
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Chloé Astruc | + 1471 word(s) | 1471 | 2021-11-24 07:35:34 | | | |
| 2 | Nora Tang | Meta information modification | 1471 | 2021-12-13 01:55:22 | | |
Video Upload Options
The main components of calves’ GIM are Ruminococcaceae and Lachnospiraceae (40%) (Firmicutes phylum), and Bacteroidaceae (15%) (Bacteroides phylum), followed by Enterobacterales (25%) (Proteobacteria phylum), which decreases during GIM maturation (5%), whereas Prevotellaceae increases (20%) (Bacteroidetes phylum). The composition of feedcolostrum and GIM in neonate calves is similar, and GIM’s evolution occurs rapidly during the first 10 weeks of life. Amounts of ARGs were found higher in calves than in adult animals reared in the same environment. Living conditions, such as wet soil and the number of cattle residing in the farm (>500), were risk factors for colonization with cefotaxime (third generation cephalosporin, GC) resistant bacteria. A decrease of Enterobacterales during the first weeks of life has been associated with a general decrease in ARGs abundance in calves, with breed influencing the abundance of certain ARGs and ampC gene (copy number).
1. Effect of Waste Milk Feeding on Calves’ GIM
2. Therapeutic Concentration of Antibiotics in Calves
2.1. Beta-Lactams
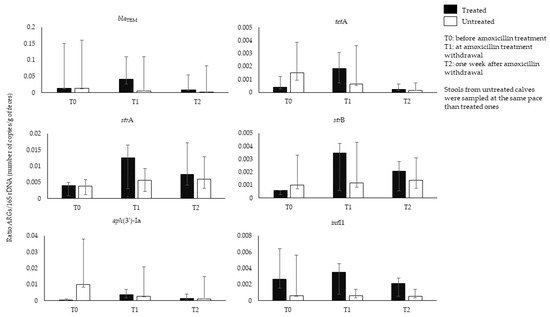
2.2. Original Data on the Analysis of Amoxicillin Effects on Calves’ GIM
2.3. Macrolides
2.4. Tetracyclines
2.5. Other Antibiotics
References
- Penati, M.; Sala, G.; Biscarini, F.; Boccardo, A.; Bronzo, V.; Castiglioni, B.; Cremonesi, P.; Moroni, P.; Pravettoni, D.; Addis, M.F. Feeding pre-weaned calves with waste milk containing antibiotic residues is related to a higher incidence of diarrhea and alterations in the fecal microbiota. Front. Vet. Sci. 2021, 8, 650150.
- Dupouy, V.; Madec, J.-Y.; Wucher, J.; Arpaillange, N.; Métayer, V.; Roques, B.; Bousquet-Mélou, A.; Haenni, M. Selection of ESBL-producing Escherichia coli in the gut of calves experimentally fed with milk containing antibiotic residues. Vet. Microbiol. 2021, 257, 109049.
- Maynou, G.; Chester-Jones, H.; Bach, A.; Terré, M. Feeding pasteurized waste milk to preweaned dairy calves changes fecal and upper respiratory tract microbiota. Front. Vet. Sci. 2019, 6, 159.
- Pereira, R.V.V.; Carroll, L.; Lima, S.; Foditsch, C.; Siler, J.D.; Bicalho, R.C.; Warnick, L.D. Impacts of feeding preweaned calves milk containing drug residues on the functional profile of the fecal microbiota. Sci. Rep. 2018, 8, 554.
- Van Vleck Pereira, R.; Lima, S.; Siler, J.D.; Foditsch, C.; Warnick, L.D.; Bicalho, R.C. Ingestion of milk containing very low concentration of antimicrobials: Longitudinal effect on fecal microbiota composition in preweaned calves. PLoS ONE 2016, 11, e0147525.
- Grønvold, A.-M.R.; Mao, Y.; L’Abée-Lund, T.M.; Sørum, H.; Sivertsen, T.; Yannarell, A.C.; Mackie, R.I. Fecal microbiota of calves in the clinical setting: Effect of penicillin treatment. Vet. Microbiol. 2011, 153, 354–360.
- Ma, T.; Villot, C.; Renaud, D.; Skidmore, A.; Chevaux, E.; Steele, M.; Guan, L.L. Linking perturbations to temporal changes in diversity, stability, and compositions of neonatal calf gut microbiota: Prediction of diarrhea. ISME J. 2020, 14, 2223–2235.
- Jarrige, N.; Cazeau, G.; Bosquet, G.; Bastien, J.; Benoit, F.; Gay, E. Effects of antimicrobial exposure on the antimicrobial resistance of Escherichia coli in the digestive flora of dairy calves. Prev. Vet. Med. 2020, 185, 105177.
- Rochegüe, T.; Haenni, M.; Cazeau, G.; Metayer, V.; Madec, J.-Y.; Ferry, T.; Lupo, A. An inventory of 44 qPCR assays using hydrolysis probes operating with a unique amplification condition for the detection and quantification of antibiotic resistance genes. Diagn. Microbiol. Infect. Dis. 2021, 100, 115328.
- Martin, C.C.; Baccili, C.C.; Avila-Campos, M.J.; Hurley, D.J.; Gomes, V. Effect of prophylactic use of tulathromycin on gut bacterial populations, inflammatory profile and diarrhea in newborn Holstein calves. Res. Vet. Sci. 2021, 136, 268–276.
- Hagiya, H.; Kimura, K.; Nishi, I.; Yamamoto, N.; Yoshida, H.; Akeda, Y.; Tomono, K. Desulfovibrio desulfuricans bacteremia: A case report and literature review. Anaerobe 2018, 49, 112–115.
- Foditsch, C.; Pereira, R.; Siler, J.D.; Altier, C.; Warnick, L.D. Effects of treatment with enrofloxacin or tulathromycin on fecal microbiota composition and genetic function of dairy calves. PLoS ONE 2019, 14, 1–18.
- Bringhenti, L.; Pallu, M.; Silva, J.; Tomazi, T.; Tomazi, A.C.; Rodrigues, M.X.; Duarte, L.M.; Bilby, T.R.; Bicalho, R.C. Effect of metaphylactic administration of tildipirosin on the incidence of pneumonia and otitis and on the upper respiratory tract and fecal microbiome of preweaning Holstein calves. J. Dairy Sci. 2021, 104, 6020–6038.
- Massot, M.; Haenni, M.; Nguyen, T.T.; Madec, J.-Y.; Mentré, F.; Denamur, E. Temporal dynamics of the fecal microbiota in veal calves in a 6-month field trial. Anim. Microbiome 2020, 2, 32.
- Keijser, B.J.F.; Agamennone, V.; Broek, T.J.V.D.; Caspers, M.; Van De Braak, A.; Bomers, R.; Havekes, M.; Schoen, E.; Van Baak, M.; Mioch, D.; et al. Dose-dependent impact of oxytetracycline on the veal calf microbiome and resistome. BMC Genom. 2019, 20, 65.
- Oultram, J.; Phipps, E.; Teixeira, A.G.V.; Foditsch, C.; Bicalho, M.L.; Machado, V.S.; Bicalho, R.C.; Oikonomou, G. Effects of antibiotics (oxytetracycline, florfenicol or tulathromycin) on neonatal calves’ faecal microbial diversity. Vet. Rec. 2015, 177, 598.
- Thames, C.H.; Pruden, A.; James, R.E.; Ray, P.P.; Knowlton, K. Excretion of antibiotic resistance genes by dairy calves fed milk replacers with varying doses of antibiotics. Front. Microbiol. 2012, 3, 139.
- Lhermie, G.; Dupouy, V.; El Garch, F.; Ravinet, N.; Toutain, P.L.; Bousquet-Mélou, A.; Seegers, H.; Assié, S. Impact of low and high doses of marbofloxacin on the selection of resistant Enterobacteriaceae in the commensal gut flora of young cattle: Discussion of data from 2 study populations. Foodborne Pathog. Dis. 2017, 14, 152–159.
- Dobrzanska, D.A.; Lamaudière, M.T.F.; Rollason, J.; Acton, L.; Duncan, M.; Compton, S.; Simms, J.; Weedall, G.D.; Morozov, I.Y. Preventive antibiotic treatment of calves: Emergence of dysbiosis causing propagation of obese state-associated and mobile multidrug resistance-carrying bacteria. Microb. Biotechnol. 2020, 13, 669–682.





