Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marcin Dębowski | + 3519 word(s) | 3519 | 2021-12-08 03:22:20 | | | |
| 2 | Bruce Ren | Meta information modification | 3519 | 2021-12-10 08:53:20 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Dębowski, M. Biogas Production from Maize Silage after Acid-Heat Pretreatment. Encyclopedia. Available online: https://encyclopedia.pub/entry/16973 (accessed on 07 February 2026).
Dębowski M. Biogas Production from Maize Silage after Acid-Heat Pretreatment. Encyclopedia. Available at: https://encyclopedia.pub/entry/16973. Accessed February 07, 2026.
Dębowski, Marcin. "Biogas Production from Maize Silage after Acid-Heat Pretreatment" Encyclopedia, https://encyclopedia.pub/entry/16973 (accessed February 07, 2026).
Dębowski, M. (2021, December 10). Biogas Production from Maize Silage after Acid-Heat Pretreatment. In Encyclopedia. https://encyclopedia.pub/entry/16973
Dębowski, Marcin. "Biogas Production from Maize Silage after Acid-Heat Pretreatment." Encyclopedia. Web. 10 December, 2021.
Copy Citation
One of the most effective technologies involving the use of lignocellulosic biomass is the production of biofuels, including methane-rich biogas. In order to increase the amount of gas produced, it is necessary to optimize the fermentation process, for example, by substrate pretreatment.
microwave
pretreatment
acid pretreatment
methane fermentation
biogas
lignocellulose
1. Introduction
A detailed objective of the “Polish Energy Policy until 2040” is the development of renewable energy sources, including biogas technologies in energy and transport. The document emphasizes that biomass is the only renewable source of a raw material character. Energy use of biomass—both thermal and anaerobic (biogas), in biogas plants, and for the production of biofuels—will increase, and the energy sector should use, in particular, waste biomass [1]. Currently, according to the register of agricultural biogas plants of the Agricultural Market Agency, in 2021 the number of agricultural biogas plants in Poland is 108. According to the presented data, agricultural biogas is becoming an increasingly important participant in the Polish energy market [2].
The use of renewable energy sources is important, not only because it allows for reducing the imported energy volumes, but also because it paves the way for the development of diversified energetics based on locally-available resources [3]. Lignocellulosic biomass represents a cheap, easily-available, and proliferative raw material [4]. Biofuels produced from biomass can offer a solution to the problem of dependence on petroleum and help reduce CO2 emissions to the atmosphere. Reducing the amounts of greenhouse gases released into the atmosphere will contribute significantly to counteracting the global warming effect. One of the most effective technologies involving the use of lignocellulosic biomass is the production of biofuels, including methane-rich biogas [5]. However, in order to use the biogas generated in biogas plants for energy and transport purposes, it is necessary to upgrade the biogas to the quality of biomethane, as a fuel for vehicles. The biogas has to undergo a subsequent treatment of halogenated siloxanes, ammonia, and volatile organic compounds, as well as the separation of gases, mainly CO2. The trends in recent years in Polish industrial practice are the separation of CO2 by absorption, adsorption, or biofiltration [6].
Due to the high oxygen to carbon ratio, lignocellulose is a weaker source of energy than fossil fuels; hence, the goal of methane fermentation is to reduce this ratio [7][8][9]. The advantage of anaerobic digestion lies in the by-products it generates, some of which are valuable chemical compounds, such as organic acids, vanillin, fatty acid methyl esters, fertilizers, and nutrients. In order to increase the amount of gas produced, it is necessary to optimize the fermentation process by, e.g., substrate pretreatment. Despite the fact that these treatment methods have been addressed in scientific research for many years, there is still a justified need to find an efficient, environmentally friendly, relatively cheap, and simple pretreatment process that would not pose a risk to the formation of large amounts of inhibitor [10].
The commonly applied pre-treatment techniques can be divided into physical, physicochemical, chemical, and biological ones [11][12]. Comparing the methods of pretreatment, attention should be paid to the effects of their operation, especially the degree of delignification, reduction of cellulose crystallinity, destruction of hemicelluloses, increase in the available surface area, and formation of fermentation inhibitors [13]. The deployment of chemical and chemical–thermal disintegration methods of lignocellulosic biomass carries a risk of the formation of fermentation inhibitors, such as furan derivatives and phenolic compounds. Furan derivatives, i.e., furfural and 5-hydroxymethylfurfural (5-HMF), are formed by the dehydration of pentoses and hexoses, respectively. In turn, multiple phenolic compounds (e.g., vanillin, 4-hydroxybenzaldehyde) are generated during lignin breakdown. These compounds are potent inhibitors of methane production [14][15].
Furan and phenolic compounds elicit toxic effects by inhibiting the enzymatic hydrolysis of fermentation bacteria, and contribute to the formation of reactive oxygen species (ROS), which affect cell metabolism and apoptosis. Furfural and 5-HMF inhibit cell growth, induce DNA damage, and inhibit some enzymes of the glycolysis pathway [16]. Phenolic compounds cause damage to bacterial cells by modifying cellular membrane permeability, and thereby causing the leakage of intracellular components and the inactivation of enzymatic systems [17]. It has also been observed that the low-molecular-weight phenolics are more toxic than those having a higher molecular weight [18]. Microorganisms differ between each other in their capability to adapt to and grow in an environment rich in toxic substances. They have also developed various adaptation mechanisms, the task of which is to avoid or repair damages. When it comes to the inhibitors formed during lignocellulose treatment, there are some reports about certain mechanisms of molecular adaptation and activities of certain bacterial groups. Selected bacterial species have been proved capable of converting cis-unsaturated fatty acids into trans-fatty acids at a cellular membrane level [19]. The structural difference in the surface of Gram-positive and Gram-negative bacterial cells affects their resistance to the effect of toxic substances; however, this effect is mainly species-dependent [20]. Certain bacteria can directly convert or damage furan compounds by using them as an additional carbon source, this capability has especially been observed in the case of Gram-negatives aerobes. Under anaerobic conditions, some bacteria can convert furfural and 5-HMF to furfuryl compounds and HMF alcohols [21].
Pretreatment may also affect the fertilization value of the digestate. Chemical pretreatment can affect the quality of the digestate. There are literature reports on the influence of KOH and NaOH on the fertilization value of the digestate. The literature shows that the use of KOH during pretreatment increases the amount of potassium and ammonia in the post-fermentation, while the addition of NaOH during the treatment may adversely affect the salinity of the soil, due to a significant increase in sodium in the post-fermentation [22].
The pros and cons of the most frequently applied pretreatment methods are collated in Table 1.
| Pretreatment Method | Advantages | Disadvantages |
|---|---|---|
| Mechanical disintegration | Cellulose crystallinity reduction | Energy consumption exceeding the amount of energy produced |
| Steam explosion | Hemicellulose degradation; lignin transformation; high cost-effectiveness | Damage to a part of xylans; incomplete disruption of the lignin–carbohydrate matrix; formation of inhibitors |
| Ammonia fiber explosion | Increasing the specific surface area; removal of lignin and cellulose; lack of inhibitors | No effects in the case of high-lignin substrates |
| Effects of carbon dioxide | Increasing the specific surface area; cost-effectiveness; no inhibitors | No effect on lignin and hemicellulose |
| Acidic | Hydrolysis of hemicellulose to xylose and other sugars; lignin structure modification | High costs; potential corrosion-inducing effect; formation of inhibitors |
| Alkaline | Removal of lignin and hemicellulose; increasing the specific surface area | Time-consuming |
| Biological | Degradation of lignin and hemicellulose; low energy requirement | Very slow course of hydrolysis |
A less popular method entails microwave–chemical pretreatment, which merges the advantages of thermal and chemical methods. Previous investigations have proven microwave treatment to be more effective than pretreatment via conventional heating [24][25]. Microwave radiation represents a method of electromagnetic energy conversion into heat energy. The capability of microwaves for the volumetric and direct heating of materials enables accelerating the course of the reaction and affecting properties of the heated materials. The selectivity of microwaves towards more polar substances leads to uneven heating of the material’s interior, as a result of which, the lignocellulosic complex is degraded. Microwave treatment entails thermal and non-thermal effects elicited by the microwave radiation in the presence of water. The thermal effects consist in the production of internal heat by radiation, due to a local temperature increase, resulting in the formation of so-called rapid local heating. The non-thermal activity of microwaves induces vibrations of polar bonds, resulting in their disruption and in increased rates of chemical and physical processes. The high radiation energy leads to structural changes of the lignocellulosic biomass, increases the specific surface area, reduces the polymerization degree and crystallinity of cellulose, and increases the rate of hemicellulose and lignin hydrolysis. Similar effects are observed in the case of microwave treatments aided with chemical agents. Microwave radiation can be deployed during pretreatment involving the use of acids [26][27][28] Acid pretreatment leads to the formation of monomeric sugars (xylose, mannose, arabinose, galactose, and glucose), followed by the depolymerization of cellulose. Cellulose is broken down to form celooligosaccharides, which are then hydrolyzed to monomeric sugars [29][30][31]. A microwave-pretreated substrate features a higher rate of hydrolysis and a high content of soluble sugars in the hydrolysate [26][27][28][32]. Zhu et al. researched microwave-assisted sodium hydroxide and sulfuric acid pretreatment of lignocellulosic biomass. Microwave pretreatment was more efficient than conventional heating [28] According to the literature, the effect of using microwave radiation on increasing the amount of secreted sugars is impressive, while the effect of microwave treatment on biogas production is not entirely clear [31]. Jackowiak achieved a 68% increase in biogas production from switchgrass and 28% for wheat straw using microwave treatment [33][34]. Similarly, Kan et al., for brewer’s spent grain after the use of microwaves, reported a 52% increase in the amount of biogas. Sapci, on the other hand, did not record an increase in biogas production when using microwave treatment in a temperature range from 200 to 300 °C [35].
In Poland, methane fermentation of plant biomass is responsible for 13–32% of the produced biogas. Maize is a popular raw material for the production of biogas, according to the estimates of the Polish Corn Producers Association, the acreage of maize production in Poland in 2018 amounted to approximately 1,191,000 ha. This means that it is one of the most popular plants in the country. The advantage of maize is that it is characterized by high yields per hectare and good susceptibility to ensilage, and it guarantees stable biogas production [36]. Although maize is such a common source of biomass, literature reports on the impact of microwave or a combined (microwave-chemical) treatment on the methane fermentation process of maize silage are very scarce. Most of the available reports focus on the use of thermal treatments based on conventional heating.
2. Biogas Production from Maize Silage after Acid-Heat Pretreatment
In the present study, the substrate was first mechanically disintegrated and then subjected to the coupled hydrothermal and acid treatment. In order to establish the most effective method of maize silage conditioning, various doses of chemical reagents, added to the substrate, were tested and then the samples were either microwave- or conventionally-heated at a temperature of 150 °C for 20 min. The temperature and duration of heating were established in the preliminary research and supported by literature data, indicating that lignin and hemicellulose begin to degrade at pretreatment temperatures of 150–180 °C [37]. Gregg and Saddler proved that heat treatments result, not only in hemicellulose destabilization, but also in lignin dissolution [38]. In turn, Brownell and Saddler recommended avoiding temperatures over 250 °C during heat treatment [39].
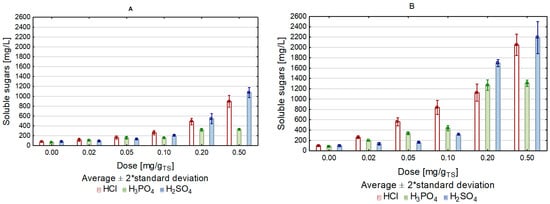
The amount of soluble sugars released to the solution as a result of hydrothermal conditioning with each tested dose of acids was higher than that determined after conventional heating. Biomass pretreatment with sulfuric acid caused a greater release of sugars during the microwave than conventional heating (Figure 1). This was particularly true for the microwave heating with the highest tested dose of H2SO4 (0.4 g/gTS), when the glucose concentration in the solution was 2.04 times higher compared to the treatment variant with conventional heating. In the case of the control sample microwave-heated without acid, the concentration of released glucose reached 96.3 ± 8.08 mg/L, and increased to 2205.7 ± 40.18 mg/L in the best acid dose variant (0.4 g/gTS), which accounted for 11.57% of the theoretical hydrolysis yield and was the highest value noted among all values determined for all acid doses and types (Table 2). Gabhane et al. [40] studied acid pretreatment of banana wastes. They used different power sources: microwave, ultra-sonification, and autoclaving. The highest sugar yield was observed in the acid–microwave pretreatment, as in the presented results of the authors’ research. Vasconcelos et al. studied a sugarcane biomass pretreatment with diluted H3PO4, and the maximum saccharification was recorded during the pretreatment at 186℃ for 8 min [41]. Kaur and Phutela [42], Lag et al. [43], and Binod et al. [27] also noted an increase in the release of sugars after microwave treatments of paddy straw, coconut husks, and sugarcane, respectively.

Figure 1. Content of soluble sugars depending on the heating method and acid dose ((A)—conventional, (B)—microwave).
Table 2. Hydrolysis yield depending on heating method and acid dose.
| Acid Dose (g/gTS) | Hydrolysis Yield (Addition of HCl) (%) | Hydrolysis Yield (Addition of H3PO4) (%) | Hydrolysis Yield (Addition of H2SO4) (%) |
|---|---|---|---|
| Conventional heating | |||
| 0.0 | 0.41 ± 0.00 | 0.35 ± 0.02 | 0.42 ± 0.04 |
| 0.02 | 0.63 ± 0.11 | 0.57 ± 0.04 | 0.48 ± 0.04 |
| 0.05 | 0.86 ± 0.10 | 0.84 ± 0.10 | 0.69 ± 0.02 |
| 0.1 | 1.38 ± 0.14 | 0.82 ± 0.05 | 1.07 ± 0.03 |
| 0.2 | 2.58 ± 0.22 | 1.69 ± 0.09 | 2.89 ± 0.27 |
| 0.4 | 4.75 ± 0.38 | 1.71 ± 0.07 | 5.70 ± 0.27 |
| Microwave heating | |||
| 0.0 | 0.51 ± 0.02 | 0.45 ± 0.02 | 0.51 ± 0.04 |
| 0.02 | 1.45 ± 0.07 | 1.01 ± 0.03 | 0.72 ± 0.06 |
| 0.05 | 2.96 ± 0.25 | 1.78 ± 0.08 | 0.82 ± 0.03 |
| 0.1 | 4.44 ± 0.44 | 2.30 ± 0.17 | 1.64 ± 0.02 |
| 0.2 | 5.95 ± 0.53 | 6.72 ± 0.33 | 8.98 ± 0.19 |
| 0.4 | 10.84 ± 0.66 | 6.91 ± 0.20 | 11.57 ± 0.22 |
When deploying thermal hydrolysis, attention should be paid to the possibility of adverse effects, such as the production of phenolic and furan compounds that elicit toxic effects and inhibit the growth of methanogenic bacteria [44]. Diaz et al., who investigated the effectiveness of bioethanol production from sunflower stems, determined the contents of non-saccharide products of thermal pretreatment, including furfural and 5–HMF. They demonstrated an increase in the contents of the analyzed compounds at pretreatment temperatures above 190 °C; however, they reported that their content in the hydrolysate was relatively low compared to the predicted content computed based on the loss of pentoses in the substrate [45]. 5–HMF and furfural were also detected during acidic pretreatment of lignocellulosic biomass by Manlau et al., in their study addressing the fermentation of sunflower stems. They analyzed eight variants of substrate pretreatment, including acidic pretreatment with HCl and FeCl3. During the hydrothermal pretreatment conducted at 170 °C for 1 h without chemical agents, furfural was released in an amount of 0.7 g/100 gTS. Its concentration increased in the pretreatment variants with chemical agents added. The pretreatment conducted under the same conditions but with HCl caused furfural and 5–HMF release at 4.1/100 gTS and 0.4 g/100 gTS, respectively; whereas, 5–HMF was not detected in the substrate subjected to hydrothermal pretreatment without acid addition. The hydrothermal pretreatment with FeCl3 caused furfural and 5–HMF release in the quantities: 2.4 g/100 gTS and 0.3 g/100 gTS, respectively [46]. Many scientists have proven the formation of adverse substances during acidic pretreatment of various types of biomass, e.g., cassava, rice husks, rice straw, spruce, sugar cane, microalgae, and macroalgae. High-temperature pretreatment conducted at 120–240 °C using H2SO4, HCl, or H3PO4 was reported to contribute to the release of 0.1–13.32 g/L of furfural and 0–4.3 g/L of 5–HMF in the mentioned plants [15].
When comparing the present study results with the literature during the experiment, the soluble sugars/furans and soluble sugars/furans and phenols ratio after the addition of acids in doses from 0.02 to 0.1 g/gTS was low, which indicated a large amount of inhibitory compounds, with a small amount of released soluble sugars. In the case of using larger amounts of acids, the ratio increased, the maximum was 3.77 for H2SO4, in an amount of 0.2 g/gTS. (Table 3). As Bondesson et al. reported, the soluble sugars/furans ratio achieved during heat treatment of corn stover at 200 °C ranged from 2 to 10.4. A significantly higher value of this ratio, i.e., 26.8, was reported after acidic pretreatment of spruce at 150 °C in the presence of H2SO4 [47]. The literature indicates that the use of dilute acids for pretreatment is too low for furfural and 5-HMF, as was the case in the described experiment, in which 10% acid was used [23][48][49].
Table 3. Generation of by-products, depending on heating method and acid dose.
| Dose | HCl | H3PO4 | H2SO4 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Soluble Sugars (mg/L) | By-Products Concentration (mg/L) | Σ Soluble Sugars/Σ Furans | Σ Soluble Sugars/Σ Furans and Phenols | Soluble Sugars (mg/L) | By-Products Concentration (mg/L) | Σ Soluble Sugars/Σ Furans | Σ Soluble Sugars/Σ Furans and Phenols | Soluble Sugars (mg/L) | By-Products Concentration (mg/L) | Σ Soluble Sugars/Σ Furans | Σ Soluble Sugars/Σ Furans and Phenols | |||||||
| Furfural | 5-HMF | Phenols | Furfural | 5-HMF | Phenols | Furfural | 5-HMF | Phenols | ||||||||||
| Conventional heating | ||||||||||||||||||
| 0 | 78.7 | 70 | 32 | 23 | 0.77 | 0.63 | 65.3 | 75 | 33 | 21 | 0.60 | 0.51 | 81.2 | 69 | 35 | 20 | 0.78 | 0.65 |
| 0.02 | 118.1 | 98 | 45 | 22 | 0.83 | 0.72 | 109.5 | 101 | 28 | 25 | 0.85 | 0.71 | 92.3 | 98 | 42 | 30 | 0.66 | 0.54 |
| 0.05 | 163.3 | 110 | 48 | 56 | 1.03 | 0.76 | 159.3 | 120 | 48 | 48 | 0.95 | 0.74 | 130.3 | 115 | 57 | 55 | 0.76 | 0.57 |
| 0.1 | 260.7 | 290 | 65 | 89 | 0.73 | 0.59 | 155.7 | 305 | 46 | 65 | 0.44 | 0.37 | 202.4 | 305 | 63 | 78 | 0.55 | 0.45 |
| 0.2 | 487.7 | 330 | 112 | 110 | 1.10 | 0.88 | 318.7 | 535 | 138 | 183 | 0.47 | 0.37 | 545.7 | 358 | 115 | 98 | 1.15 | 0.96 |
| 0.4 | 897.3 | 482 | 185 | 158 | 1.35 | 1.09 | 323.1 | 702 | 215 | 335 | 0.35 | 0.26 | 1076.7 | 498 | 171 | 134 | 1.61 | 1.34 |
| Microwave heating | ||||||||||||||||||
| 0 | 98.2 | 78 | 35 | 28 | 1.24 | 0.70 | 85.7 | 81 | 34 | 26 | 0.75 | 0.61 | 96.3 | 77 | 31 | 24 | 0.89 | 0.73 |
| 0.02 | 275.7 | 118 | 42 | 28 | 1.69 | 1.47 | 192.3 | 105 | 27 | 25 | 1.46 | 1.22 | 135.3 | 98 | 45 | 27 | 0.95 | 0.80 |
| 0.05 | 559.3 | 250 | 56 | 65 | 2.07 | 1.51 | 337.8 | 116 | 46 | 41 | 2.09 | 1.66 | 156.7 | 104 | 59 | 48 | 0.96 | 0.74 |
| 0.1 | 838.0 | 308 | 120 | 89 | 1.47 | 1.62 | 435.2 | 317 | 57 | 67 | 1.16 | 0.99 | 310.2 | 287 | 67 | 81 | 0.88 | 0.71 |
| 0.2 | 1124.3 | 310 | 152 | 125 | 1.12 | 1.92 | 127.0 | 448 | 171 | 191 | 2.05 | 1.57 | 1696.8 | 322 | 128 | 89 | 3.77 | 3.15 |
| 0.4 | 2049.5 | 450 | 190 | 168 | 1.26 | 2.54 | 1306.7 | 698 | 204 | 328 | 1.45 | 1.34 | 2186.7 | 502 | 198 | 155 | 3.12 | 2.56 |
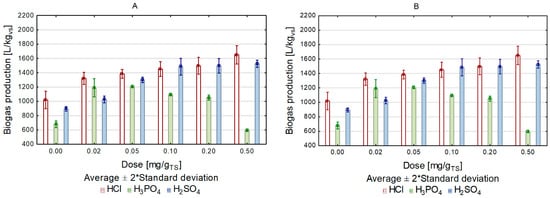
The use of increasing doses of H2SO4 and HCl contributed to the increased concentration of soluble sugars and increased biogas production in the respirometric tests (Figure 1 and Figure 2). This means that the acid doses applied did not result in the release of inhibitors in the amounts likely to significantly hamper methane fermentation. Opposite observations were made during substrate pretreatment with H3PO4. Despite a proportional increase in glucose concentration in the pretreated solutions to the acid doses applied, the amount of biogas produced in the respirometric tests increased only at acid doses of up to 0.05 g/gTS in the variant with microwave heating and at acid doses of up to 0.02 g/gTS in that with conventional heating. The use of higher acid doses caused a successive decrease in methane fermentation yield, with the highest dose applied resulting in the lowest biogas production for both heating variants. In the case of conventional heating, the fermentation yield decreased by 48.8%, whereas in the case of microwave heating it decreased by 44.9% (Figure 2). The decrease in biogas production yield was due to the formation of phenolic compounds, furfural, and 5-hydroxymethylfurfural (5-HMF). In the case of the sample pretreated with the highest acid dose, the content of furan compounds increased to 917 mg/L in the conventional heating variant and to 902 mg/L in the microwave heating variant. Contents of phenolic compounds in the samples from the respective variants reached 335 mg/L and 328 mg/L (Table 3).

Figure 2. Biogas production depending on the heating method and acid dose ((A)—conventional, (B)—microwave).
Most studies on the thermal pretreatment of lignocellulose show an increase in BMP (biochemical methane potential). Thomas et al. tested BMP from Miscanthus after non-temperature pretreatment with NaOH and obtained a 55% increase in BMP [50]. Siddhu tested the BMP of steam-exploded corn stover and, compared with the control sample, received a BMP increase of 56% [51]. Microwave researchers also obtained an increase in BMP: Jackowiak, using microwaves at 260 °C and a pressure of 33 bar obtained an increase of 28% with switchgrass [31], while Kainthola et al. noted an increase in the amount of methane produced by 100 NmL CH4/gVS [52]. The authors of the study noted an increase in the amount of biogas generated in each of the tested microwave-heated variants compared to conventionally heated samples. The biggest difference in the amount of biogas between conventional and microwave heating was noted during the conditioning of the substrate with HCl, for a dose of 0.2 g/gTS (Figure 2).
Monlau et al. investigated sunflower oil cake conversion in the methane fermentation process and assessed the effect of acid-heat pretreatment on biogas production and solubilization of total organic carbon, sugars, and protein. They demonstrated a higher methane production for the sample pretreated with dilute sulfuric(VI) acid than for the control sample (195 mL/gTS). The highest yield (302 mL/gTS) was obtained after acidic pretreatment at a temperature of 170 °C [53]. The highest volume of biogas in our research, exceeding 1800 L/kgVS, was produced using HCl in the highest tested dose, whereas biogas production using the same dose of H2SO4 was 10% lower (Figure 2).
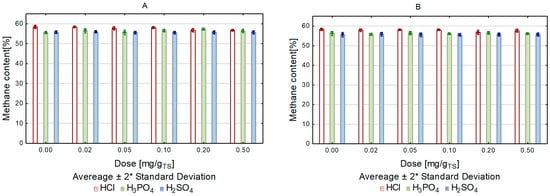
Comparing the two heating methods used, and affecting the tested substrate for the same time with each of the tested variants, it can be concluded that microwave heating resulted in a greater volume of biogas being produced in the methane fermentation process. The same correlation was noted in the case of using acids. In all variants of substrate pretreatment with acids, the volume of biogas in the samples exposed to microwave heating was higher than in those exposed to conventional heating. The greatest biogas volume was produced in the variant with HCl used in the highest dose (Figure 2). The highest value of the biogas production rate (r) was recorded in the variant with HCl in a dose of 0.2 g/gTS, r = 0.22 L/d (Table 4). The content of methane in biogas in the individual experimental variants did not differ significantly and fluctuated between 55 and 58% (Figure 3). Reports on the use of microwave treatment to intensify the methane fermentation of lignocellulosic biomass have been inconclusive in confirming its effectiveness. The author of one of these reports, Sapci, studied the production of biogas from barley straw, spring wheat, winter wheat, and oats. He deployed microwave radiation at temperatures of 200 °C and 300 °C as a pretreatment method, and subjected the pretreated substrate to methane fermentation under mesophilic conditions for 60 days. The results of his research showed that the microwave treatment did not improve the fermentation yield, and that the increase in process temperature suppressed biogas production [35]. Completely different research results were obtained by Jackowiak et al., who focused their study on optimizing wheat straw methane fermentation by deploying a microwave process [33].

Figure 3. Methane content of biogas, depending on heating method and acid dose ((A)—conventional, (B)—microwave).
Table 4. Biogas production rate depending on heating method and acid dose.
| Acid Dose (g/gvs) |
Reaction Rate Constant | Biogas Production Rate | ||||
|---|---|---|---|---|---|---|
| Addition of HCl k (d−1) |
Addition of H3PO4 k (d−1) |
Addition of H2SO4 k (d−1) |
Addition of HCl r (L/d) |
Addition of H3PO4 r (L/d) |
Addition of H2SO4 r (L/d) |
|
| Conventional heating | ||||||
| 0.0 | 0.27 | 0.27 | 0.21 | 0.12 | 0.08 | 0.09 |
| 0.02 | 0.25 | 0.22 | 0.26 | 0.15 | 0.12 | 0.12 |
| 0.05 | 0.23 | 0.2 | 0.23 | 0.14 | 0.11 | 0.14 |
| 0.1 | 0.25 | 0.23 | 0.23 | 0.16 | 0.11 | 0.16 |
| 0.2 | 0.24 | 0.24 | 0.21 | 0.16 | 0.13 | 0.14 |
| 0.4 | 0.2 | 0.27 | 0.2 | 0.15 | 0.08 | 0.14 |
| Microwave heating | ||||||
| 0.0 | 0.27 | 0.27 | 0.29 | 0.14 | 0.09 | 0.13 |
| 0.02 | 0.26 | 0.17 | 0.27 | 0.17 | 0.09 | 0.13 |
| 0.05 | 0.21 | 0.18 | 0.25 | 0.15 | 0.13 | 0.15 |
| 0.1 | 0.22 | 0.17 | 0.21 | 0.16 | 0.98 | 0.18 |
| 0.2 | 0.25 | 0.23 | 0.23 | 0.22 | 0.13 | 0.17 |
| 0.4 | 0.2 | 0.27 | 0.16 | 0.17 | 0.09 | 0.13 |
Comparing microwave heating to conventional heating, different behaviors and properties were noted in the case of many biological reactions, which, according to scientists, shows the non-thermal effects of microwave radiation [54][55]. In the case of the presented research, the nonthermal effect of microwaves was evident in the difference in the amount of biogas produced between the use of microwave and conventional heating. For each of the tested acids in each dose variant, microwave heating caused an increase in biogas production over conventional heating; according to the theory of the non-terminating microwave effect, microwave activation took place, which increased the speed of the processes. The maximum difference in biogas production between microwave and conventionally heated variants was 19%, during substrate conditioning with HCl, for a dose of 0.2 g/gTS (Figure 2).
References
- Dziennik Ustaw. Available online: https://www.dziennikustaw.gov.pl/M2021000026401.pdf (accessed on 17 November 2021).
- Krajowy Rejestr Wytwórców Biogazu. Available online: https://www.kowr.gov.pl/uploads/pliki/oze/biogaz/rejestr%20wytw%C3%B3rc%C3%B3w%20biogazu%20rolniczego%20z%20dnia%2016.04.2021%20r.pdf (accessed on 17 November 2021).
- Kazimierowicz, J.; Dzienis, L.; Dębowski, M.; Zieliński, M. Optimisation of methane fermentation as a valorisation method for food waste products. Biomass Bioenergy 2021, 144, 105913.
- Dębowski, M.; Kisielewska, M.; Kazimierowicz, J.; Rudnicka, A.; Dudek, M.; Romanowska-Duda, Z.; Zieliński, M. The effects of Microalgae Biomass Co-Substrate on Biogas Production from the Common Agricultural Biogas Plants Feedstock. Energies 2020, 13, 2186.
- Kazimierowicz, J.; Dzienis, L. Giant miscanthus as a substrate for biogas production. J. Ecol. Eng. 2015, 16, 139–142.
- Piechota, G. Multi-Step Biogas Quality Improving by Adsorptive Packed Column System as Application to Biomethane Upgrading. J. Environ. Chem. Eng. 2021, 9, 105944.
- Li, P.; Sakuragi, K.; Makino, H. Extraction Techniques in Sustainable Biofuel Production: A Concise Review. Fuel Process. Technol. 2019, 193, 295–303.
- Aguilar-Reynosa, A.; Romaní, A.; Rodríguez-Jasso, R.M.; Aguilar, C.N.; Garrote, G.; Ruiz, H.A. Microwave Heating Processing as Alternative of Pretreatment in Second-Generation Biorefinery: An Overview. Energy Convers. Manag. 2017, 136, 50–65.
- Alalwan, H.A.; Alminshid, A.H.; Aljaafari, H.A.S. Promising Evolution of Biofuel Generations. Subject Review. Renew. Energy Focus 2019, 28, 127–139.
- Wang, D.; Shen, F.; Yang, G.; Zhang, Y.; Deng, S.; Zhang, J.; Zeng, Y.; Luo, T.; Mei, Z. Can Hydrothermal Pretreatment Improve Anaerobic Digestion for Biogas from Lignocellulosic Biomass? Bioresour. Technol. 2018, 249, 117–124.
- Kazimierowicz, J.; Bartkowska, I.; Walery, M. Effect of Low-Temperature Conditioning of Excess Dairy Sewage Sludge with the Use of Solidified Carbon Dioxide on the Efficiency of Methane Fermentation. Energies 2021, 14, 150.
- Sindhu, R.; Binod, P.; Pandey, A. Biological Pretreatment of Lignocellulosic Biomass—An Overview. Bioresour. Technol. 2016, 199, 76–82.
- Hendriks, A.T.W.M.; Zeeman, G. Pretreatments to Enhance the Digestibility of Lignocellulosic Biomass. Bioresour. Technol. 2009, 100, 10–18.
- Jönsson, L.J.; Martín, C. Pretreatment of Lignocellulose: Formation of Inhibitory by-Products and Strategies for Minimizing Their Effects. Bioresour. Technol. 2016, 199, 103–112.
- Monlau, F.; Sambusiti, C.; Barakat, A.; Quéméneur, M.; Trably, E.; Steyer, J.P.; Carrère, H. Do Furanic and Phenolic Compounds of Lignocellulosic and Algae Biomass Hydrolyzate Inhibit Anaerobic Mixed Cultures? A Comprehensive Review. Biotechnol. Adv. 2014, 32, 934–951.
- Almeida, J.; Bertilsson, M.; Gorwa-Grauslund, M.F.; Gorsich, S.; Lidén, G. Metabolic Effects of Furaldehydes and Impacts on Biotechnological Processes. Appl. Microbiol. Biotechnol. 2009, 82, 625–638.
- Hierholtzer, A.; Chatellard, L.; Kierans, M.; Akunna, J.C.; Collier, P.J. The Impact and Mode of Action of Phenolic Compounds Extracted from Brown Seaweed on Mixed Anaerobic Microbial Cultures. J. Appl. Microbiol. 2013, 114, 964–973.
- Mills, T.Y.; Sandoval, N.R.; Gill, R.T. Cellulosic Hydrolysate Toxicity and Tolerance Mechanisms in Escherichia Coli. Biotechnol. Biofuels 2009, 2, 26.
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of Lignocellulosic Hydrolysates. II: Inhibitors and Mechanisms of Inhibition. Bioresour. Technol. 2000, 74, 25–33.
- Cueva, C.; Mingo, S.; Muñoz-González, I.; Bustos, I.; Requena, T.; del Campo, R.; Martín-Álvarez, P.; Bartolomé, B.; Moreno-Arribas, M.V. Antibacterial Activity of Wine Phenolic Compounds and Oenological Extracts against Potential Respiratory Pathogens. Lett. Appl. Microbiol. 2012, 54, 557–563.
- Zhang, Y.; Ujor, V.; Wick, M.; Ezeji, T. Identification, Purification and Characterization of Furfural Transforming Enzymes from Clostridium Beijerinckii NCIMB 8052. Anaerobe 2015, 33, 124–131.
- Elalami, D.; Monlau, F.; Carrere, H.; Abdelouahdi, K.; Oukarroum, A.; Zeroual, Y.; Barakat, A. Effect of Coupling Alkaline Pretreatment and Sewage Sludge Co-Digestion on Methane Production and Fertilizer Potential of Digestate. Sci. Total Environ. 2020, 743, 140670.
- Sołowski, G.; Konkol, I.; Cenian, A. Production of Hydrogen and Methane from Lignocellulose Waste by Fermentation. A Review of Chemical Pretreatment for Enhancing the Efficiency of the Digestion Process. J. Clean. Prod. 2020, 267, 121721.
- Balat, M. Production of Bioethanol from Lignocellulosic Materials via the Biochemical Pathway: A Review. Energy Convers. Manag. 2011, 52, 858–875.
- Du, Z.; Zheng, T.; Wang, P.; Hao, L.; Wang, Y. Fast Microwave-Assisted Preparation of a Low-Cost and Recyclable Carboxyl Modified Lignocellulose-Biomass Jute Fiber for Enhanced Heavy Metal Removal from Water. Bioresour. Technol. 2016, 201, 41–49.
- Nowicka, A.; Zieliński, M.; Dębowski, M.; Dudek, M.; Rusanowska, P. Progress in the Production of Biogas from Virginia Mallow after Alkaline-Heat Pretreatment. Biomass Bioenergy 2019, 126, 174–180.
- Binod, P.; Satyanagalakshmi, K.; Sindhu, R.; Janu, K.U.; Sukumaran, R.K.; Pandey, A. Short Duration Microwave Assisted Pretreatment Enhances the Enzymatic Saccharification and Fermentable Sugar Yield from Sugarcane Bagasse. Renew. Energy 2012, 37, 109–116.
- Zhu, Z.; Rezende, C.A.; Simister, R.; McQueen-Mason, S.J.; Macquarrie, D.J.; Polikarpov, I.; Gomez, L.D. Efficient Sugar Production from Sugarcane Bagasse by Microwave Assisted Acid and Alkali Pretreatment. Biomass Bioenergy 2016, 93, 269–278.
- Lorenci Woiciechowski, A.; Dalmas Neto, C.J.; Porto de Souza Vandenberghe, L.; de Carvalho Neto, D.P.; Novak Sydney, A.C.; Letti, L.A.J.; Karp, S.G.; Zevallos Torres, L.A.; Soccol, C.R. Lignocellulosic Biomass: Acid and Alkaline Pretreatments and Their Effects on Biomass Recalcitrance—Conventional Processing and Recent Advances. Bioresour. Technol. 2020, 304, 122848.
- Solarte-Toro, J.C.; Romero-García, J.M.; Martínez-Patiño, J.C.; Ruiz-Ramos, E.; Castro-Galiano, E.; Cardona-Alzate, C.A. Acid Pretreatment of Lignocellulosic Biomass for Energy Vectors Production: A Review Focused on Operational Conditions and Techno-Economic Assessment for Bioethanol Production. Renew. Sustain. Energy Rev. 2019, 107, 587–601.
- Bichot, A.; Lerosty, M.; Méchin, V.; Bernet, N.; Delgenès, J.P.; García-Bernet, D. Evaluation of Chemical-Free Microwave Pretreatment on Methane Yield of Two Grass Biomass with Contrasted Parietal Content. Energy Convers. Manag. 2021, 229, 113746.
- Naik, G.P.; Poonia, A.K.; Chaudhari, P.K. Pretreatment of Lignocellulosic Agricultural Waste for Delignification, Rapid Hydrolysis, and Enhanced Biogas Production: A Review. J. Indian Chem. Soc. 2021, 98, 100147.
- Jackowiak, D.; Bassard, D.; Pauss, A.; Ribeiro, T. Optimisation of a Microwave Pretreatment of Wheat Straw for Methane Production. Bioresour. Technol. 2011, 102, 6750–6756.
- Jackowiak, D.; Frigon, J.C.; Ribeiro, T.; Pauss, A.; Guiot, S. Enhancing Solubilisation and Methane Production Kinetic of Switchgrass by Microwave Pretreatment. Bioresour. Technol. 2011, 102, 3535–3540.
- Sapci, Z. The Effect of Microwave Pretreatment on Biogas Production from Agricultural Straws. Bioresour. Technol. 2013, 128, 487–494.
- Jankowski, K.J.; Dubis, B.; Sokólski, M.M.; Załuski, D.; Bórawski, P.; Szempliński, W. Productivity and Energy Balance of Maize and Sorghum Grown for Biogas in a Large-Area Farm in Poland: An 11-Year Field Experiment. Ind. Crop. Prod. 2020, 148, 112326.
- Garrote, G.; Domínguez, H.; Parajó, J.C. Hydrothermal Processing of Lignocellulosic Materials. Holz als roh-und Werkst. 1999, 57, 191–202.
- Gregg, D.; Saddler, J.N. A Techno-Economic Assessment of the Pretreatment and Fractionation Steps of a Biomass-to-Ethanol Process. Appl. Biochem. Biotechnol. 1996, 57, 711–727.
- Brownell, H.; Yu, E.; Saddler, J. Steam-Explosion Pretreatment of Wood: Effect of Chip Size, Acid, Moisture Content and Pressure Drop. Biotechnol. Bioeng. 1986, 28, 792–801.
- Gabhane, J.; Prince William, S.P.M.; Gadhe, A.; Rath, R.; Vaidya, A.N.; Wate, S. Pretreatment of Banana Agricultural Waste for Bio-Ethanol Production: Individual and Interactive Effects of Acid and Alkali Pretreatments with Autoclaving, Microwave Heating and Ultrasonication. Waste Manag. 2014, 34, 498–503.
- de Vasconcelos, S.M.; Santos, A.M.P.; Rocha, G.J.M.; Souto-Maior, A.M. Diluted Phosphoric Acid Pretreatment for Production of Fermentable Sugars in a Sugarcane-Based Biorefinery. Bioresour. Technol. 2013, 135, 46–52.
- Kaur, K.; Phutela, U.G. Enhancement of Paddy Straw Digestibility and Biogas Production by Sodium Hydroxide-Microwave Pretreatment. Renew. Energy 2016, 92, 178–184.
- Laghari, S.; Isa, M.; Laghari, A. Delignification of Coconut Husk by Microwave Assisted Chemical Pretreatment. Adv. Environ. Biol. 2015, 9, 1–5.
- Gossett, J.M.; Stuckey, D.C.; Owen, W.F.; McCarty, P.L. Heat Treatment and Anaerobic Digestion of Refuse. J. Environ. Eng. Div. 1982, 108, 437–454.
- Díaz, M.J.; Cara, C.; Ruiz, E.; Pérez-Bonilla, M.; Castro, E. Hydrothermal Pre-Treatment and Enzymatic Hydrolysis of Sunflower Stalks. Fuel 2011, 90, 3225–3229.
- Monlau, F.; Sambusiti, C.; Barakat, A.; Guo, X.M.; Latrille, E.; Trably, E.; Steyer, J.-P.; Carrere, H. Predictive Models of Biohydrogen and Biomethane Production Based on the Compositional and Structural Features of Lignocellulosic Materials. Environ. Sci. Technol. 2012, 46, 12217–12225.
- Larsson, S. Ethanol from Lignocellulose-Fermentation Inhibitors, Detoxification and Genetic Engineering of Sacchwomyces Cerevkiae for Enhanced Resistance. Ph.D. Thesis, Lund University, Lund, Sweden, 2000.
- Wyman, C.E.; Dale, B.E.; Elander, R.T.; Holtzapple, M.; Ladisch, M.R.; Lee, Y.Y. Coordinated Development of Leading Biomass Pretreatment Technologies. Bioresour. Technol. 2005, 96, 1959–1966.
- Rashid, N.; Lee, K.; Mahmood, Q. Bio-Hydrogen Production by Chlorella Vulgaris under Diverse Photoperiods. Bioresour. Technol. 2011, 102, 2101–2104.
- Thomas, H.L.; Arnoult, S.; Brancourt-Hulmel, M.; Carrère, H. Methane Production Variability According to Miscanthus Genotype and Alkaline Pretreatments at High Solid Content. Bioenergy Res. 2019, 12, 325–337.
- Siddhu, M.A.H.; Li, J.; Zhang, J.; Huang, Y.; Wang, W.; Chen, C.; Liu, G. Improve the Anaerobic Biodegradability by Copretreatment of Thermal Alkali and Steam Explosion of Lignocellulosic Waste. BioMed Res. Int. 2016, 2016, 2786598.
- Kainthola, J.; Shariq, M.; Kalamdhad, A.S.; Goud, V.V. Enhanced Methane Potential of Rice Straw with Microwave Assisted Pretreatment and Its Kinetic Analysis. J. Environ. Manag. 2019, 232, 188–196.
- Monlau, F.; Latrille, E.; da Costa, A.C.; Steyer, J.P.; Carrère, H. Enhancement of Methane Production from Sunflower Oil Cakes by Dilute Acid Pretreatment. Appl. Energy 2013, 102, 1105–1113.
- Dębicki, P.; Styłba, S. Oddziaływania Środowiskowe Pół Elektromagnetycznych: Aspekty Fizyczne, Techniczne i Prawne; Akademia Morska: Szczecin, Poland, 2010.
- Kazimierowicz, J.; Zieliński, M.; Dębowski, M. Influence of the Heating Method on the Efficiency of Biomethane Production from Expired Food Products. Fermentation 2021, 7, 12.
More
Information
Subjects:
Energy & Fuels
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
923
Entry Collection:
Environmental Sciences
Revisions:
2 times
(View History)
Update Date:
10 Dec 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




