
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Gabriel Aguirre-Álvarez | + 2395 word(s) | 2395 | 2021-12-08 05:20:23 | | | |
| 2 | Peter Tang | Meta information modification | 2395 | 2021-12-10 04:08:38 | | |
Video Upload Options
Hydrolyzed collagen (HC) is a group of peptides with low molecular weight (3–6 KDa) that can be obtained by enzymatic action in acid or alkaline media at a specific incubation temperature. HC can be extracted from different sources such as bovine or porcine. Recently research has shown good properties of the HC found in skin, scale, and bones from marine sources. Type and source of extraction are the main factors that affect HC properties, such as molecular weight of the peptide chain, solubility, and functional activity. HC is widely used in several industries including food, pharmaceutical, cosmetic, biomedical, and leather industries.
1. Introduction
2. Hydrolyzed Collagen: Extraction and Properties
2.1. Extraction and Structure of Hydrolyzed Collagen
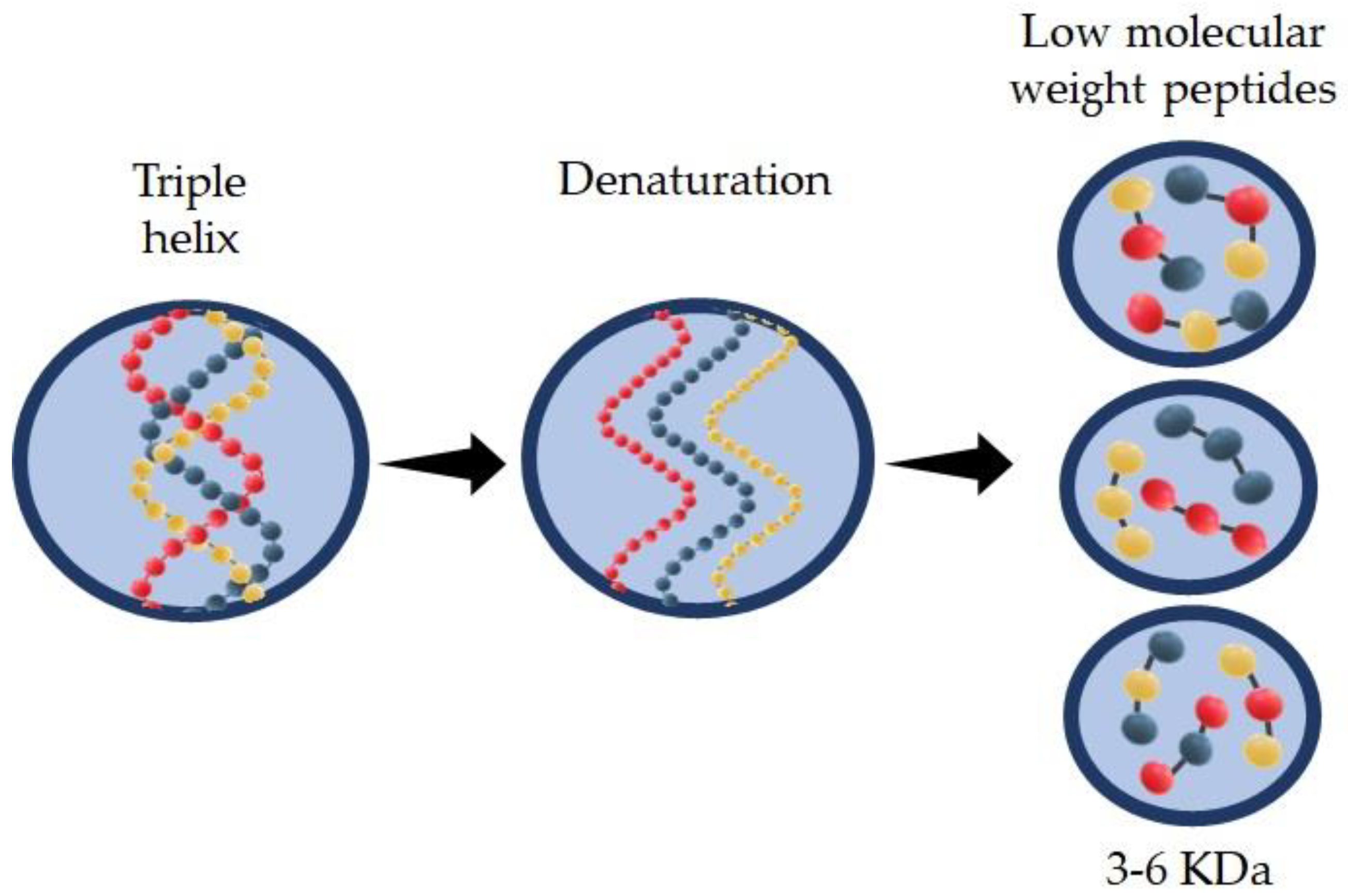
2.2. Techniques for HC Molecular Weight Measurements
2.3. Hydrolyzed Collagen Properties
|
Properties |
Type of Collagen |
Reference |
|
|---|---|---|---|
|
Native |
Hydrolyzed |
||
|
Molecular weight (Mw) |
~300 KDa |
3–6 KDa |
|
|
Isoelectric point (pI) |
7.0–8.3 |
3.68–5.7 |
|
|
Viscosity |
High |
Low (0 Cp) |
|
|
Film formation |
Yes |
No |
|
3. Hydrolyzed Collagen: Sources and Applications
3.1. Sources
3.1.1. Bovine
3.1.2. Porcine
3.1.3. Marine
3.1.4. Alternative Sources
3.2. Applications
3.2.1. Oral Collagen Supplementation
3.2.2. Food Industry
3.2.3. Biomaterials
References
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.J.A.M. The collagen suprafamily: From biosynthesis to advanced biomaterial development. Adv. Mater. 2019, 31, 1801651.
- Gelse, K.; Pöschl, E.; Aigner, T. Collagens—structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546.
- Schrieber, R.; Gareis, H. Gelatine Handbook. Theory and Industrial Practice; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007; pp. 45–117.
- Ketnawa, S.; Benjakul, S.; Martínez-Alvarez, O.; Rawdkuen, S. Fish skin gelatin hydrolysates produced by visceral peptidase and bovine trypsin: Bioactivity and stability. Food Chem. 2017, 215, 383–390.
- Thuanthong, M.; De Gobba, C.; Sirinupong, N.; Youravong, W.; Otte, J. Purification and characterization of angiotensin-converting enzyme-inhibitory peptides from Nile tilapia (Oreochromis niloticus) skin gelatine produced by an enzymatic membrane reactor. J. Funct. Foods 2017, 36, 243–254.
- Hong, H.; Chaplot, S.; Chalamaiah, M.; Roy, B.C.; Bruce, H.L.; Wu, J. Removing cross-linked telopeptides enhances the production of low-molecular-weight collagen peptides from spent hens. J. Agric. Food Chem. 2017, 65, 7491–7499.
- Cheung, I.W.Y.; Li-Chan, E.C.Y. Enzymatic production of protein hydrolysates from steelhead (Oncorhynchus mykiss) skin gelatin as inhibitors of dipeptidyl-peptidase IV and angiotensin-I converting enzyme. J. Funct. Foods 2017, 28, 254–264.
- Barzideh, Z.; Latiff, A.A.; Gan, C.-Y.; Abedin, M.Z.; Alias, A.K. ACE inhibitory and antioxidant activities of collagen hydrolysates from the ribbon jellyfish (Chrysaora sp.). Food Technol. Biotechnol. 2014, 52, 495–504.
- Bilek, S.E.; Bayram, S.K. Fruit juice drink production containing hydrolyzed collagen. J. Funct. Foods 2015, 14, 562–569.
- Offengenden, M.; Chakrabarti, S.; Wu, J. Chicken collagen hydrolysates differentially mediate anti-inflammatory activity and type I collagen synthesis on human dermal fibroblasts. Food Sci. Hum. Wellness 2018, 7, 138–147.
- Masuda, R.; Dazai, Y.; Mima, T.; Koide, T. Structure-activity relationships and action mechanisms of collagen-like antimicrobial peptides. Pept. Sci. 2017, 108, e22931.
- Chi, C.-F.; Cao, Z.-H.; Wang, B.; Hu, F.-Y.; Li, Z.-R.; Zhang, B. Antioxidant and functional properties of collagen hydrolysates from spanish mackerel skin as influenced by average molecular weight. Molecules 2014, 19, 11211–11230.
- Onuh, J.O.; Girgih, A.T.; Aluko, R.E.; Aliani, M. In vitro antioxidant properties of chicken skin enzymatic protein hydrolysates and membrane fractions. Food Chem. 2014, 150, 366–373.
- Lin, Y.-J.; Le, G.-W.; Wang, J.-Y.; Li, Y.-X.; Shi, Y.-H.; Sun, J. Antioxidative peptides derived from enzyme hydrolysis of bone collagen after microwave assisted acid pre-treatment and nitrogen protection. Int. J. Mol. Sci. 2010, 11, 4297–4308.
- León-López, A.; Fuentes-Jiménez, L.; Hernández-Fuentes, A.D.; Campos-Montiel, R.G.; Aguirre-Álvarez, G. Hydrolysed collagen from sheepskins as a source of functional peptides with antioxidant activity. Int. J. Mol. Sci. 2019, 20, 3931.
- Hong, H.; Fan, H.; Chalamaiah, M.; Wu, J. Preparation of low-molecular-weight, collagen hydrolysates (peptides): Current progress, challenges, and future perspectives. Food Chem. 2019, 301, 125222.
- Elavarasan, K.; Shamasundar, B.; Badii, F.; Howell, N. Angiotensin I-converting enzyme (ACE) inhibitory activity and structural properties of oven-and freeze-dried protein hydrolysate from fresh water fish (Cirrhinus mrigala). Food Chem. 2016, 206, 210–216.
- Powell, T.; Bowra, S.; Cooper, H.J. Subcritical water hydrolysis of peptides: Amino acid side-chain modifications. J. Am. Soc. Mass Spectrom. 2017, 28, 1775–1786.
- Jo, Y.-J.; Kim, J.-H.; Jung, K.-H.; Min, S.-G.; Chun, J.-Y. Effect of sub-and super-critical water treatment on physicochemical properties of porcine skin. Korean J. Food Sci. Anim. Resour. 2015, 35, 35.
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680.
- Schägger, H. Tricine–sds-page. Nat. Protoc. 2006, 1, 16.
- Haider, S.R.; Reid, H.J.; Sharp, B.L. Tricine-sds-page. In Protein Electrophoresis: Methods and Protocols; Kurien, B.T., Scofield, R.H., Eds.; Humana Press: New York, NY, USA, 2012; Volume 869, pp. 81–91.
- Karas, M.; Hillenkamp, F. Laser desorption ionization of proteins with molecular masses exceeding 10,000 daltons. Anal. Chem. 1988, 60, 2299–2301.
- Hema, G.; Joshy, C.; Shyni, K.; Chatterjee, N.S.; Ninan, G.; Mathew, S. Optimization of process parameters for the production of collagen peptides from fish skin (Epinephelus malabaricus) using response surface methodology and its characterization. J. Food Sci. Technol. 2017, 54, 488–496.
- Lecchi, P.; Olson, M.; Brancia, F.L. The role of esterification on detection of protonated and deprotonated peptide ions in matrix assisted laser desorption/ionization (MALDI) mass spectrometry (MS). J. Am. Soc. Mass Spectrom. 2005, 16, 1269–1274.
- Pataridis, S.; Eckhardt, A.; Mikulikova, K.; Sedlakova, P.; Mikšík, I. Identification of collagen types in tissues using HPLC-MS/MS. J. Sep. Sci. 2008, 31, 3483–3488.
- Zhang, G.; Sun, A.; Li, W.; Liu, T.; Su, Z. Mass spectrometric analysis of enzymatic digestion of denatured collagen for identification of collagen type. J. Chromatogr. A 2006, 1114, 274–277.
- Mikulíková, K.; Eckhardt, A.; Pataridis, S.; Mikšík, I. Study of posttranslational non-enzymatic modifications of collagen using capillary electrophoresis/mass spectrometry and high performance liquid chromatography/mass spectrometry. J. Chromatogr. A 2007, 1155, 125–133.
- Zhang, Y.; Zhang, Y.; Liu, X.; Huang, L.; Chen, Z.; Cheng, J. Influence of hydrolysis behaviour and microfluidisation on the functionality and structural properties of collagen hydrolysates. Food Chem. 2017, 227, 211–218.
- Li, Z.; Wang, B.; Chi, C.; Gong, Y.; Luo, H.; Ding, G. Influence of average molecular weight on antioxidant and functional properties of cartilage collagen hydrolysates from Sphyrna lewini, Dasyatis akjei and Raja porosa. Food Res. Int. 2013, 51, 283–293.
- Sun Pan, B.; En Chen, H.O.A.; Sung, W.C. Molecular and thermal characteristics of acid-soluble collagen from orbicular batfish: Effects of deep-sea water culturing. Int. J. Food Prop. 2018, 21, 1080–1090.
- Chen, J.; Li, L.; Yi, R.; Xu, N.; Gao, R.; Hong, B. Extraction and characterization of acid-soluble collagen from scales and skin of tilapia (Oreochromis niloticus). Lwt-Food Sci. Technol. 2016, 66, 453–459.
- Abdollahi, M.; Rezaei, M.; Jafarpour, A.; Undeland, I. Sequential extraction of gel-forming proteins, collagen and collagen hydrolysate from gutted silver carp (Hypophthalmichthys molitrix), a biorefinery approach. Food Chem. 2018, 242, 568–578.
- Kezwoń, A.; Chromińska, I.; Frączyk, T.; Wojciechowski, K. Effect of enzymatic hydrolysis on surface activity and surface rheology of type I collagen. Colloids Surf. B: Biointerfaces 2016, 137, 60–69.
- Wang, J.; Luo, D.; Liang, M.; Zhang, T.; Yin, X.; Zhang, Y.; Yang, X.; Liu, W. Spectrum-effect relationships between high-performance liquid chromatography (HPLC) fingerprints and the antioxidant and anti-inflammatory activities of collagen peptides. Molecules 2018, 23, 3257.
- Wang, L.; Jiang, Y.; Wang, X.; Zhou, J.; Cui, H.; Xu, W.; He, Y.; Ma, H.; Gao, R. Effect of oral administration of collagen hydrolysates from Nile tilapia on the chronologically aged skin. J. Funct. Foods 2018, 44, 112–117.
- Chi, C.; Hu, F.; Li, Z.; Wang, B.; Luo, H. Influence of different hydrolysis processes by trypsin on the physicochemical, antioxidant, and functional properties of collagen hydrolysates from Sphyrna lewini, Dasyatis akjei, and Raja porosa. J. Aquat. Food Prod. Technol. 2016, 25, 616–632.
- Fauzi, M.B.; Lokanathan, Y.; Aminuddin, B.S.; Ruszymah, B.H.I.; Chowdhury, S.R. Ovine tendon collagen: Extraction, characterisation and fabrication of thin films for tissue engineering applications. Mater. Sci. Eng. C 2016, 68, 163–171.
- Ramadass, S.K.; Perumal, S.; Gopinath, A.; Nisal, A.; Subramanian, S.; Madhan, B. Sol–gel assisted fabrication of collagen hydrolysate composite scaffold: A novel therapeutic alternative to the traditional collagen scaffold. Acs Appl. Mater. Interfaces 2014, 6, 15015–15025.
- Zhang, Y.; Olsen, K.; Grossi, A.; Otte, J. Effect of pretreatment on enzymatic hydrolysis of bovine collagen and formation of ACE-inhibitory peptides. Food Chem. 2013, 141, 2343–2354.
- Lima, C.A.; Campos, J.F.; Lima Filho, J.L.; Converti, A.; da Cunha, M.G.C.; Porto, A.L. Antimicrobial and radical scavenging properties of bovine collagen hydrolysates produced by Penicillium aurantiogriseum URM 4622 collagenase. J. Food Sci. Technol. 2015, 52, 4459–4466.
- O’Sullivan, S.M.; Lafarga, T.; Hayes, M.; O’Brien, N.M. Bioactivity of bovine lung hydrolysates prepared using papain, pepsin, and Alcalase. J. Food Biochem. 2017, 41, e12406.
- Fu, Y.; Young, J.F.; Løkke, M.M.; Lametsch, R.; Aluko, R.E.; Therkildsen, M. Revalorisation of bovine collagen as a potential precursor of angiotensin I-converting enzyme (ACE) inhibitory peptides based on in silico and in vitro protein digestions. J. Funct. Foods 2016, 24, 196–206.
- Choi, D.; Min, S.G.; Jo, Y.J. Functionality of porcine skin hydrolysates produced by hydrothermal processing for liposomal delivery system. J. Food Biochem. 2018, 42, e12464.
- O’Keeffe, M.B.; Norris, R.; Alashi, M.A.; Aluko, R.E.; FitzGerald, R.J. Peptide identification in a porcine gelatin prolyl endoproteinase hydrolysate with angiotensin converting enzyme (ACE) inhibitory and hypotensive activity. J. Funct. Foods 2017, 34, 77–88.
- Yazaki, M.; Ito, Y.; Yamada, M.; Goulas, S.; Teramoto, S.; Nakaya, M.-a.; Ohno, S.; Yamaguchi, K. Oral ingestion of collagen hydrolysate leads to the transportation of highly concentrated Gly-Pro-Hyp and its hydrolyzed form of Pro-Hyp into the bloodstream and skin. J. Agric. Food Chem. 2017, 65, 2315–2322.
- Min, S.-G.; Jo, Y.-J.; Park, S.H. Potential application of static hydrothermal processing to produce the protein hydrolysates from porcine skin by-products. Lwt-Food Sci. Technol. 2017, 83, 18–25.
- Dandagi, G.L.; Byahatti, S.M. An insight into the swine-influenza A (H1N1) virus infection in humans. Lung India 2011, 28, 34–38.
- Bradley, R. Bovine spongiform encephalopathy (BSE): The current situation and research. Eur. J. Epidemiol. 1991, 7, 532–544.
- Regenstein, J.M.; Chaudry, M.M.; Regenstein, C.E. The kosher and halal food laws. Compr. Rev. Food Sci. Food Saf. 2003, 2, 111–127.
- Silvipriya, K.; Kumar, K.K.; Bhat, A.; Kumar, B.D.; John, A.; Lakshmanan, P. Collagen: Animal sources and biomedical application. J. Appl. Pharm. Sci. 2015, 5, 123–127.
- Felician, F.F.; Xia, C.; Qi, W.; Xu, H. Collagen from marine biological sources and medical applications. Chem. Biodivers. 2018, 15, e1700557.
- Pati, F.; Adhikari, B.; Dhara, S. Isolation and characterization of fish scale collagen of higher thermal stability. Bioresour. Technol. 2010, 101, 3737–3742.
- Sanchez, A.; Blanco, M.; Correa, B.; Perez-Martin, R.; Sotelo, C. Effect of fish collagen hydrolysates on type I collagen mRNA levels of human dermal fibroblast culture. Mar. Drugs 2018, 16, 144.
- Chen, J.; Li, L.; Yi, R.; Gao, R.; He, J. Release kinetics of Tilapia scale collagen I peptides during tryptic hydrolysis. Food Hydrocoll. 2018, 77, 931–936.
- Das, J.; Dey, P.; Chakraborty, T.; Saleem, K.; Nagendra, R.; Banerjee, P. Utilization of marine industry waste derived collagen hydrolysate as peroxide inhibition agents in lipid-based food. J. Food Process. Preserv. 2018, 42, e13430.
- Villamil, O.; Váquiro, H.; Solanilla, J.F. Fish viscera protein hydrolysates: Production, potential applications and functional and bioactive properties. Food Chem. 2017, 224, 160–171.
- Ahmed, R.; Chun, B.-S. Subcritical water hydrolysis for the production of bioactive peptides from tuna skin collagen. J. Supercrit. Fluids 2018, 141, 88–96.
- Sabeena Farvin, K.H.; Andersen, L.L.; Otte, J.; Nielsen, H.H.; Jessen, F.; Jacobsen, C. Antioxidant activity of cod (Gadus morhua) protein hydrolysates: Fractionation and characterisation of peptide fractions. Food Chem. 2016, 204, 409–419.
- Liu, C.; Ma, X.; Che, S.; Wang, C.; Li, B. The effect of hydrolysis with neutrase on molecular weight, functional properties, and antioxidant activities of Alaska pollock protein isolate. J. Ocean. Univ. China 2018, 17, 1423–1431.
- Tao, J.; Zhao, Y.-Q.; Chi, C.-F.; Wang, B. Bioactive peptides from cartilage protein hydrolysate of spotless smoothhound and their antioxidant activity in vitro. Mar. Drugs 2018, 16, 100.
- Saiga, A.; Iwai, K.; Hayakawa, T.; Takahata, Y.; Kitamura, S.; Nishimura, T.; Morimatsu, F. Angiotensin I-converting enzyme-inhibitory peptides obtained from chicken collagen hydrolysate. J. Agric. Food Chem 2008, 56, 9586–9591.
- Zhao, Y.; Wang, Z.; Zhang, J.; Su, T. Extraction and characterization of collagen hydrolysates from the skin of Rana chensinensis. 3 Biotech. 2018, 8, 181.
- Dhakal, D.; Koomsap, P.; Lamichhane, A.; Sadiq, M.B.; Anal, A.K. Optimization of collagen extraction from chicken feet by papain hydrolysis and synthesis of chicken feet collagen based biopolymeric fibres. Food Biosci. 2018, 23, 23–30.
- Soladoye, O.P.; Saldo, J.; Peiro, L.; Rovira, A.; Mor-Mur, M. Antioxidant and angiotensin 1 converting enzyme inhibitory functions from chicken collagen hydrolysates. J. Nutr. Food Sci. 2015, 5, 1–9.
- Venkatesan, J.; Anil, S.; Kim, S.-K.; Shim, M. Marine fish proteins and peptides for cosmeceuticals: A review. Mar. Drugs 2017, 15, 143.
- Hashim, P.; Sofberi, M.; Ridzwan, M.; Bakar, J.; Mat Hashim, D. Collagen in food and beverage industries. Int. Food Res. J. 2015, 22, 1–8.
- Varani, J.; Dame, M.K.; Rittie, L.; Fligiel, S.E.G.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Decreased collagen production in chronologically aged skin: Roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am. J. Pathol 2006, 168, 1861–1868.
- Baumann, L. Skin ageing and its treatment. J. Pathol. 2007, 211, 241–251.
- Hays, N.P.; Kim, H.; Wells, A.M.; Kajkenova, O.; Evans, W.J. Effects of whey and fortified collagen hydrolysate protein supplements on nitrogen balance and body composition in older women. J. Am. Diet. Assoc. 2009, 109, 1082–1087.
- Zorrilla García, A.E. El envejecimiento y el estrés oxidativo. Rev. Cuba. De Investig. Biomédicas 2002, 21, 178–185.
- Gómez-Guillén, M.C.; Giménez, B.; López-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827.
- Najafian, L.; Babji, A.S. A review of fish-derived antioxidant and antimicrobial peptides: Their production, assessment, and applications. Peptides 2012, 33, 178–185.
- Santana, R.C.; Perrechil, F.A.; Sato, A.C.K.; Cunha, R.L. Emulsifying properties of collagen fibers: Effect of pH, protein concentration and homogenization pressure. Food Hydrocoll. 2011, 25, 604–612.
- Guo, L.; Harnedy, P.A.; Zhang, L.; Li, B.; Zhang, Z.; Hou, H.; Zhao, X.; FitzGerald, R.J. In vitro assessment of the multifunctional bioactive potential of Alaska pollock skin collagen following simulated gastrointestinal digestion. J. Sci. Food Agric. 2015, 95, 1514–1520.
- Pal, G.K.; Suresh, P.V. Sustainable valorisation of seafood by-products: Recovery of collagen and development of collagen-based novel functional food ingredients. Innov. Food Sci. Emerg. Technol. 2016, 37, 201–215.
- Wang, W.; Chen, M.; Wu, J.; Wang, S. Hypothermia protection effect of antifreeze peptides from pigskin collagen on freeze-dried Streptococcus thermophiles and its possible action mechanism. Lwt-Food Sci. Technol. 2015, 63, 878–885.
- Sousa, S.C.; Fragoso, S.P.; Penna, C.R.A.; Arcanjo, N.M.O.; Silva, F.A.P.; Ferreira, V.C.S.; Barreto, M.D.S.; Araújo, Í.B.S. Quality parameters of frankfurter-type sausages with partial replacement of fat by hydrolyzed collagen. Lwt-Food Sci. Technol. 2017, 76, 320–325.
- Ibrahim, F.N.; Ismail-Fitry, M.R.; Yusoff, M.M.; Shukri, R. Effects of Fish Collagen Hydrolysate (FCH) as fat replacer in the production of buffalo patties. J. Adv. Res. Appl. Sci. Eng. Technol. 2018, 11, 108–117.
- Prestes, R.C.; Carneiro, E.B.B.; Demiate, I.M. Hydrolyzed collagen, modified starch and guar gum addition in turkey ham. Ciência Rural 2012, 42, 1307–1313.
- Gerhardt, Â.; Monteiro, B.W.; Gennari, A.; Lehn, D.N.; Souza, C.F.V.d. Características físico-químicas e sensoriais de bebidas lácteas fermentadas utilizando soro de ricota e colágeno hidrolisado. Physicochemical and sensory characteristics of fermented dairy drink using ricotta cheese whey and hydrolyzed collagen. Rev. Do Inst. De Laticínios Cândido Tostes 2013, 68, 41–50.
- Da Mata Rigoto, J.; Ribeiro, T.H.S.; Stevanato, N.; Sampaio, A.R.; Ruiz, S.P.; Bolanho, B.C. Effect of açaí pulp, cheese whey, and hydrolysate collagen on the characteristics of dairy beverages containing probiotic bacteria. J. Food Process. Eng. 2019, 42, e12953.
- Benjakul, S.; Chantakun, K.; Karnjanapratum, S. Impact of retort process on characteristics and bioactivities of herbal soup based on hydrolyzed collagen from seabass skin. J. Food Sci. Technol. 2018, 55, 3779–3791.
- Zhang, Q.-X.; Fu, R.-J.; Yao, K.; Jia, D.-Y.; He, Q.; Chi, Y.-L. Clarification effect of collagen hydrolysate clarifier on chrysanthemum beverage. LWT 2018, 91, 70–76.
- Fu, R.; Yao, K.; Zhang, Q.; Jia, D.; Zhao, J.; Chi, Y. Collagen hydrolysates of skin shavings prepared by enzymatic hydrolysis as a natural flocculant and their flocculating property. Appl. Biochem. Biotechnol. 2017, 182, 55–66.
- Ramshaw, J.A. Biomedical applications of collagens. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2016, 104, 665–675.
- Zeugolis, D.I.; Paul, R.G.; Attenburrow, G. Factors influencing the properties of reconstituted collagen fibers prior to self-assembly: Animal species and collagen extraction method. J. Biomed. Mater. Res. Part. A 2008, 86, 892–904.
- Pei, Y.; Yang, J.; Liu, P.; Xu, M.; Zhang, X.; Zhang, L. Fabrication, properties and bioapplications of cellulose/collagen hydrolysate composite films. Carbohydr. Polym. 2013, 92, 1752–1760.
- Ficai, A.; Albu, M.G.; Birsan, M.; Sonmez, M.; Ficai, D.; Trandafir, V.; Andronescu, E. Collagen hydrolysate based collagen/hydroxyapatite composite materials. J. Mol. Struct. 2013, 1037, 154–159.
- Ocak, B. Film-forming ability of collagen hydrolysate extracted from leather solid wastes with chitosan. Environ. Sci. Pollut. Res. 2018, 25, 4643–4655.




