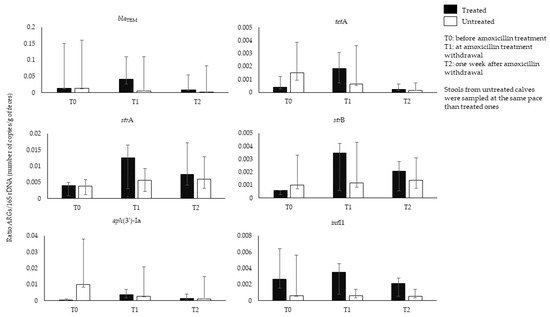
History of amoxicillin therapy has been associated to the rise of resistance in
E.
coli isolates from calves’ feces [
53]. To our knowledge, longitudinal studies analyzing the effects of amoxicillin on calves’ GIM were lacking. We thus prospectively collected feces from calves (n = 16) aged from 5 to 26 days, belonging to five breeds (Charolais/Montbeliard, Montbeliard, Prim’ Holstein, Charolais/Prim’ Holstein, Limousin/Montbeliard), and resident in different farms (n = 7) in the Rhône-Alpes region (France), during the period October 2018–March 2020. Eleven out of 16 calves were suffering from omphalitis (umbilical cord infection) and were treated with IMI of amoxicillin (Longamox
®, 15 mg/kg) for a duration varying between 4 and 16 days. The remaining five calves did not receive antibiotic treatment, and their feces were sampled at the same pace of the treated ones. The abundance of 41
ARGs,
intI1/2/3, and of 16S rDNA was analyzed by qPCR [
54]. Seventeen out of 41 investigated
ARGs were found in the feces of all calves before amoxicillin treatment. The
blaTEM gene and bacterial abundance were comparable between the treated and untreated group before treatment (ratio
blaTEM/16S rDNA: 0.013 in both groups). At the end of amoxicillin treatment (T1), the amount of
blaTEM increased in treated calves (
blaTEM/16S rDNA ratio: 0.040) along with other
ARGs (
tetA,
strA and
strB), and
intI1, index of class 1 integrons. These data suggest co-selection by amoxicillin treatment of
ARGs related to other antibiotic classes and potential multidrug development (
Figure 1). A decrease of all
ARGs was observed 1 week after amoxicillin withdrawal (T2) (
blaTEM/16S rDNA ratio: 0.008). The amount of
blaTEM constantly decreased in the untreated group (
blaTEM/16S rDNA ratio: T1, 0.005; T2: 0.002). The difference observed in the amount of
ARGs at pretreatment and post-treatment, or between treated and untreated group, was not statistically significant (Wilcoxon Mann Whitney or Wilcoxon signed-rank test,
p > 0.05). Several factors could confound the effect of the amoxicillin treatment on the
blaTEM amount, for instance variation of the
blaTEM gene amount among individuals, probably due to variation of calves’ age, which ranged from 5 to 26 days. Age is a determinant for GIM composition and
ARGs amount at early life. In addition, calves were distributed in seven commercial farms probably contributing to the difference in
ARGs content as well, because of different farm management. Environmental exposure of all calves to
ARGs cannot be excluded, as calves of the untreated and treated group lived in the same farm, thus probably influencing the level of difference of
blaTEM amount between the two groups. For a better understanding of antibiotics action on the GIM and selection of
ARGs, experiments in environmentally controlled set-up would be a benefit for avoiding confounding factors influencing GIM composition and
ARGs variation further than antibiotic action. However, studies in commercial farms are necessary to model antibiotic therapies effects in a real-life environment and evaluate
ARGs propagation to other hosts or in the farm environment.