflammatory cytokines MIF Macrophage inflammatory protein-1b (MIP-1b)/chemokine ligand 4 (CCL4), TNF-α, and IL-17 |
| Decreased | TGF-ß, IL-10 Tregs |
IL-4, IL-10 | Treg CD8+ cells |
Immunosuppressive cytokines (VEGF, TGF-β1, TGF-β2 IL-4, and IL-10) |
DAMPs: danger-associated molecular patterns; HSPs: heat shock proteins; HMGB: high-mobility group box; GPC3: glypican-3; APCs: antigen--presenting cells; vW: von Willebrand; PAI-1: plasminogen activator inhibitor-1; FGF-19: fibroblast growth factor-19; HGF: hepatocyte growth factor; DCs: dendritic cells; MIF: macrophage migration inhibitory factor; MIP-1b: macrophage inflammatory protein-1b; MHC: major histocompatibility complex.
This entry is adapted from the peer-reviewed paper 10.3390/cancers13225797
This entry is offline, you can click here to edit this entry!
