Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mingqing Li | + 1477 word(s) | 1477 | 2021-12-03 04:17:04 | | | |
| 2 | Rita Xu | Meta information modification | 1477 | 2021-12-14 03:31:36 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Li, M. Matrix Metalloproteinases in Endometriosis. Encyclopedia. Available online: https://encyclopedia.pub/entry/17052 (accessed on 15 January 2026).
Li M. Matrix Metalloproteinases in Endometriosis. Encyclopedia. Available at: https://encyclopedia.pub/entry/17052. Accessed January 15, 2026.
Li, Mingqing. "Matrix Metalloproteinases in Endometriosis" Encyclopedia, https://encyclopedia.pub/entry/17052 (accessed January 15, 2026).
Li, M. (2021, December 13). Matrix Metalloproteinases in Endometriosis. In Encyclopedia. https://encyclopedia.pub/entry/17052
Li, Mingqing. "Matrix Metalloproteinases in Endometriosis." Encyclopedia. Web. 13 December, 2021.
Copy Citation
Endometriosis is a condition that is influenced by hormones and involves stroma and glands being found outside the uterus; there are increases in proliferation, invasion, internal bleeding, and fibrosis.
matrix metalloproteinases
endometriosis
function
1. Introduction
Endometriosis is a benign, hormone-dependent disease characterized by the presence of endometrial glands and stroma outside the uterus and affects at least 10–15% of women of reproductive age [1]. Several characteristics, such as infertility, dysmenorrhea, and dyspareunia, are associated with endometriosis [2]. Moreover, estrogen, matrix remodeling, inflammation, and oxidative stress have been shown to be involved in endometriosis progression from early to advanced stages [3].
Based on the hypothesis of the retrograde transplantation theory proposed by Sampson in 1927, we recognize the significance of matrix regrading and matrix remolding. Endometriosis is established when endometrial cells (influenced by hormone fluctuations) break off and travel via the fallopian tubes to new sites, where they implant and grow. The extracellular matrix (ECM) is a complex network of macromolecular structures, such as collagens, proteoglycans, glycoproteins, and elastin [4]. The matrix metalloprotease (MMP) family is devoted to maintaining ECM homeostasis, and the dysregulation of its expression leads to the disease. Since the 1960s, the MMP family has drawn much attention, and its functions have been further determined [5] and systematically reviewed. [6].
2. The MMP Family
MMPs belong to a large family of calcium-dependent, zinc-containing endopeptidases that are known for their ability to cleave nonmatrix proteins, as well as several ECM constituents (e.g., collagens, proteoglycan, and glycoproteins). In 1949, MMPs were discovered to be depolymerizing enzymes that could promote the proliferation of malignant cells by remolding connective tissue stroma such as blood vessels [7]. A decade later, the first vertebrate MMP was isolated and identified to act as a collagenase [5]. At least 28 MMPs have been identified in mammals to date [8].
2.1. Classification of MMPs
How MMPs should be classified remains controversial. A previous report classified them into the following five groups based on their substrate-specificity and location: collagenases, gelatinases, stromelysins, matrilysins, and membrane-type metalloproteinases [9]. However, Garcia-Fernandez et al. and Kapoor et al. both divided MMPs into six groups depending on their different substrates. Compared with the previous five groups, the latter researchers added an “other MMPs” group, which included those that did not fit into any other category [7][10]. Beyond that, MMPs can also be divided into two groups: secreted MMPs and anchor MMPs. Of the known MMPs, MMP14, 15, 16, 17, 23, 24, and 25 are membrane-anchored [11][12].
2.2. Structure of MMPs
The sophisticated structure of MMPs has been known for some time and has been described in significant detail [13]. The compositions of most MMPs are the same: they involve a pro-peptide domain, a signal peptide, a cysteine switch motif, a catalytic domain, and a hemopexin-like domain [7]. However, some MMPs have different structural characteristics that enable them to perform special activities. For example, MMP2 and MMP9 can interact with collagen via three fibronectin type-II domains. Moreover, MMP7, MMP23, and MMP26 have no C-terminal hemopexin-like domain, which usually consists of 190 amino acids. Additionally, MMP23 is the only MMP that possesses a cysteine-rich and Ig domain. Some researchers have designed MMP inhibitors according to the specific structures. Ilomastat (GM-6001), a first-generation collagen peptidomimetic, is a broad-spectrum MMP inhibitor [14][15]. However, due to its poor bioavailability, clinical trials of ilomastat have failed. However, Tanomastat (BAY 12-9566) has been shown to potently and selectively inhibit MMP13, gelatinase A, and gelatinase B. It is an analog of biphenyl non-peptide butanoic acid and was first developed by Bayer, Inc. (Leverkusen, Germany) [16]. However, no anti-MMP inhibitors with few side effects and strong specificity have been used in clinical anti-tumor therapy to date.
2.3. The Expression of MMPs (Natural MMP Inhibitors)
MMPs are generally poorly expressed in humans due to their specific endogenous inhibitors, known as the tissue inhibitor of metalloproteinase (TIMP) family, which includes four known proteins: TIMP-1, 2, 3, and 4 [17]. TIMPs can bind to the catalytic domains of MMPs with a 1:1 stoichiometric ratio and then block their enzymatic activity [18][19]. The TIMP family includes proteins with specific substrates; for example, TIMP-1 can only regulate the membrane-type MMPs, but the other three members have wider ranges of biological activity [9]. In addition to MMPs, TIMPs can suppress other enzymes, such as those of the disintegrin and metalloproteinase (ADAM) family [20][21][22].
3. MMP Expression in Endometriosis
3.1. Summary of Clinical Studies
Aside from MMP7, which is only expressed in epithelial endometrial cells, MMPs are present in both the stromal and epithelial tissue compartments of the endometrium [23]. As an invasive disorder, endometriosis involves increased MMP activity. Several studies have reported that the levels of MMPs are elevated in the ectopic tissue, peritoneal fluid, or sera of patients with endometriosis, especially MMP2 and MMP9 [24][25][26]. In addition, Borghese et al. shared gene expression profiles of eutopic. vs. ectopic endometrium in 2008 and provided a list of more than 5600 genes related to endometriosis. The overexpressed extracellular matrix genes (such as MMP23 and MMP26) showed significant expression differences [27]. However, the potential mechanism of the dysregulation of MMP23 and MMP26 expression still needs further research. Considering their key roles in endometriosis, MMPs have been considered potential therapeutic targets for this disease. As presented in Table 1, accumulating evidence has demonstrated that MMPs play significant roles in promoting the development of endometriosis.
Table 1. Changes in MMPs in endometriosis.
| Classification | MMPs | Location | Change (Endometriosis. vs. Control) | Reference |
|---|---|---|---|---|
| Collagenases | MMP1 | Eutopic endometrium Peripheral blood |
up down |
[28] [29] |
| MMP13 | Ectopic endometrium Peritoneal fluid |
up down |
[30] [31] |
|
| Gelatinases | MMP2 | Ectopic endometrium Eutopic endometrium Peripheral blood Peritoneal fluid |
up up down ns up up |
[24][32][33][34][35] [36] [37] [25][34] [24][38] [24][38] |
| MMP9 | Ectopic endometrium Eutopic endometrium Peripheral blood |
up ns down up |
[32][33][39] [25] [28] [40] |
|
| Stromelysins | MMP3 | Ectopic endometrium Eutopic endometrium Peripheral blood |
up down ns |
[34][35][41][42] [34] [29] |
| MMP10 | Ectopic endometrium | up | [35] | |
| MMP11 | Ectopic endometrium Eutopic endometrium |
up down |
[43][44] [34] |
|
| Matrilysins | MMP7 | Ectopic endometrium Peripheral blood |
up up |
[43][45][46] [45] |
| MMP26 | Ectopic endometrium | up | [27] | |
| Membrane-type MMPs | MT1-MMP | Ectopic endometrium Eutopic endometrium Peritoneal fluid |
up up down |
[33][37] [36] [31] |
| MT5-MMP | Eutopic endometrium | up | [47] | |
| Other MMPs | MMP12 | Ectopic endometrium | up | [30] |
| MMP23 | Ectopic endometrium | up | [27] |
MMP, matrix metalloproteinase; ns, no significant difference.
3.2. Complexity of MMP Regulation in Endometriosis
Over the past few years, evidence has shown that MMPs play an important role in the mechanisms involved in the occurrence and treatment of endometriosis. The epidermal growth factor receptor (EGFR)-MMP7 signaling pathway has been shown to be involved in the regulation of epithelial-mesenchymal transition (EMT) during the progression of endometriosis [45]. Moreover, chloride channel-3 (CIC3) and stress-induced phosphoprotein 1 enhance the activity of MMP9, while microRNA-33b has the opposite effect [48][49][50]. The fibrinogen alpha chain is upregulated and affects MMP2 in endometriosis [51]. Moreover, it has been shown that leptin promotes cell migration and invasion and that the cyclooxygenase-prostaglandin E2 (PGE2)-pAKT axis can promote angiogenesis via MMP2 [37][52]. In an in vivo trial, Shu et al. observed that the silencing of aquaporin 1, a water-channel protein, could influence the expression of invasion-related factors (MMP2, MMP9, TIMP1, and TIMP2), alleviating the progression of endometriosis in a mouse model [53]. Lipoxin A4 (LXA4) is a lipid medium that is widely involved in the establishment of endometriosis [54][55]. Moreover, LAX4 can suppress estrogen-mediated EMT via binding to its receptor and can inhibit the activities of MMP2 and MMP9 [56].
It is well known that the microenvironment of patients with endometriosis is inflammatory. Interleukin (IL)-2 and IL-27 synergistically inhibit MMP9 expression by maintaining the balance of interferon (IFN)-γ and IL-10, thereby improving the invasive ability of endometriosis cells [57]. Lin et al. reported that IL-34, through activating signal transducer and activator of transcription 6 (STAT6), promoted the expression of MMP9 in endometriosis in vitro and in vivo via the colony-stimulating factor 1 receptor/Janus kinase 3/STAT6 pathway [58]. Moreover, IL-37 affects downstream MMP9 expression via a variety of signaling pathways and regulates the biological behavior of endometrial stromal cells [59]. MMP2 and MMP9 can be regarded as the most typical downstream biomarkers in the progression of endometriosis. Furthermore, as shown in Figure 1, various extracellular factors, such as estrogen [60], cytokines [57][58][59], iron overload [61], and environmental contaminants [62][63], contribute to the regulation of MMPs expression.
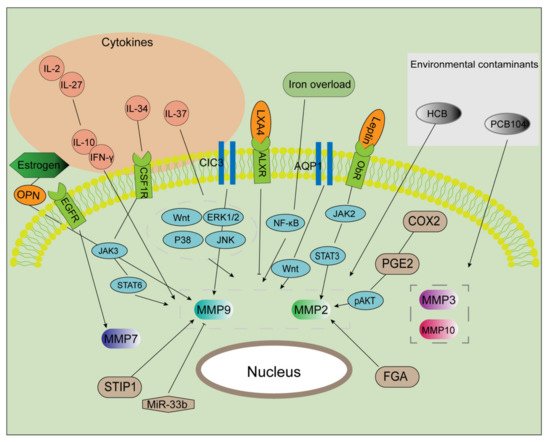
Figure 1. Multiple factors regulate MMP activities. After exposure to certain environmental contaminants (e.g., PCB104 and HCB), the expression of MMPs (MMP3, 10, 2, and 9) is markedly enhanced. IL-37 upregulates the expression of MMPs via multiple signaling pathways. IL-2 and IL-27 were found to maintain the balance of IL-10 and IFN-γ, promoting MMP2 and MMP9 expression and then inducing cell invasion and proliferation. IL-34 binds to CSF1R, which activated the JAK/STAT6 pathway in an autocrine manner. Estrogen induces MMP9 expression via the OPN. CIC3 and STIR1 improve the activity of MMP9, while miR-33b inhibits it. AQP1 promotes the expression of MMP2 and 9 via the Wnt signaling pathway. The COX2/PGE2/pAKT axis, as well as the leptin/JAK2/STAT3 axis, serves as a significant regulator in increasing MMP2 expression. Additionally, MMP2 is a target of FGA and LXA4. MMP7 is a downstream component in the EGFR-mediated signaling pathway. Iron markedly increases EMT and MMP2/9 activities in endometriosis.
MMP, matrix metalloproteinase; PCB104, polychlorinated biphenyl 104; HCB, hexachlorobenzene; CSF1R, colony-stimulating factor 1 receptor; OPN, osteopontin; CIC3, chloride channel-3; STIR1, stress-induced phosphoprotein 1; miR, microRNA; AQP1, aquaporin 1; FGA, fibrinogen alpha chain; COX2, cyclooxygenase 2; PGE2, prostaglandin E2; p, phosphorylated; JAK2, Janus kinase 2; STAT3, signal transducer and activator of transcription 3; LXA4, lipoxin A4; EMT, epithelial-mesenchymal transition.
References
- Samimi, M.; Pourhanifeh, M.H.; Mehdizadehkashi, A.; Eftekhar, T.; Asemi, Z. The role of inflammation, oxidative stress, angiogenesis, and apoptosis in the pathophysiology of endometriosis: Basic science and new insights based on gene expression. J. Cell. Physiol. 2019, 234, 19384–19392.
- Farland, L.V.; Prescott, J.; Sasamoto, N.; Tobias, D.K.; Gaskins, A.J.; Stuart, J.J.; Carusi, D.A.; Chavarro, J.E.; Horne, A.W.; Rich-Edwards, J.W.; et al. Endometriosis and Risk of Adverse Pregnancy Outcomes. Obstet. Gynecol. 2019, 134, 527–536.
- Zondervan, K.T.; Becker, C.M.; Koga, K.; Missmer, S.A.; Taylor, R.N.; Vigano, P. Endometriosis. Nat. Rev. Dis. Primers 2018, 4, 9.
- Tsang, K.Y.; Cheung, M.C.; Chan, D.; Cheah, K.S. The developmental roles of the extracellular matrix: Beyond structure to regulation. Cell Tissue Res. 2010, 339, 93–110.
- Gross, J.; Lapiere, C.M. Collagenolytic activity in amphibian tissues: A tissue culture assay. Proc. Natl. Acad. Sci. USA 1962, 48, 1014–1022.
- Pitsos, M.; Kanakas, N. The role of matrix metalloproteinases in the pathogenesis of endometriosis. Reprod. Sci. 2009, 16, 717–726.
- Kapoor, C.; Vaidya, S.; Wadhwan, V.; Kaur, G.; Pathak, A. Seesaw of matrix metalloproteinases (MMPs). J. Cancer Res. Ther. 2016, 12, 28–35.
- Quintero-Fabian, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argaez, V.; Lara-Riegos, J.; Ramirez-Camacho, M.A.; Alvarez-Sanchez, M.E. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front. Oncol. 2019, 9, 1370.
- Balkowiec, M.; Maksym, R.B.; Wlodarski, P.K. The bimodal role of matrix metalloproteinases and their inhibitors in etiology and pathogenesis of endometriosis (Review). Mol. Med. Rep. 2018, 18, 3123–3136.
- Garcia-Fernandez, N.; Jacobs-Cacha, C.; Mora-Gutierrez, J.M.; Vergara, A.; Orbe, J.; Soler, M.J. Matrix Metalloproteinases in Diabetic Kidney Disease. J. Clin. Med. 2020, 9, 472.
- Stawowczyk, M.; Wellenstein, M.D.; Lee, S.B.; Yomtoubian, S.; Durrans, A.; Choi, H.; Narula, N.; Altorki, N.K.; Gao, D.; Mittal, V. Matrix Metalloproteinase 14 promotes lung cancer by cleavage of Heparin-Binding EGF-like Growth Factor. Neoplasia 2017, 19, 55–64.
- Wang, X.; Khalil, R.A. Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease. Adv. Pharmacol. 2018, 81, 241–330.
- Fischer, T.; Riedl, R. Inhibitory Antibodies Designed for Matrix Metalloproteinase Modulation. Molecules 2019, 24, 2265.
- Agren, M.S.; Mirastschijski, U.; Karlsmark, T.; Saarialho-Kere, U.K. Topical synthetic inhibitor of matrix metalloproteinases delays epidermal regeneration of human wounds. Exp. Dermatol. 2001, 10, 337–348.
- Hao, J.L.; Nagano, T.; Nakamura, M.; Kumagai, N.; Mishima, H.; Nishida, T. Effect of galardin on collagen degradation by Pseudomonas aeruginosa. Exp. Eye Res. 1999, 69, 595–601.
- Gatto, C.; Rieppi, M.; Borsotti, P.; Innocenti, S.; Ceruti, R.; Drudis, T.; Scanziani, E.; Casazza, A.M.; Taraboletti, G.; Giavazzi, R. BAY 12-9566, a novel inhibitor of matrix metalloproteinases with antiangiogenic activity. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 1999, 5, 3603–3607.
- Lockhart, A.C.; Braun, R.D.; Yu, D.; Ross, J.R.; Dewhirst, M.W.; Humphrey, J.S.; Thompson, S.; Williams, K.M.; Klitzman, B.; Yuan, F.; et al. Reduction of wound angiogenesis in patients treated with BMS-275291, a broad spectrum matrix metalloproteinase inhibitor. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2003, 9, 586–593.
- Egeblad, M.; Werb, Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer 2002, 2, 161–174.
- Zakiyanov, O.; Kalousova, M.; Zima, T.; Tesar, V. Matrix Metalloproteinases in Renal Diseases: A Critical Appraisal. Kidney Blood Press. Res. 2019, 44, 298–330.
- Rai, G.P.; Baird, S.K. Tissue inhibitor of matrix metalloproteinase-3 has both anti-metastatic and anti-tumourigenic properties. Clin. Exp. Metastasis 2020, 37, 69–76.
- Fujii, T.; Duarte, S.; Lee, E.; Ke, B.; Busuttil, R.W.; Coito, A.J. Tissue Inhibitor of Metalloproteinase 3 Deficiency Disrupts the Hepatocyte E-Cadherin/beta-Catenin Complex and Induces Cell Death in Liver Ischemia/Reperfusion Injury. Liver Transplant. 2020, 26, 113–126.
- Opdenakker, G.; Abu El-Asrar, A. Metalloproteinases mediate diabetes-induced retinal neuropathy and vasculopathy. Cell. Mol. Life Sci. 2019, 76, 3157–3166.
- Rodgers, W.H.; Osteen, K.G.; Matrisian, L.M.; Navre, M.; Giudice, L.C.; Gorstein, F. Expression and localization of matrilysin, a matrix metalloproteinase, in human endometrium during the reproductive cycle. Am. J. Obstet. Gynecol. 1993, 168, 253–260.
- Sui, X.; Li, Y.; Sun, Y.; Li, C.; Li, X.; Zhang, G. Expression and significance of autophagy genes LC3, Beclin1 and MMP-2 in endometriosis. Exp. Ther. Med. 2018, 16, 1958–1962.
- Szymanowski, K.; Mikolajczyk, M.; Wirstlein, P.; Dera-Szymanowska, A. Matrix metalloproteinase-2 (MMP-2), MMP-9, tissue inhibitor of matrix metalloproteinases (TIMP-1) and transforming growth factor-beta2 (TGF-beta2) expression in eutopic endometrium of women with peritoneal endometriosis. Ann. Agric. Environ. Med. 2016, 23, 649–653.
- Kodarahmian, M.; Amidi, F.; Moini, A.; Kashani, L.; Shabani Nashtaei, M.; Pazhohan, A.; Bahramrezai, M.; Berenjian, S.; Sobhani, A. The modulating effects of Resveratrol on the expression of MMP-2 and MMP-9 in endometriosis women: A randomized exploratory trial. Off. J. Int. Soc. Gynecol. Endocrinol. 2019, 35, 719–726.
- Borghese, B.; Mondon, F.; Noël, J.C.; Fayt, I.; Mignot, T.M.; Vaiman, D.; Chapron, C. Gene expression profile for ectopic versus eutopic endometrium provides new insights into endometriosis oncogenic potential. Mol. Endocrinol. 2008, 22, 2557–2562.
- Pino, M.; Galleguillos, C.; Torres, M.; Sovino, H.; Fuentes, A.; Boric, M.A.; Johnson, M.C. Association between MMP1 and MMP9 activities and ICAM1 cleavage induced by tumor necrosis factor in stromal cell cultures from eutopic endometria of women with endometriosis. Reproduction 2009, 138, 837–847.
- Shan, K.; Ying, W.; Jian-Hui, Z.; Wei, G.; Na, W.; Yan, L. The function of the SNP in the MMP1 and MMP3 promoter in susceptibility to endometriosis in China. Mol. Hum. Reprod. 2005, 11, 423–427.
- Borghese, B.; Chiche, J.D.; Vernerey, D.; Chenot, C.; Mir, O.; Bijaoui, G.; Bonaiti-Pellie, C.; Chapron, C. Genetic polymorphisms of matrix metalloproteinase 12 and 13 genes are implicated in endometriosis progression. Hum. Reprod. 2008, 23, 1207–1213.
- Laudanski, P.; Szamatowicz, J.; Ramel, P. Matrix metalloproteinase-13 and membrane type-1 matrix metalloproteinase in peritoneal fluid of women with endometriosis. Gynecol. Endocrinol. 2005, 21, 106–110.
- Weigel, M.T.; Kramer, J.; Schem, C.; Wenners, A.; Alkatout, I.; Jonat, W.; Maass, N.; Mundhenke, C. Differential expression of MMP-2, MMP-9 and PCNA in endometriosis and endometrial carcinoma. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 160, 74–78.
- Ueda, M.; Yamashita, Y.; Takehara, M.; Terai, Y.; Kumagai, K.; Ueki, K.; Kanda, K.; Hung, Y.C.; Ueki, M. Gene expression of adhesion molecules and matrix metalloproteinases in endometriosis. Off. J. Int. Soc. Gynecol. Endocrinol. 2002, 16, 391–402.
- Uzan, C.; Cortez, A.; Dufournet, C.; Fauvet, R.; Siffroi, J.P.; Daraï, E. Eutopic endometrium and peritoneal, ovarian and bowel endometriotic tissues express a different profile of matrix metalloproteinases-2, -3 and -11, and of tissue inhibitor metalloproteinases-1 and -2. Virchows Arch. 2004, 445, 603–609.
- Luddi, A.; Marrocco, C.; Governini, L.; Semplici, B.; Pavone, V.; Luisi, S.; Petraglia, F.; Piomboni, P. Expression of Matrix Metalloproteinases and Their Inhibitors in Endometrium: High Levels in Endometriotic Lesions. Int. J. Mol. Sci. 2020, 21, 2840.
- Chung, H.W.; Lee, J.Y.; Moon, H.S.; Hur, S.E.; Park, M.H.; Wen, Y.; Polan, M.L. Matrix metalloproteinase-2, membranous type 1 matrix metalloproteinase, and tissue inhibitor of metalloproteinase-2 expression in ectopic and eutopic endometrium. Fertil. Steril. 2002, 78, 787–795.
- Jana, S.; Chatterjee, K.; Ray, A.K.; DasMahapatra, P.; Swarnakar, S. Regulation of Matrix Metalloproteinase-2 Activity by COX-2-PGE2-pAKT Axis Promotes Angiogenesis in Endometriosis. PLoS ONE 2016, 11, e0163540.
- Huang, H.F.; Hong, L.H.; Tan, Y.; Sheng, J.Z. Matrix metalloproteinase 2 is associated with changes in steroid hormones in the sera and peritoneal fluid of patients with endometriosis. Fertil. Steril. 2004, 81, 1235–1239.
- Paul, S.; Sharma, A.V.; Mahapatra, P.D.; Bhattacharya, P.; Reiter, R.J.; Swarnakar, S. Role of melatonin in regulating matrix metalloproteinase-9 via tissue inhibitors of metalloproteinase-1 during protection against endometriosis. J. Pineal Res. 2008, 44, 439–449.
- Bostanci Durmus, A.; Dincer Cengiz, S.; Yilmaz, H.; Candar, T.; Gursoy, A.Y.; Sinem Caglar, G. The levels of matrix metalloproteinase-9 and neutrophil gelatinase-associated lipocalin in different stages of endometriosis. J. Inst. Obstet. Gynaecol. 2019, 39, 991–995.
- Jana, S.; Paul, S.; Swarnakar, S. Curcumin as anti-endometriotic agent: Implication of MMP-3 and intrinsic apoptotic pathway. Biochem. Pharmacol. 2012, 83, 797–804.
- Lv, X.; Chen, P.; Liu, W. Down regulation of MiR-93 contributes to endometriosis through targeting MMP3 and VEGFA. Am. J. Cancer Res. 2015, 5, 1706–1717.
- Vallvé-Juanico, J.; López-Gil, C.; Ponomarenko, J.; Melnychuk, T.; Castellví, J.; Ballesteros, A.; Colás, E.; Gil-Moreno, A.; Santamaria Costa, X. External validation of putative biomarkers in eutopic endometrium of women with endometriosis using NanoString technology. J. Assist. Reprod. Genet. 2020, 37, 2981–2987.
- Feng, X.; Qi, L.; Xu, X.; Feng, Y.; Gong, X.; Aili, A.; Chen, Y.; Xue, Z.; Xue, J.; Tong, X. Analysis of differences in the transcriptomic profiles of eutopic and ectopic endometriums in women with ovarian endometriosis. PeerJ 2021, 9, e11045.
- Chatterjee, K.; Jana, S.; DasMahapatra, P.; Swarnakar, S. EGFR-mediated matrix metalloproteinase-7 up-regulation promotes epithelial-mesenchymal transition via ERK1-AP1 axis during ovarian endometriosis progression. FASEB J. 2018, 32, 4560–4572.
- Itoh, H.; Mogami, H.; Bou Nemer, L.; Word, L.; Rogers, D.; Miller, R.; Word, R.A. Endometrial stromal cell attachment and matrix homeostasis in abdominal wall endometriomas. Hum. Reprod. 2018, 33, 280–291.
- Gaetje, R.; Holtrich, U.; Engels, K.; Kourtis, K.; Cikrit, E.; Kissler, S.; Rody, A.; Karn, T.; Kaufmann, M. Expression of membrane-type 5 matrix metalloproteinase in human endometrium and endometriosis. Gynecol. Endocrinol. 2007, 23, 567–573.
- Guan, Y.T.; Huang, Y.Q.; Wu, J.B.; Deng, Z.Q.; Wang, Y.; Lai, Z.Y.; Wang, H.B.; Sun, X.X.; Zhu, Y.L.; Du, M.M.; et al. Overexpression of chloride channel-3 is associated with the increased migration and invasion ability of ectopic endometrial cells from patients with endometriosis. Hum. Reprod. 2016, 31, 986–998.
- Wang, H.S.; Tsai, C.L.; Chang, P.Y.; Chao, A.; Wu, R.C.; Chen, S.H.; Wang, C.J.; Yen, C.F.; Lee, Y.S.; Wang, T.H. Positive associations between upregulated levels of stress-induced phosphoprotein 1 and matrix metalloproteinase-9 in endometriosis/adenomyosis. PLoS ONE 2018, 13, e0190573.
- Yang, W.W.; Hong, L.; Xu, X.X.; Wang, Q.; Huang, J.L.; Jiang, L. Regulation of miR-33b on endometriosis and expression of related factors. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2027–2033.
- Chen, Y.; Li, H.; Cheng, H.Y.; Rui-Qiong, M.; Ye, X.; Cui, H.; Hong-Lan, Z.; Chang, X.H. Fibrinogen alpha chain is up-regulated and affects the pathogenesis of endometriosis. Reprod. Biomed. Online 2019, 39, 893–904.
- Ahn, J.H.; Choi, Y.S.; Choi, J.H. Leptin promotes human endometriotic cell migration and invasion by up-regulating MMP-2 through the JAK2/STAT3 signaling pathway. Mol. Hum. Reprod. 2015, 21, 792–802.
- Shu, C.; Shu, Y.; Gao, Y.; Chi, H.; Han, J. Inhibitory effect of AQP1 silencing on adhesion and angiogenesis in ectopic endometrial cells of mice with endometriosis through activating the Wnt signaling pathway. Cell Cycle 2019, 18, 2026–2039.
- Chen, S.; Wu, R.F.; Su, L.; Zhou, W.D.; Zhu, M.B.; Chen, Q.H. Lipoxin A4 regulates expression of the estrogen receptor and inhibits 17beta-estradiol induced p38 mitogen-activated protein kinase phosphorylation in human endometriotic stromal cells. Fertil. Steril. 2014, 102, 264–271.
- Wu, R.; Zhou, W.; Chen, S.; Shi, Y.; Su, L.; Zhu, M.; Chen, Q.; Chen, Q. Lipoxin A4 suppresses the development of endometriosis in an ALX receptor-dependent manner via the p38 MAPK pathway. Br. J. Pharmacol. 2014, 171, 4927–4940.
- Wu, R.F.; Huang, Z.X.; Ran, J.; Dai, S.J.; Lin, D.C.; Ng, T.W.; Chen, Q.X.; Chen, Q.H. Lipoxin A4 Suppresses Estrogen-Induced Epithelial-Mesenchymal Transition via ALXR-Dependent Manner in Endometriosis. Reprod. Sci. 2018, 25, 566–578.
- Qiu, X.M.; Lai, Z.Z.; Ha, S.Y.; Yang, H.L.; Liu, L.B.; Wang, Y.; Shi, J.W.; Ruan, L.Y.; Ye, J.F.; Wu, J.N.; et al. IL-2 and IL-27 synergistically promote growth and invasion of endometriotic stromal cells by maintaining the balance of IFN-γ and IL-10 in endometriosis. Reproduction 2020, 159, 251–260.
- Lin, K.; Ma, J.; Peng, Y.; Sun, M.; Xu, K.; Wu, R.; Lin, J. Autocrine Production of Interleukin-34 Promotes the Development of Endometriosis through CSF1R/JAK3/STAT6 signaling. Sci. Rep. 2019, 9, 16781.
- Jiang, J.; Yu, K.; Jiang, Z.; Xue, M. IL-37 affects the occurrence and development of endometriosis by regulating the biological behavior of endometrial stromal cells through multiple signaling pathways. Biol. Chem. 2018, 399, 1325–1337.
- Yang, M.; Jiang, C.; Chen, H.; Nian, Y.; Bai, Z.; Ha, C. The involvement of osteopontin and matrix metalloproteinase- 9 in the migration of endometrial epithelial cells in patients with endometriosis. Reprod. Biol. Endocrinol. 2015, 13, 95.
- Woo, J.H.; Choi, Y.S.; Choi, J.H. Iron-Storage Protein Ferritin Is Upregulated in Endometriosis and Iron Overload Contributes to a Migratory Phenotype. Biomedicines 2020, 8, 454.
- Chiappini, F.; Sanchez, M.; Miret, N.; Cocca, C.; Zotta, E.; Ceballos, L.; Pontillo, C.; Bilotas, M.; Randi, A. Exposure to environmental concentrations of hexachlorobenzene induces alterations associated with endometriosis progression in a rat model. Food Chem. Toxicol. 2019, 123, 151–161.
- Hu, T.; Yao, M.; Fu, X.; Chen, C.; Wu, R. Polychlorinated biphenyl 104 promotes migration of endometrial stromal cells in endometriosis. Toxicol. Lett. 2018, 290, 19–28.
More
Information
Subjects:
Obstetrics & Gynaecology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
882
Revisions:
2 times
(View History)
Update Date:
14 Dec 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




