Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Deepak Timalsina | + 1523 word(s) | 1523 | 2021-12-01 02:18:18 | | | |
| 2 | Bruce Ren | Meta information modification | 1523 | 2021-12-09 10:16:41 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Timalsina, D. Eclipta prostrata (L.)L.(Asteraceae): Ethnomedicinal Uses and Chemical Constituents. Encyclopedia. Available online: https://encyclopedia.pub/entry/16926 (accessed on 12 January 2026).
Timalsina D. Eclipta prostrata (L.)L.(Asteraceae): Ethnomedicinal Uses and Chemical Constituents. Encyclopedia. Available at: https://encyclopedia.pub/entry/16926. Accessed January 12, 2026.
Timalsina, Deepak. "Eclipta prostrata (L.)L.(Asteraceae): Ethnomedicinal Uses and Chemical Constituents" Encyclopedia, https://encyclopedia.pub/entry/16926 (accessed January 12, 2026).
Timalsina, D. (2021, December 09). Eclipta prostrata (L.)L.(Asteraceae): Ethnomedicinal Uses and Chemical Constituents. In Encyclopedia. https://encyclopedia.pub/entry/16926
Timalsina, Deepak. "Eclipta prostrata (L.)L.(Asteraceae): Ethnomedicinal Uses and Chemical Constituents." Encyclopedia. Web. 09 December, 2021.
Copy Citation
Eclipta prostrata (L.) L. (Syn.: Eclipta alba (L.) Hassak, Family: Asteraceae) is an important medicinal plant in the tropical and subtropical regions. It is widely used in treating various diseases of skin, liver and stomach in India, Nepal, Bangladesh, and other countries.
Eclipta prostrata
Eclipta alba
ecalbasaponin
hepatoprotective
wedelolactone
1. Introduction
The use of plants in traditional medicines covers a wide range of therapeutic uses to treat the infection as well as many chronic diseases [1][2][3][4]. Many people still rely on the traditional medicine and healthcare because of their wider cultural acceptance and affordability [5]. The plant based bioactive compounds have been an important source of modern drugs discovery and development [6]. Hence, the medicinal value of various plants should be explored with their pharmacological significance and potential application in different products.
Eclipta prostrata (L.) L. (Syn.: Eclipta alba (L.) Hassak, Family: Asteraceae) is commonly known as False daisy or Ink plant in English and locally known as Bhringraj, Bhumiraj, Aali jhar, and Nash jhar in Nepali language (Figure 1) [7][8]. E. prostrata is a medium-sized, branched, annual herb-bearing white flower natively found in the tropical and subtropical regions of the world [9][10]. It grows mostly in moist sites such as swamp edges, river or lake banks and edge of rice-fields and easily propagated and spread throughout China, India, Nepal, Brazil and other parts of the world [8][11][12][13]. It is widely distributed in tropical and sub-tropical regions of Asia, Africa, and South America (Figure 2) [14][15]. Traditionally, it is used to treat different skin problems such as wounds, hair loss prevention, and dermatitis. The leaves are used to treat snakebite in India, China, and Brazil. The mixture of leaf juice and honey is used to cure catarrh in infants [16][17]. The juice of E. prostrata is taken orally or applied locally to promote hair growth [18].

Figure 1. Photographs of Eclipta prostrata (Photos by Basu Dev Neupane, used with permission).
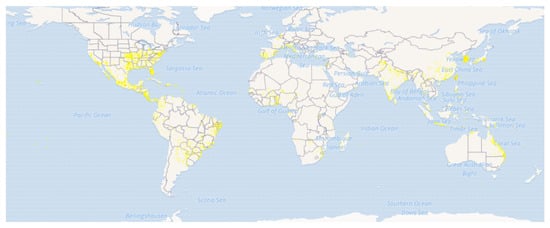
Figure 2. Distribution map of Eclipta prostrata. (Source: GBIF, https://www.gbif.org/species/5384950 (accessed on 1 November 2021) [15]).
Various research articles have been published regarding the chemical constituents and biological activities of different plant parts of E. prostrata. Critical analysis of these published scientific studies would provide the detailed understanding about the potential use of E. prostrata as medicine, cosmetic, and other formulations along with highlighting the gaps in research.
2. Ethnomedicinal Uses
This plant is widely used in different regions of India for the treatment of skin problems, hepatic problems such as jaundice, gastrointestinal problems, respiratory problems such as asthma, and other symptoms such as fever, hair loss and whitening of hair, cuts, and wounds, spleen enlargement, etc. [19][20]. The leaf juice is used with honey to cure catarrh in infants, shoot juice and mustard oil is taken together for diarrhea and dysentery, and the whole plant is used for the treatment of symptoms related to hepatitis, itching, hemoptysis, bleeding, hematuria, diarrhea, and diphtheria [16]. The leaves and shoots are used in preventing infection in wounds and its treatment in Nepal [7][8][11][21]. Some ethnic groups in South American countries use it to treat snakebites [22]. In Ayurveda, it is used for its revitalizing and anti-aging properties [23]. Many ethnic groups of Bangladesh use it for the treatment of jaundice [24][25]. The plant juice has been used to control, kill, and inhibit the growth of diseases carrying vectors such as mosquito [26][27]. Additionally, it is also used to treat different types of symptoms such as acidity, alopecia [28], gingivitis, fever, body pain, asthma, bronchitis, burns, constipation, wounds, wrinkles, edema, pimples, and other skin diseases [29][30][31][32].
3. Bioactive Chemical Constituents
Eclipta prostrata contains a wide range of active phytoconstituents, which includes coumestan derivatives, triterpene saponins, steroidal saponins, triterpenes, steroids, steroidal alkaloids, flavonoids, phenolic acids, thiophene derivatives and many other compounds. Most of the chemical analysis are reported for whole plant or aerial parts. The detailed list of these compounds is given in Table 1 and Table 2. The structures of main coumestan derivatives, triterpene saponins and flavonoids are represented in Figure 3, Figure 4 and Figure 5, respectively.
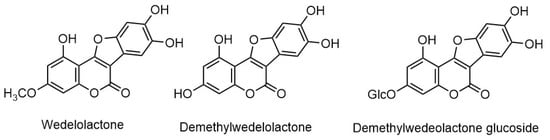
Figure 3. Structures of major coumestan derivatives.
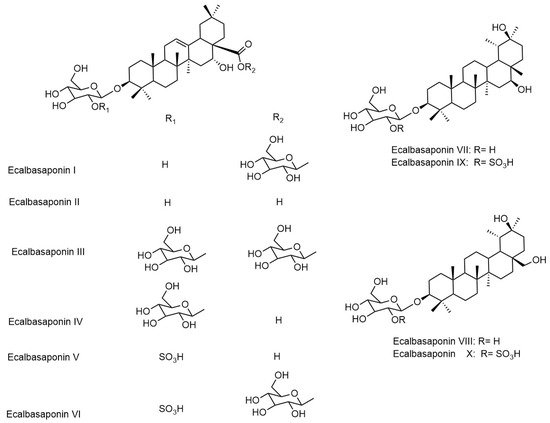
Figure 4. Structures of main triterpene saponins.
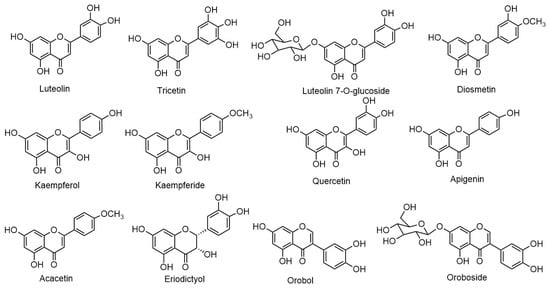
Figure 5. Structures of main flavonoids.
Table 1. Coumestan, steroid and triterpene derivatives and related compounds from various parts of E. prostrata.
| Chemical Compounds (Class/Constituents) | Plant Parts | References |
|---|---|---|
| Coumestan derivatives | ||
| Wedelolactone | Leaves | [21][33][34][35][36] |
| Demethylwedelolactone | Leaves | [35][36] |
| Isodemethylwedelolactone | Whole plant | [36] |
| Strychnolactone | Whole plant | [36] |
| Demethylwedelolactone glucoside | Aerial Parts | [37] |
| Steroidal and triterpene saponins, steroidal alkaloids, steroids and triterpenoids | ||
| Eclalbasaponins I | Whole plant | [34][38][39] |
| Eclalbasaponins II | Whole plant | [34][38][39] |
| Eclalbasaponins III | Whole plant | [34][38][39] |
| Eclalbasaponins IV | Whole plant | [38][39] |
| Eclalbasaponins V | Whole plant | [34][38][39] |
| Eclalbasaponins VI | Whole plant | [38][39] |
| Eclalbasaponins VII | Whole plant | [38] |
| Eclalbasaponins VIII | Whole plant | [38] |
| Eclalbasaponins IX | Whole plant | [38] |
| Eclalbasaponins X | Whole plant | [38] |
| Eclalbasaponin XI | Whole plant | [38] |
| Eclalbasaponin XII | Whole plant | [38] |
| Eclalbasaponin XIII | Whole plant | [38] |
| Eclalbasaponin A | Whole plant | [40] |
| Eclalbasaponin B | Whole plant | [40] |
| Eclalbasaponin C | Whole plant | [40] |
| Eclalbasaponin D | Whole plant | [40] |
| Echinocystic acid | Whole plant | [40] |
| Echinocystic acid-3-O-(6-O-acetyl)-β-D-glucopyranoside | Aerial parts | [41] |
| Eclalbatin | Aerial Parts | [42][43] |
| 3β,25-Dihydroxy-23E-lemmaphyll-8,23-diene | Whole plant | [44] |
| 16α-Hydroxy-olean-12-en-3-on-28,21β-olide | Whole plant | [44] |
| 3β-Hydroxy-17-epi-28-norolean-12-en-16-one 3-O-β-D-glucopyranoside | Whole plant | [44] |
| 3β-O-(6-O-Crotonyl-β-D-glucopyranosyl)-16α-hydroxy-olean-12-en-28-oic acid 28-O-β-D-glucopyranosyl ester | Whole plant | [44] |
| 3-O-(2-O-Acetyl-β-D-glucopyranosyl) oleanolic acid-28-O-(β-D-glucopyranosyl) ester | Aerial parts | [45] |
| 3-O-(6-O-Acetyl-β-D-glucopyranosyl) oleanolic acid-28-O-(β-D-glucopyranosyl) ester | Aerial parts | [45] |
| 3-O-(β-D-Glucopyranosyl) oleanolic acid-28-O-(6-O-acetyl-β-D-glucopyranosyl) ester | Aerial parts | [45] |
| 3β,16β,29-Trihydroxy oleanane-12-ene-3-O-β-D-glucopyranoside | Aerial parts | [46] |
| 3,28-di-O-β-D-Glucopyranosyl-3β,16β-dihydroxy oleanane-12-ene-28-oleanlic acid | Aerial parts | [46] |
| 3-O-β-D-Glucopyranosyl-(1-2)-β-D-glucopyranosyl oleanlic-18- ene acid-28-O-β-D-glucopyranoside | Aerial parts | [46] |
| (20S)(25S)-22,26-Imino-cholesta-5,22(N)-dien-3β-ol (Verazine) | Leaves | [47] |
| 20-epi-3-Dehydroxy-3-oxo-5,6-dihydro-4,5-dehydroverazine | Leaves | [32][47] |
| (20R)-20-Pyridyl-cholesta-5-ene-3β,23-diol (Ecliptalbine) | Leaves | [47] |
| (20R)-25β-Hydroxyverazine | Leaves | [47] |
| 20-epi-4β-Hydroxyverazine | Leaves | [47] |
| 20-epi-25β-Hydroxyverazin | Leaves | [47] |
| 4β-Hydroxyverazine | Leaves | [47] |
| 25β-Hydroxyverazine | Leaves | [47] |
| Lanost-5,24-dien-3β-ol-18, 21-olide -3β- yl tetradecanoate | Whole plant | [48] |
| α-Amyrin | Whole plant | [43] |
| Ursolic acid | Whole plant | [43] |
| Oleanolic acid | Whole plant | [43] |
| 3-Oxo-16α-hydroxy-olean-12-en-28-oic acid | Aerial parts | [49] |
| Machaeroceric acid | Aerial parts | [34] |
| Silphioside C | Whole plant | [50] |
| β-Sitosterol | Whole plant | [36] |
| Stigmasterol | Leaves/Stems | [40] |
| Stigmasterol-3-O-glucoside | Aerial parts/leaves/Stems | [19][34][40] |
| 3-O-(6′-O-Palmitoyl-β-D-glucopyranosyl) stigmasterol | Whole plant | [50] |
| Daucosterol | Leaves/Stems | [40] |
Table 2. Flavonoids, phenolic acids, substituted thiophines, and other compounds present in the E. prostrata.
| Flavonoids | ||
| Luteolin | Aerial parts | [34][41][51] |
| Tricetin | Aerial parts | [34] |
| Luteolin-7-O-β-D-glucoside | Aerial parts | [34][41][51] |
| Diosmetin | Aerial parts | [52] |
| Skullcapflavone Ⅱ | Whole plant | [51] |
| Kaempferol | Whole plant | [51] |
| Kaempferol-7-O-α-D-rhamnoside | Aerial parts | [34] |
| Kaempferide | Whole plant | [51] |
| Quercetin | Aerial parts | [51][53] |
| Quercetin-3-O-β-D-glucoside | Aerial parts | [34] |
| Apigenin | Aerial parts | [34][41][51] |
| Acacetin | Whole plant | [51] |
| Acacetin-7-O-rutinoside | Whole plant | [51] |
| Eriodictyol | Whole plant | [50] |
| Pyracanthoside | Whole plant | [50] |
| Hesperetin-7-O-β-D-glucoside | Aerial parts | [34] |
| 3′-Hydroxybiochanin A | Aerial Parts | [37][49] |
| Orobol (isoluteolin) | Whole plant | [31][34] |
| 7-O-Methylorobol-4′-O-β-D-glucopyranoside | Aerial Parts | [34][49][50] |
| 7-Dihydroxyl-3′, 6′-dimethoxylisoflavone-7-O-glucoside | Whole plant | [51] |
| 3′-O-Methylorobol | Aerial parts | [50][52] |
| Pratensein | Aerial parts | [37][49][50] |
| Pratensein-7-O-β-D-glucopyranoside | Aerial parts | [41][50] |
| Oroboside (Orobol-7-O-β-D-glucoside) | Whole plant | [34][37][50][51] |
| Phenolic acids | ||
| Protocatechuic acid | Leaves/Steam/Whole plant | [34][36][40][43] |
| 4-Hydroxybenzoic acid | Leaves/Steam | [34][40][43] |
| Vanillic acid | Aerial parts | [34] |
| Syringic acid | Aerial parts | [34] |
| Chlorogenic acid | Aerial parts | [34] |
| Syringic acid | Aerial parts | [34] |
| Tachinoside | Whole plant | [50] |
| Coniferylaldehyde | Whole plant | [50] |
| Leonuriside A | Whole plant | [50] |
| Caffeic acid | Whole plant | [50] |
| Ferulic acid ethyl ester | Whole plant | [50] |
| Caffeic acid ethyl ester | Whole plant | [50] |
| Lignin | ||
| Ecliptalignin A | ||
| Coumarins | ||
| Psoralen | Whole plant | [51] |
| Isopsoralen | Whole plant | [51] |
| Polyacetylinic compounds | ||
| (5E)-Hendeca-1,5- dien-7,9-diyne-diol-4-O-β-D-glucopyranoside | Stem | [54] |
| (5E)-Trideca-1,5-dien-7,9,11-triyne-3,4-diol-4-O-β-D-glucopyranoside | Stem | [46][54] |
| 3-O-β-D-Glucopyranosyl1-hydroxy-4E,6E-tetradecene,8,10,12-triyne | Stem | [46][54] |
| 2-O-β-D-Glucosyltrideca-3E,11E-dien5,7,9-triyne-1,2,13-triol | Stem | [54] |
| 2-O-β-D-Glucosyltrideca-3E,11E-dien-5,7,9-triyne-1,2-diol | Stem | [54] |
| 2-O-β-D-Glucosyltrideca-3E,11Z-dien-5,7,9-triyne3–1,2-diol | Stem | [54] |
| Substituted thiophenes | ||
| 5-Hydroxymethyl-(2,2′:5′,2″)-terthienyl tiglate | Whole plant | [55] |
| 5-Hydroxymethyl-(2,2′:5′,2″)-terthienyl agelate | Whole plant | [55] |
| 5-Hydroxymethyl-(2,2′:5′,2″)-terthienyl acetate | Whole plant | [55] |
| 5-Formyl-(2, 2:5, 2″)-terthiophene (Ecliptal) | Whole plant | [56] |
| 5-Hydroxymethyl-(2, 2: 5, 2″)-terthiophene (α-terthienylmethanol) | Whole plant | [56] |
| 5-Methoxy-(2, 2:5, 2″)-terthiophene | Whole plant | [56] |
| 3′-Methoxy-2,2′:5′,2″-terthiophene | Aerial parts | [41] |
| 5-(3″,4″-Dihydroxy-1″-butynyl)-2,2′-bithiophene | Aerial parts | [41] |
| α-Terthienyl | Aerial parts | [41] |
| α-Formylterthienyl | Whole plant | [54] |
| α-Terthienyl methanol | Whole plant | [41][54][56] |
| 3′-Methoxy-2,2′:5′,2″-terthiophene | Aerial parts | [41] |
| 4-(2,2′-Bithiophen-5-yl)but-3-yne-1,2-diol | Aerial parts | [57] |
| Arctinol B | Aerial parts | [57] |
| 2-(Penta-1,3-diynyl)-5-(3,4-dihydroxy-but-1-ynyl)-thiophene | Aerial parts | [57] |
| 6-Methoxy-arctinol-b | Aerial parts | [57] |
| 5-[l-(4-Hydroxybut-l-ynyl)]-2,20 -bithiophene-50 -carbaldehyde | Aerial parts | [57] |
| 5-Hydroxymethyl- (2,2′:5′,2′’-terthienyl) | Aerial parts | [57] |
| 5′-Hydroxymethyl-5-(3-butene-1-ynyl)-2,2′ -bithiophene | Aerial parts | [46][57] |
| 3′-Hydroxy-2,2′:5′,2′’ terthiophene-3′-O-β-D-glucopyranoside | Aerial parts | [57] |
| Ecliprostin A | Aerial parts | [58] |
| Ecliprostin B | Aerial parts | [58] |
| Ecliprostin C | Aerial parts | [58] |
| Alkaloids | ||
| Crinumaquine | Whole plant | [51] |
| 2,3,9,12-Tetramethoxyprotoberberine | Whole plant | [51] |
| Lignans | ||
| Pinoresinol-4-O-β-D-glucopyranoside | Whole plant | [50] |
| 4,4′-Dimethoxy-3′-hydroxy-7,9′:7′,9-diepoxylignan-3-O-β-D-glucopyranoside | Whole plant | [50] |
| Syringaresinol-4′-O-β-D-glucopyroside | Whole plant | [50] |
| Lanicepside A | Whole plant | [50] |
| Longifloroside | Whole plant | [50] |
| Other compounds | ||
| 1-O-Octadecanoyl-2-O-(9Z,12Z-octadecadienoyl)-3-O-[α-D-galactopyranosyl- (1′′→6′)-O-β-D-galactopyranosyl]glycerol | Whole plant | [50] |
| (2S)-3-O-α-D-Galactopyranosyl-(1′′→6′)-β-D-galactopyranosyl-1,2-di-O-[(9Z,12Z,15Z)-octadeca-9,12,15-trienoyl]-sn-glycerol | Whole plant | [50] |
| 1-O-(9Z,12Z,15Z-Octadecatrienoyl)-2-O-hexadecanoyl-3-O-[α-D-galactopyranosyl-(1′′→6′)-O-β-D-galactopyranosyl]glycerol | Whole plant | [50] |
| 1-O-(β-D-glucopyranosyl)- (2S,3S,4R,8Z)-2N-[(2′R)-2′-hydroxytetracosanoyl]-8-(Z)-octadecene-1,3,4-triol | Whole plant | [50] |
| (2S,3S,4R,10E)-2-[(2′R)-2′- Hydroxytetracosanoylamino]-10-octadecene-1,3,4-triol | Whole plant | [50] |
| (3S,5R,6S,7E,9R)-3-Hydroxy-5,6-epoxy-β-ionyl-3-O-β-D-glucopyranoside | Whole plant | [50] |
| Euodionoside A | Whole plant | [50] |
| Junipeionoloside | Whole plant | [50] |
| Calaliukiuenoside | Whole plant | [50] |
| rel-(1S,2S,3S,4R,6R)-1,6-Epoxy-menthane-2,3-diol-3-O-β-D-glucopyranoside | Aerial parts | [46] |
| rel-(1S,2S,3S,4R,6R)-3-O-(6-O-caffeoyl-β-D-glucopyranosyl)-1,6-epoxy menthane-2,3-diol | Aerial parts | [46] |
| Siliphioside E | Aerial parts | [46] |
| (2E,6E)- 2,6,10-trimethyl-2,6,11-dodecatriene-1,10-diol-1-O-β-D-glucopyranoside | Aerial parts | [46] |
| (2S)-1-O-Stearoyl-3-O-β-D-galactopyranosyl-sn-glycerol | Aerial parts | [34] |
| (2S)-3-O-(9Z,12Z-Octadecadienoyl) glyceryl-O-β-D-galactopyranoside | Aerial parts | [34] |
| Bidensmenthoside A | Whole plant | [50] |
| Bidensmenthoside B | Whole plant | [50] |
| 11β,17-Dihydroxy-beyer-15-ene | Whole plant | [44] |
| 4β- Hydroxy-guai-10(14),11(13)-dien-12-oic acid | Whole plant | [44] |
References
- Ambu, G.; Chaudhary, R.P.; Mariotti, M.; Cornara, L. Traditional Uses of Medicinal Plants by Ethnic People in the Kavrepalanchok District, Central Nepal. Plants 2020, 9, 759.
- Kunwar, R.M.; Bussmann, R.W. Ethnobotany in the Nepal Himalaya. J. Ethnobiol. Ethnomed. 2008, 4, 24.
- Fitzgerald, M.; Heinrich, M.; Booker, A. Medicinal plant analysis: A historical and regional discussion of emergent complex techniques. Front. Pharmacol. 2020, 10, 1480.
- David, B.; Wolfender, J.L.; Dias, D.A. The pharmaceutical industry and natural products: Historical status and new trends. Phytochem. Rev. 2015, 14, 299–315.
- Khanal, A.; Devkota, H.P.; Kaundinnyayana, S.; Gyawali, P.; Ananda, R.; Adhikari, R. Culinary Herbs and Spices in Nepal: A Review of Their Traditional Uses, Chemical Constituents, and Pharmacological Activities. Ethnobot. Res. Appl. 2021, 21, 1–18.
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614.
- Manandhar, N.P. Plants and People of Nepal; Timber Press: Portland, OR, USA, 2002.
- Sherchan, J.; Poudel, P.; Sapkota, B.; Jan, H.A.; Bussmann, R.W. Eclipta prostrata (L.) L. Asteraceae. In Ethnobotany of the Himalayas; Kunwar, R.M., Sher, H., Bussmann, R.W., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–19. ISBN 978-3-030-45597-2.
- Uddin, M.N.; Rahman, M.A.; Ahmed, N.U.; Rana, M.S.; Akter, R.; Chowdhury, A.M.A. Antioxidant, Cytotoxic and Antimicrobial Properties of Eclipta alba Ethanol Extract. Int. J. Biol. Med. Res. 2010, 4, 341–346.
- Baskaran, P.; Jayabalan, N. An Efficient Micropropagation System for Eclipta alba—A Valuable Medicinal Herb. In Vitro Cell. Dev. Biol. Plant 2005, 41, 532–539.
- Adhikari, M.; Thapa, R.; Kunwar, R.M.; Devkota, H.P.; Poudel, P. Ethnomedicinal Uses of Plant Resources in the Machhapuchchhre Rural Municipality of Kaski District, Nepal. Medicines 2019, 6, 69.
- Mansoorali, K.P.; Prakash, T.; Kotresha, D.; Prabhu, K.; Rama Rao, N. Cerebroprotective Effect of Eclipta alba against Global Model of Cerebral Ischemia Induced Oxidative Stress in Rats. Phytomedicine 2012, 19, 1108–1116.
- Gupta, A.; Kumar, A.; Kumar, D.; Nandan, S.; Shankar, K.; Varshney, S.; Rajan, S.; Srivastava, A.; Gupta, S.; Kanojiya, S.; et al. Ethyl Acetate Fraction of Eclipta alba: A Potential Phytopharmaceutical Targeting Adipocyte Differentiation. Biomed. Pharmacother. 2017, 96, 572–583.
- Hussain, I.; Khan, N.; Ullah, R.; Ahmed, S.; Khan, F.A.; Yaz, S. Phytochemical, Physiochemical and Anti-Fungal Activity of Eclipta alba. Afr. J. Pharm. Pharmacol. 2011, 5, 2150–2155.
- Global Biodiversity Information Facility Secretariat (GBIF). Eclipta prostrata (L.) L. Available online: https://www.gbif.org/species/5384950 (accessed on 1 November 2021).
- Bakht, J.; Islam, A.; Ali, H.; Tayyab, M.; Shafi, M. Antimicrobial Potentials of Eclipta alba by Disc Diffusion Method. Afr. J. Biotechnol. 2011, 10, 7658–7667.
- Jayathirtha, M.G.; Mishra, S.H. Preliminary Immunomodulatory Activities of Methanol Extracts of Eclipta alba and Centella asiatica. Phytomedicine 2004, 11, 361–365.
- Datta, K.; Singh, A.T.; Mukherjee, A.; Bhat, B.; Ramesh, B.; Burman, A.C. Eclipta alba Extract with Potential for Hair Growth Promoting Activity. J. Ethnopharmacol. 2009, 124, 450–456.
- Jahan, R.; Al-Nahain, A.; Majumder, S.; Rahmatullah, M. Ethnopharmacological Significance of Eclipta alba (L.) Hassk. (Asteraceae). Int. Sch. Res. Not. 2014, 2014, 385969.
- Dalal, S.; Kataria, S.; Sastry, K.; Rana, S.V.S. Phytochemical Screening of Methanolic Extract and Antibacterial Activity of Active Principles of Hepatoprotective Herb, Eclipta alba. Ethnobot. Leafl. 2010, 2010, 3.
- Gautam, T.P. Indigenous Uses of Some Medicinal Plants in Panchthar District, Nepal. Nepal. J. Biosci. 2011, 1, 125–130.
- Diogo, L.C.; Fernandes, R.S.; Marcussi, S.; Menaldo, D.L.; Roberto, P.G.; Matrangulo, P.V.F.; Pereira, P.S.; França, S.C.; Giuliatti, S.; Soares, A.M.; et al. Inhibition of Snake Venoms and Phospholipases A2 by Extracts from Native and Genetically Modified Eclipta alba: Isolation of Active Coumestans. Basic Clin. Pharmacol. Toxicol. 2009, 104, 293–299.
- Puri, H.S. Rasayana: Ayurvedic Herbs for Longevity and Rejuvenation. J. Altern. Complement. Med. 2003, 9, 331–332.
- Rai, M.B. Medicinal Plants of Tehrathum District, Eastern Nepal. Our Nat. 2003, 1, 42–48.
- Badgujar, S.B.; Patil, M.B. Ethnomedicines for Jaundice Used in Tribal Areas of North Maharashtra. Ind. J. Nat. Prod. Resour 2008, 7, 79–81.
- Govindarajan, M.; Karuppannan, P. Mosquito Larvicidal and Ovicidal Properties of Eclipta alba (L.) Hassk (Asteraceae) against Chikungunya Vector, Aedes aegypti (Linn.) (Diptera: Culicidae). Asian Pac. J. Trop. Med. 2011, 4, 24–28.
- Rajith, N.P.; Ramachandran, V.S. Ethnomedicines of Kurichyas, Kannur District, Western Ghats, Kerala. Indian J. Nat. Prod. Resour. 2010, 1, 249–253.
- Roy, R.K.; Thakur, M.; Dixit, V.K. Hair Growth Promoting Activity of Eclipta alba in Male Albino Rats. Arch. Dermatol. Res. 2008, 300, 357–364.
- Khan, A.V.; Khan, A.A. Ethnomedicinal Uses of Eclipta prostrata Linn. Indian J. Trad. Knowl. 2008, 7, 316–320.
- Kumari, C.S.; Govindasamy, S.; Sukumar, E. Lipid Lowering Activity of Eclipta prostrata in Experimental Hyperlipidemia. J. Ethnopharmacol. 2006, 105, 332–335.
- Tewtrakul, S.; Subhadhirasakul, S.; Cheenpracha, S.; Karalai, C. HIV-1 Protease and HIV-1 Integrase Inhibitory Substances from Eclipta prostrata. Phytother. Res. 2007, 21, 1092–1095.
- Neeraja, P.V.; Margaret, E. Eclipta alba (L.) Hassk: A Valuable Medicinal Herb. Int. J. Curr. Pharm. Rev. Res. 2011, 2, 188–197.
- Kaushik-Basu, N.; Bopda-Waffo, A.; Talele, T.T.; Basu, A.; Costa, P.R.R.; da Silva, A.J.M.; Sarafianos, S.G.; Noël, F. Identification and Characterization of Coumestans as Novel HCV NS5B Polymerase Inhibitors. Nucleic Acids Res. 2008, 36, 1482–1496.
- Le, D.D.; Nguyen, D.H.; Ma, E.S.; Lee, J.H.; Min, B.S.; Choi, J.S.; Woo, M.H. PTP1B Inhibitory and Anti-Inflammatory Properties of Constituents from Eclipta prostrata L. Biol. Pharm. Bull. 2021, 44, 298–304.
- Wagner, H.; Geyer, B.; Kiso, Y.; Hikino, H.; Rao, G. Coumestans as the Main Active Principles of the Liver Drugs Eclipta alba and Wedelia calendulacea. Planta Med. 1986, 52, 370–374.
- Zhang, J.S.; Guo, Q.M. Studies on the chemical constituents of Eclipta prostrata (L). Yao Xue Xue Bao 2001, 36, 34–37.
- Feng, L.; Zhai, Y.-Y.; Xu, J.; Yao, W.-F.; Cao, Y.-D.; Cheng, F.-F.; Bao, B.-H.; Zhang, L. A Review on Traditional Uses, Phytochemistry and Pharmacology of Eclipta prostrata (L.) L. J. Ethnopharmacol. 2019, 245, 112109.
- Yahara, S.; Ding, N.; Nohara, T.; Masuda, K.; Ageta, H. Taraxastane Glycosides from Eclipta alba. Phytochemistry 1997, 44, 131–135.
- Yahara, S.; Ding, N.; Nohara, T. Oleanane Glycosides from Eclipta alba. Chem. Pharm. Bull. 1994, 42, 1336–1338.
- Zhang, M.; Chen, Y.Y.; Di, X.H.; Liu, M. Isolation and identification of ecliptasaponin D from Eclipta alba (L.) Hassk. Yao Xue Xue Bao 1997, 32, 633–634.
- Kim, D.-I.; Lee, S.-H.; Choi, J.-H.; Lillehoj, H.S.; Yu, M.-H.; Lee, G.-S. The Butanol Fraction of Eclipta prostrata (Linn) Effectively Reduces Serum Lipid Levels and Improves Antioxidant Activities in CD Rats. Nutr. Res. 2008, 28, 550–554.
- Khanna, V.G.; Kannabiran, K. Anticancer-Cytotoxic Activity of Saponins Isolated from the Leaves of Gymnema Sylvestre and Eclipta prostrata on HeLa Cells. Int. J. Green Pharm. 2009, 3, 227–229.
- Upadhyay, R.K.; Pandey, M.B.; Jha, R.N.; Pandey, V.B. Eclalbatin, a Triterpene Saponin from Eclipta alba. J. Asian Nat. Prod. Res. 2001, 3, 213–217.
- Yu, S.-J.; Yu, J.-H.; Yu, Z.-P.; Yan, X.; Zhang, J.-S.; Sun, J.; Zhang, H. Bioactive Terpenoid Constituents from Eclipta prostrata. Phytochemistry 2020, 170, 112192.
- Xi, F.-M.; Li, C.-T.; Mi, J.-L.; Wu, Z.-J.; Chen, W.-S. Three New Olean-Type Triterpenoid Saponins from Aerial Parts of Eclipta prostrata (L.). Nat. Prod. Res. 2014, 28, 35–40.
- Xi, F.-M.; Li, C.-T.; Han, J.; Yu, S.-S.; Wu, Z.-J.; Chen, W.-S. Thiophenes, Polyacetylenes and Terpenes from the Aerial Parts of Eclipta prostrata. Bioorg. Med. Chem. 2014, 22, 6515–6522.
- Abdel-Kader, M.S.; Bahler, B.D.; Malone, S.; Werkhoven, M.C.M.; van Troon, F.; David, ⊥., II; Wisse, J.H.; Bursuker, I.; Neddermann, K.M.; Mamber, S.W.; et al. DNA-Damaging Steroidal Alkaloids from Eclipta alba from the Suriname Rainforest. J. Nat. Prod. 1998, 61, 1202–1208.
- Sethiya, N.; Tomer, K.; Singh, V.; Kumar, M.; Jaiswal, D.; Yadav, I.; Singh, H.; Chandra, D.; Jain, D. Isolation and Characterization of New Lanosteriod from Ethanolic Extract of Eclipta alba Linn. J. Pharm. Res. 2009, 2, 1635–1637.
- Han, L.; Zhao, J.; Zhang, Y.; Kojo, A.; Liu, E.; Wang, T. Chemical Constituents from Dried Aerial Parts of Eclipta prostrata. Chin. Herb. Med. 2013, 5, 313–316.
- Xiong, H.-P.; Xi, F.-M.; Chen, W.-S.; Lu, W.-Q.; Wu, Z.-J. Chemical Constituents of Eclipta prostrata. Chem. Nat. Compd. 2021, 57, 166–168.
- Li, W.; Pang, X.; Han, L.-F.; Zhou, Y.; Cui, Y.-M. Chemical constituents of Eclipta prostrata. China J. Chin. Mater. Medica 2018, 43, 3498–3505.
- Lee, M.K.; Ha, N.R.; Yang, H.; Sung, S.H.; Kim, Y.C. Stimulatory Constituents of Eclipta prostrata on Mouse Osteoblast Differentiation. Phytother. Res. 2009, 23, 129–131.
- Zhao, Y.; Peng, L.; Lu, W.; Wang, Y.; Huang, X.; Gong, C.; He, L.; Hong, J.; Wu, S.; Jin, X. Effect of Eclipta prostrata on Lipid Metabolism in Hyperlipidemic Animals. Exp. Gerontol. 2015, 62, 37–44.
- Meng, X.; Li, B.-B.; Lin, X.; Jiang, Y.-Y.; Zhang, L.; Li, H.-Z.; Cui, L. New Polyacetylenes Glycoside from Eclipta Prostrate with DGAT Inhibitory Activity. J. Asian Nat. Prod. Res. 2019, 21, 501–506.
- Tewtrakul, S.; Subhadhirasakul, S.; Tansakul, P.; Cheenpracha, S.; Karalai, C. Antiinflammatory Constituents from Eclipta prostrata Using RAW264.7 Macrophage Cells. Phytother. Res. 2011, 25, 1313–1316.
- Lee, J.-S.; Ahn, J.-H.; Cho, Y.-J.; Kim, H.-Y.; Yang, Y.-I.; Lee, K.-T.; Jang, D.-S.; Choi, J.-H. α-Terthienylmethanol, Isolated from Eclipta prostrata, Induces Apoptosis by Generating Reactive Oxygen Species via NADPH Oxidase in Human Endometrial Cancer Cells. J. Ethnopharmacol. 2015, 169, 426–434.
- Yu, S.-J.; Zhang, J.-S.; He, H.; Yu, J.-H.; Bao, J.; Zhang, H. Thiophene Enantiomers from the Aerial Parts of Eclipta prostrata. J. Asian Nat. Prod. Res. 2021, 23, 745–753.
- Yu, S.-J.; Yu, J.-H.; He, F.; Bao, J.; Zhang, J.-S.; Wang, Y.-Y.; Zhang, H. New Antibacterial Thiophenes from Eclipta prostrata. Fitoterapia 2020, 142, 104471.
More
Information
Subjects:
Tropical Medicine
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
4.0K
Entry Collection:
Gastrointestinal Disease
Revisions:
2 times
(View History)
Update Date:
09 Dec 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




