
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Chen Yan | + 3708 word(s) | 3708 | 2021-11-29 05:19:25 | | | |
| 2 | Peter Tang | Meta information modification | 3708 | 2021-12-08 03:37:04 | | |
Video Upload Options
Chitosan is a nontoxic natural antimicrobial polymer and is approved by GRAS (Generally Recognized as Safe by the United States Food and Drug Administration). Chitosan and chitosan derivatives can kill microbes by neutralizing negative charges on the microbial surface. Besides, chemical modifications give chitosan derivatives better water solubility and antimicrobial property.
1. Introduction
2. General Properties of Chitosan

3. Chemical Modification of Chitosan

|
Polymer |
Preparation |
Biological Activities |
Citation |
|---|---|---|---|
|
Hydroxypropyl chitosan |
Reacting with propylene epoxide under alkaline medium (NaOH) |
Water solubility, film-forming property, antibacterial property |
|
|
Thioglycolic Chitosan |
Reacting with 1-ethyl-3-(3-dimethyl aminopropyl) carbodiimide and thioglycolic acid |
High antimicrobial property and good mucoadhesive property |
|
|
N-(2-(N,N,N-trimethylammoniumyl)acetyl)-chitin |
A combination of Boc and tert-butyldimethylsilyl (TBDMS) protection strategies |
Antimicrobial property and enhancing permeation |
|
|
Carboxymethyl chitosan |
Reacting with 2-chloroacetic acid with NaOH |
Enhanced antimicrobial property and water solubility |
[39] |
|
N,N,N-trimethyl chitosan |
Reacting with methylation reagents |
Antimicrobial property and enhanced solubility in alkaline medium |
|
|
N-(2-Hydroxyl) Propyl-3- Trimethyl Ammonium Chitosan |
Reacting with glycidyl trimethyl ammonium chloride |
Antimicrobial property and good aqueous solubility in acidic, neutral, and alkaline medium |
|
|
Chitosan- polyethylene glycol-peptide (PEG)-peptide conjugate |
The chitosan was PEGylated by a carboxyl and azideterminated polyethylene glycol; peptide was conjugated onto chitosan-PEG through a click reaction |
Antimicrobial property and antibiofilm activity against Pseudomonas aeruginosa |
[56] |
|
Thiosemicarbazone O-Carboxymethyl-chitosan (TCNCMCs) derivatives |
Preparation of O-Carboxymethyl chitosan (CMCs): alkalized chitosan reacting with monochloroacetic, acetic acid, and methanol; TCNCMCs preparation: CMC reacting with ammonium hydroxide and carbon disulfide, followed by adding sodium chloroacetate and hydrazine hydrate |
High antimicrobial and antifungal properties |
[57] |
4. Action Modes of Chitosan against Pathogen Microorganisms

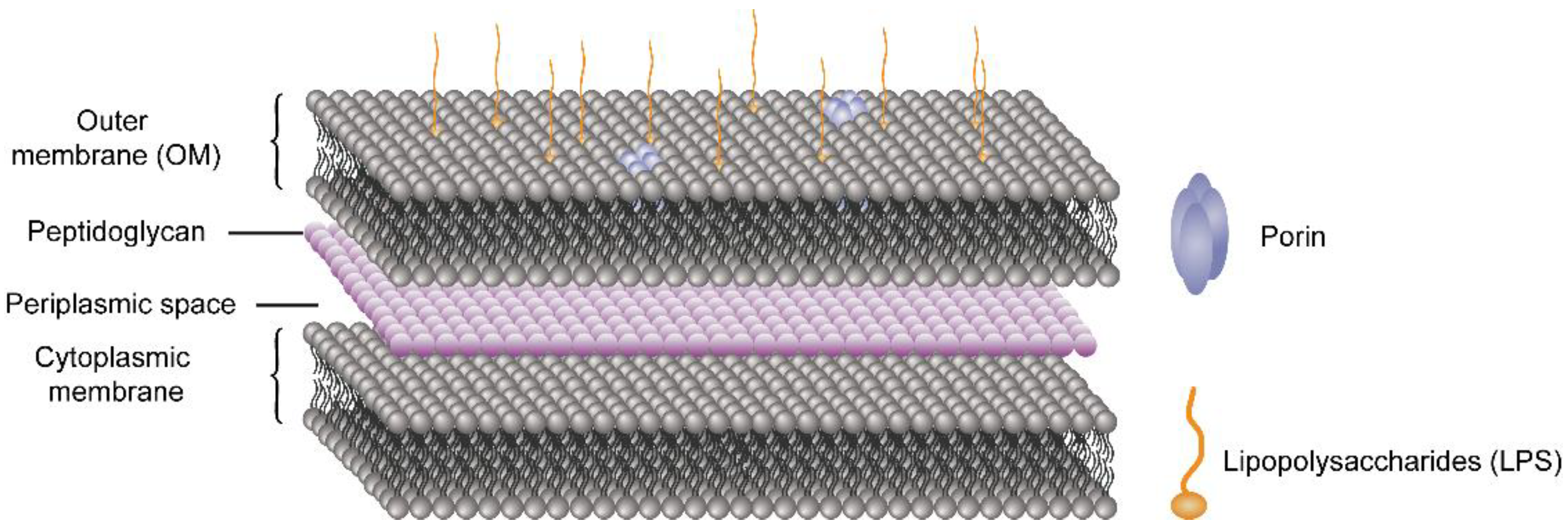
5. Current Treatment of the Enteric Infections
6. Antibiofilm Properties of Chitosan and Chitosan Derivatives
7. Gut Microbiota and Colonization Resistance against Enteric Infections
8. The Role of Chitosan in the Treatment of Enteric Infections
8.1. Chitosan as Drug Delivery System
8.2. Chitosan as Antimicrobial Agents
8.3. Chitosan Conjugation with Other Polymers or Nanoparticles
|
Chitosan Conjugation with Other Polymers and Nanoparticles |
Preparation |
Biological Activities |
Citation |
|---|---|---|---|
|
Chitosan coated PLA (poly d, l-lactic acid) nanoparticles |
Coated on the surface of PLA nanoparticles which are prepared by nanoprecipitation method |
High cornea permeation and high sustained release of 5-FU in conjunctival/corneal squamous cell carcinoma |
[136] |
|
Antibody-conjugated chitosan nanoparticles |
Preparation of chitosan nanoparticles (CNs): chitosans are dissolved in 2% acetic acid and mixed with 1% Tween 80, followed by the addition of a 10% sodium sulfate solution and centrifugation at 8200 g; Bioconjugation of IgY antibodies to CN: The CNs are dissolved in 0.1 M sodium acetate buffer, followed by an addition of antibodies, 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC), sulfo-N-hydroxysulfosuccinimide, and the centrifugation at 39,800 g |
Enhanced and specific antimicrobial activities against Shiga toxin-producing Escherichia coli (STEC) |
[133] |
|
Chitosan-based nanocomposites |
Chitosan prepared in acetic acid, silver, and copper nanoparticles are dispersed in ethanol by sonication and precipitated in an alkaline medium. |
Increased antimicrobial properties |
[137] |
|
Cranberry proanthocyanidins-chitosan composite nanoparticles (PAC-CHT NPs) |
Chitosan (CHT) is prepared in 0.5% acetic acid, filtered and degassed, followed by linking to cranberry proanthocyanidins (PAC) through hydrogen bonding. |
Higher bioactive than CHT and PAC alone. Increased bioactivity of PAC-CHT NPs against E. coli. |
[134] |
|
Antibody-loaded-mannose-modified chitosan microspheres |
Mannose-modified chitosan (MC) preparation: dissolved chitosan is treated with mannose and sodium cyanoborohydride; chitosan microsphere preparation: sodium tripolyphosphate (TPP) solution is added dropwise to MCs under 15 W sonication; antibody-loaded chitosan microsphere preparation: dispersing 5 mg of antibodies in 1.0 mL of phosphate-buffered saline (PBS) containing 30 mg of microspheres. |
Mannose-modified chitosan microspheres can serve as a promising subunit delivery system for vaccines against P. aeruginosa infection. |
[135] |
|
Chitosan-Caffeic Acid Conjugate |
Chitosan is dissolved in 2% acetic acid and reacts with 1.0 M hydrogen peroxide containing ascorbic acid. Caffeic acid is added to the mixture for 24 h at room temperature. |
Antibacterial activity of against acne-related bacteria |
[138] |
8.4. Chitosan as Prebiotics to Improve Colonization Resistance against Enteric Pathogens
References
- Saberpour, M.; Bakhshi, B.; Najar-Peerayeh, S. Evaluation of the Antimicrobial and Antibiofilm Effect of Chitosan Nanoparticles as Carrier for Supernatant of Mesenchymal Stem Cells on Multidrug-Resistant Vibrio cholerae. Infect. Drug Resist. 2020, 13, 2251–2260.
- Lim, S.C.; Knight, D.R.; Riley, T.V. Clostridium difficile and One Health. Clin. Microbiol. Infect. 2020, 26, 857–863.
- Bennett, J.E.; Dolin, R.; Blaser, M.J. Syndromes of Enteric Infection. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 9th ed.; LaRocque, R.C., Harris, J., Eds.; Saunders Elsevier: Philadelphia, PA, USA, 2019; pp. 1330–1339.
- Payne, D.C.; Vinjé, J.; Szilagyi, P.G.; Edwards, K.M.; Staat, M.A.; Weinberg, G.A.; Hall, C.B.; Chappell, J.; Bernstein, D.I.; Curns, A.T.; et al. Norovirus and medically attended gastroenteritis in U.S. children. N. Engl. J. Med. 2013, 368, 1121–1130.
- Marder, E.P.; Cieslak, P.R.; Cronquist, A.B.; Dunn, J.; Lathrop, S.; Rabatsky-Ehr, T.; Ryan, P.; Smith, K.; Tobin-D’Angelo, M.; Vugia, D.J.; et al. Incidence and Trends of Infections with Pathogens Transmitted Commonly Through Food and the Effect of Increasing Use of Culture-Independent Diagnostic Tests on Surveillance-Foodborne Diseases Active Surveillance Network, 10 U.S. Sites, 2013–2016. MMWR. Morb. Mortal. Wkly. Rep. 2017, 66, 397–403.
- Thiagarajah, J.R.; Donowitz, M.; Verkman, A.S. Secretory diarrhoea: Mechanisms and emerging therapies. Nat. Reviews. Gastroenterol. Hepatol. 2015, 12, 446–457.
- Victora, C.G.; Bryce, J.; Fontaine, O.; Monasch, R. Reducing deaths from diarrhoea through oral rehydration therapy. Bull. World Health Organ. 2000, 78, 1246–1255.
- Christopher, P.R.H.; David, K.V.; John, S.M.; Sankarapandian, V. Antibiotic therapy for Shigella dysentery. Cochrane Database Syst. Rev. 2010, 8, CD006784.
- Harris, J.B.; LaRocque, R.C.; Qadri, F.; Ryan, E.T.; Calderwood, S.B. Cholera. Lancet 2012, 379, 2466–2476.
- DuPont, H.L.; Ericsson, C.D. Prevention and treatment of traveler’s diarrhea. N. Engl. J. Med. 1993, 328, 1821–1827.
- Yan, C.; Zhang, C.; Cao, X.; Feng, B.; Li, X. Intestinal Population in Host with Metabolic Syndrome during Administration of Chitosan and Its Derivatives. Molecules 2020, 25, 5857.
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174.
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368.
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. Int. J. Mol. Sci. 2019, 20, 5889.
- Garg, U.; Chauhan, S.; Nagaich, U.; Jain, N. Current Advances in Chitosan Nanoparticles Based Drug Delivery and Targeting. Adv. Pharm. Bull. 2019, 9, 195–204.
- Mo, X.; Cen, J.; Gibson, E.; Wang, R.; Percival, S.L. An open multicenter comparative randomized clinical study on chitosan. Wound Repair Regen. 2015, 23, 518–524.
- Layek, B.; Rahman Nirzhor, S.S.; Rathi, S.; Kandimalla, K.K.; Wiedmann, T.S.; Prabha, S. Design, Development, and Characterization of Imiquimod-Loaded Chitosan Films for Topical Delivery. AAPS PharmSciTech 2019, 20, 58.
- Sahariah, P.; Másson, M. Antimicrobial Chitosan and Chitosan Derivatives: A Review of the Structure-Activity Relationship. Biomacromolecules 2017, 18, 3846–3868.
- Ahsan, S.M.; Thomas, M.; Reddy, K.K.; Sooraparaju, S.G.; Asthana, A.; Bhatnagar, I. Chitosan as biomaterial in drug delivery and tissue engineering. Int. J. Biol. Macromol. 2018, 110, 97–109.
- Vila, A.; Sánchez, A.; Janes, K.; Behrens, I.; Kissel, T.; Vila Jato, J.L.; Alonso, M.J. European journal of pharmaceutics and biopharmaceutics: Official journal of Arbeitsgemeinschaft fur. Pharm. Verfahr. e.V 2004, 57, 123–131.
- Artursson, P.; Lindmark, T.; Davis, S.S.; Illum, L. Effect of chitosan on the permeability of monolayers of intestinal epithelial cells (Caco-2). Pharm. Res. 1994, 11, 1358–1361.
- De Campos, A.M.; Sánchez, A.; Alonso, M.J. Chitosan nanoparticles: A new vehicle for the improvement of the delivery of drugs to the ocular surface. Application to cyclosporin A. Int. J. Pharm. 2001, 224, 159–168.
- Portero, A.; Remuñán-López, C.; Criado, M.T.; Alonso, M.J. Reacetylated chitosan microspheres for controlled delivery of anti-microbial agents to the gastric mucosa. J. Microencapsul. 2002, 19, 797–809.
- Al-Qadi, S.; Grenha, A.; Carrión-Recio, D.; Seijo, B.; Remuñán-López, C. Microencapsulated chitosan nanoparticles for pulmonary protein delivery: In vivo evaluation of insulin-loaded formulations. J. Control. Release: Off. J. Control. Release Soc. 2012, 157, 383–390.
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan Derivatives and Their Application in Biomedicine. Int. J. Mol. Sci. 2020, 21, 487.
- Huang, G.Q.; Zhang, Z.K.; Cheng, L.Y.; Xiao, J.X. Intestine-targeted delivery potency of O-carboxymethyl chitosan-coated layer-by-layer microcapsules: An in vitro and in vivo evaluation. Mater. Sci. Eng. C 2019, 105, 110129.
- Singh, B.; Maharjan, S.; Cho, K.H.; Cui, L.; Park, I.K.; Choi, Y.J.; Cho, C.S. Chitosan-based particulate systems for the delivery of mucosal vaccines against infectious diseases. Int. J. Biol. Macromol. 2018, 110, 54–64.
- Gao, J.; Azad, M.A.K.; Han, H.; Wan, D.; Li, T. Impact of Prebiotics on Enteric Diseases and Oxidative Stress. Curr. Pharm. Des. 2020, 26, 2630–2641.
- Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Núñez, G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017, 279, 70–89.
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Abd El-Hakim, Y.M.; Al-Sagheer, A.A. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: A review. Int. J. Biol. Macromol. 2020, 164, 2726–2744.
- Perinelli, D.R.; Fagioli, L.; Campana, R.; Lam, J.K.W.; Baffone, W.; Palmieri, G.F.; Casettari, L.; Bonacucina, G. Chitosan-based nanosystems and their exploited antimicrobial activity. Eur. J. Pharm. Sci. 2018, 117, 8–20.
- Qin, Y.; Li, P. Antimicrobial Chitosan Conjugates: Current Synthetic Strategies and Potential Applications. Int. J. Mol. Sci. 2020, 21, 499.
- Wang, Q.; Wang, M.; Li, P.; Wang, K.; Fang, L.; Ren, F.; Lu, G.; Lu, X. The interaction of chitosan and BMP-2 tuned by deacetylation degree and pH value. J. Biomed. Mater. Res. Part A 2019, 107, 769–779.
- Wang, J.; Wang, L.; Yu, H.; Zain Ul, A.; Chen, Y.; Chen, Q.; Zhou, W.; Zhang, H.; Chen, X. Recent progress on synthesis, property and application of modified chitosan: An overview. Int. J. Biol. Macromol. 2016, 88, 333–344.
- Dragland, I.S.; Rukke, H.V.; Stenhagen, I.S.; Lönn-Stensrud, J.; Kopperud, H.M. Antibacterial and Antibiofilm Effect of Low Viscosity Chitosan against Staphylococcus epidermidis. Int. J. Microbiol. 2016, 2016, 9159761.
- Simůnek, J.; Brandysová, V.; Koppová, I.; Simůnek, J., Jr. The antimicrobial action of chitosan, low molar mass chitosan, and chitooligosaccharides on human colonic bacteria. Folia Microbiol. 2012, 57, 341–345.
- Wang, Y.; Zhou, P.; Yu, J.; Pan, X.; Wang, P.; Lan, W.; Tao, S. Antimicrobial effect of chitooligosaccharides produced by chitosanase from Pseudomonas CUY8. Asia Pac. J. Clin. Nutr. 2007, 16, 174–177.
- Másson, M. Antimicrobial Properties of Chitosan and Its Derivatives. In Chitosan for Biomaterials III; Jayakumar, R., Prabaharan, M., Eds.; Springer: Cham, Switzerland, 2021; Volume 287, pp. 131–168.
- Rathinam, S.; Solodova, S.; Kristjánsdóttir, I.; Hjálmarsdóttir, M.; Másson, M. The antibacterial structure-activity relationship for common chitosan derivatives. Int. J. Biol. Macromol. 2020, 165, 1686–1693.
- Prabaharan, M.; Mano, J.F. Chitosan derivatives bearing cyclodextrin cavitiesas novel adsorbent matrices. Carbohydr. Polym. 2006, 63, 153–166.
- Zhang, K.; Zhuang, P.; Wang, Z.; Li, Y.; Jiang, Z.; Hu, Q.; Liu, M.; Zhao, Q. One-pot synthesis of chitosan-g-(PEO-PLLA-PEO) via “click” chemistry and “SET-NRC” reaction. Carbohydr. Polym. 2012, 90, 1515–1521.
- Kurita, K.; Ikeda, H.; Yoshida, Y.; Shimojoh, M.; Harata, M. Chemoselective protection of the amino groups of chitosan by controlled phthaloylation: Facile preparation of a precursor useful for chemical modifications. Biomacromolecules 2002, 3, 1–4.
- Holappa, J.; Nevalainen, T.; Soininen, P.; Másson, M.; Järvinen, T. Synthesis of novel quaternary chitosan derivatives via N-Chloroacyl-6-O-triphenylmethylchitosans. Biomacromolecules 2006, 7, 407–410.
- Kurita, K.; Hirakawa, M.; Kikuchi, S.; Yamanaka, H.; Yang, J. Trimethylsilylation of chitosan and some properties of the product. Carbohydr. Polym. 2004, 56, 333–337.
- Rúnarsson, O.V.; Malainer, C.; Holappa, J.; Sigurdsson, S.T.; Másson, M. tert-Butyldimethylsilyl O-protected chitosan and chitooligosaccharides: Useful precursors for N-modifications in common organic solvents. Carbohydr. Res. 2008, 343, 2576–2582.
- Shrestha, A.; Kishen, A. Antibacterial Nanoparticles in Endodontics: A Review. J. Endod. 2016, 42, 1417–1426.
- Khan, F.; Pham, D.T.N.; Oloketuyi, S.F.; Manivasagan, P.; Oh, J.; Kim, Y.M. Chitosan and their derivatives: Antibiofilm drugs against pathogenic bacteria. Colloids Surf. B Biointerfaces 2020, 185, 110627.
- Sparavigna, A.; Setaro, M.; Frisenda, L. Physical and microbiological properties of a new nail protective medical device. J. Plast. Dermatol. 2008, 4, 5–12.
- Geisberger, G.; Gyenge, E.B.; Hinger, D.; Käch, A.; Maake, C.; Patzke, G.R. Chitosan-Thioglycolic Acid as a Versatile Antimicrobial Agent. Biomacromolecules 2013, 14, 1010–1017.
- Kast, C.E.; Bernkop-Schnürch, A. Thiolated polymers—Thiomers: Development and in vitro evaluation of chitosan–thioglycolic acid conjugates. Biomaterials 2001, 22, 2345–2352.
- Holappa, J.; Hjálmarsdóttir, M.; Másson, M.; Rúnarsson, Ö.; Asplund, T.; Soininen, P.; Nevalainen, T.; Järvinen, T. Antimicrobial activity of chitosan N-betainates. Carbohydr. Polym. 2006, 65, 114–118.
- Holappa, J.; Nevalainen, T.; Savolainen, J.; Soininen, P.; Elomaa, M.; Safin, R.; Suvanto, S.; Pakkanen, T.; Másson, M.; Loftsson, T.; et al. Synthesis and Characterization of Chitosan N-Betainates Having Various Degrees of Substitution. Macromolecules 2004, 37, 2784–2789.
- Pardeshi, C.V.; Belgamwar, V.S. Controlled synthesis of N,N,N-trimethyl chitosan for modulated bioadhesion and nasal membrane permeability. Int. J. Biol. Macromol. 2016, 82, 933–944.
- Rúnarsson, Ö.V.; Holappa, J.; Nevalainen, T.; Hjálmarsdóttir, M.; Järvinen, T.; Loftsson, T.; Einarsson, J.M.; Jónsdóttir, S.; Valdimarsdóttir, M.; Másson, M. Antibacterial activity of methylated chitosan and chitooligomer derivatives: Synthesis and structure activity relationships. Eur. Polym. J. 2007, 43, 2660–2671.
- Xu, Y.; Du, Y.; Huang, R.; Gao, L. Preparation and modification of N-(2-hydroxyl) propyl-3-trimethyl ammonium chitosan chloride nanoparticle as a protein carrier. Biomaterials 2003, 24, 5015–5022.
- Ju, X.; Chen, J.; Zhou, M.; Zhu, M.; Li, Z.; Gao, S.; Ou, J.; Xu, D.; Wu, M.; Jiang, S.; et al. Combating Pseudomonas aeruginosa Biofilms by a Chitosan-PEG-Peptide Conjugate via Changes in Assembled Structure. ACS Appl. Mater. Interfaces 2020, 12, 13731–13738.
- Tan, W.; Li, Q.; Dong, F.; Wei, L.; Guo, Z. Synthesis, characterization, and antifungal property of chitosan ammonium salts with halogens. Int. J. Biol. Macromol. 2016, 92, 293–298.
- Brown, S.; Santa Maria, J.P., Jr.; Walker, S. Wall teichoic acids of gram-positive bacteria. Annu. Rev. Microbiol. 2013, 67, 313–336.
- Tang, C.H.; Hsu, C.J.; Yang, W.H.; Fong, Y.C. Lipoteichoic acid enhances IL-6 production in human synovial fibroblasts via TLR2 receptor, PKCdelta and c-Src dependent pathways. Biochem. Pharmacol. 2010, 79, 1648–1657.
- Khalid, S.; Piggot, T.J.; Samsudin, F. Atomistic and Coarse Grain Simulations of the Cell Envelope of Gram-Negative Bacteria: What Have We Learned? Acc. Chem. Res. 2019, 52, 180–188.
- Feng, P.; Luo, Y.; Ke, C.; Qiu, H.; Wang, W.; Zhu, Y.; Hou, R.; Xu, L.; Wu, S. Chitosan-Based Functional Materials for Skin Wound Repair: Mechanisms and Applications. Front. Bioeng. Biotechnol. 2021, 9, 650598.
- World Health Organization. Oral Rehydration Salts (ORS) a New Reduced Osmolarity Formulation; World Health Organization: Geneva, Switzerland, 2002.
- Hatchette, T.F.; Farina, D. Infectious diarrhea: When to test and when to treat. Can. Med Assoc. J. 2011, 183, 339–344.
- Mahajan, V.; Saini, S.S.; Sharma, A.; Kaur, J. Ringer’s lactate vs normal saline for children with acute diarrhea and severe dehydration: A double blind randomized controlled trial. Indian Pediatrics 2012, 49, 963–968.
- Ternhag, A.; Asikainen, T.; Giesecke, J.; Ekdahl, K. A meta-analysis on the effects of antibiotic treatment on duration of symptoms caused by infection with Campylobacter species. Clin. Infect. Dis. 2007, 44, 696–700.
- Onwuezobe, I.A.; Oshun, P.O.; Odigwe, C.C. Antimicrobials for treating symptomatic non-typhoidal Salmonella infection. Cochrane Database Syst. Rev. 2012, 11, CD001167.
- Wong, C.S.; Jelacic, S.; Habeeb, R.L.; Watkins, S.L.; Tarr, P.I. The risk of the hemolytic–uremic syndrome after antibiotic treatment of Escherichia coli O157: H7 infections. N. Engl. J. Med. 2000, 342, 1930–1936.
- Ke, C.L.; Deng, F.S.; Chuang, C.Y.; Lin, C.H. Antimicrobial Actions and Applications of Chitosan. Polymers 2021, 13, 904.
- Bhattacharya, S.P.; Bhattacharya, A.; Sen, A. A comprehensive and comparative study on the action of pentacyclic triterpenoids on Vibrio cholerae biofilms. Microb. Pathog. 2020, 149, 104493.
- Fong, J.N.; Yildiz, F.H. Biofilm matrix proteins. Microbiol. Spectr. 2015, 3, 2.
- Limoli, D.H.; Jones, C.J.; Wozniak, D.J. Bacterial extracellular polysaccharides in biofilm formation and function. Microbiol. Spectr. 2015, 3, 3.
- Rabin, N.; Zheng, Y.; Opoku-Temeng, C.; Du, Y.; Bonsu, E.; Sintim, H.O. Biofilm formation mechanisms and targets for developing antibiofilm agents. Future Med. Chem. 2015, 7, 493–512.
- Hall-Stoodley, L.; Stoodley, P. Evolving concepts in biofilm infections. Cell. Microbiol. 2009, 11, 1034–1043.
- Chen, G.; Swem, L.R.; Swem, D.L.; Stauff, D.L.; O’Loughlin, C.T.; Jeffrey, P.D.; Bassler, B.L.; Hughson, F.M. A strategy for antagonizing quorum sensing. Mol. Cell 2011, 42, 199–209.
- Hirakawa, H.; Tomita, H. Interference of bacterial cell-to-cell communication: A new concept of antimicrobial chemotherapy breaks antibiotic resistance. Front. Microbiol. 2013, 4, 114.
- Khan, F.; Khan, M.M.; Kim, Y.-M. Recent progress and future perspectives of antibiofilm drugs immobilized on nanomaterials. Curr. Pharm. Biotechnol. 2018, 19, 631–643.
- Oloketuyi, S.F.; Khan, F. Strategies for biofilm inhibition and virulence attenuation of foodborne pathogen-Escherichia coli O157: H7. Curr. Microbiol. 2017, 74, 1477–1489.
- Oloketuyi, S.F.; Khan, F. Inhibition strategies of Listeria monocytogenes biofilms—Current knowledge and future outlooks. J. Basic Microbiol. 2017, 57, 728–743.
- Rabin, N.; Zheng, Y.; Opoku-Temeng, C.; Du, Y.; Bonsu, E.; Sintim, H.O. Agents that inhibit bacterial biofilm formation. Future Med. Chem. 2015, 7, 647–671.
- Pham, D.T.N.; Khan, F.; Phan, T.T.V.; Park, S.-K.; Manivasagan, P.; Oh, J.; Kim, Y.M. Biofilm inhibition, modulation of virulence and motility properties by FeOOH nanoparticle in Pseudomonas aeruginosa. Braz. J. Microbiol. 2019, 50, 791–805.
- Khan, F.; Manivasagan, P.; Lee, J.-W.; Pham, D.T.N.; Oh, J.; Kim, Y.-M. Fucoidan-stabilized gold nanoparticle-mediated biofilm inhibition, attenuation of virulence and motility properties in Pseudomonas aeruginosa PAO1. Mar. Drugs 2019, 17, 208.
- Aleksic, I.; Petkovic, M.; Jovanovic, M.; Milivojevic, D.; Vasiljevic, B.; Nikodinovic-Runic, J.; Senerovic, L. Anti-biofilm properties of bacterial di-rhamnolipids and their semi-synthetic amide derivatives. Front. Microbiol. 2017, 8, 2454.
- Brahma, U.; Kothari, R.; Sharma, P.; Bhandari, V. Antimicrobial and anti-biofilm activity of hexadentated macrocyclic complex of copper (II) derived from thiosemicarbazide against Staphylococcus aureus. Sci. Rep. 2018, 8, 1–8.
- Omwenga, E.O.; Hensel, A.; Pereira, S.; Shitandi, A.A.; Goycoolea, F.M. Antiquorum sensing, antibiofilm formation and cytotoxicity activity of commonly used medicinal plants by inhabitants of Borabu sub-county, Nyamira County, Kenya. PLoS ONE 2017, 12, e0185722.
- Jiang, L.; Shen, C.; Long, X.; Zhang, G.; Meng, Q. Rhamnolipids elicit the same cytotoxic sensitivity between cancer cell and normal cell by reducing surface tension of culture medium. Appl. Microbiol. Biotechnol. 2014, 98, 10187–10196.
- Kashyap, P.L.; Xiang, X.; Heiden, P. Chitosan nanoparticle based delivery systems for sustainable agriculture. Int. J. Biol. Macromol. 2015, 77, 36–51.
- Youssef, A.M.; Abou-Yousef, H.; El-Sayed, S.M.; Kamel, S. Mechanical and antibacterial properties of novel high performance chitosan/nanocomposite films. Int. J. Biol. Macromol. 2015, 76, 25–32.
- Lodhi, G.; Kim, Y.S.; Hwang, J.W.; Kim, S.K.; Jeon, Y.J.; Je, J.Y.; Ahn, C.B.; Moon, S.H.; Jeon, B.T.; Park, P.J. Chitooligosaccharide and its derivatives: Preparation and biological applications. BioMed Res. Int. 2014, 2014, 654913.
- Mu, H.; Guo, F.; Niu, H.; Liu, Q.; Wang, S.; Duan, J. Chitosan improves anti-biofilm efficacy of gentamicin through facilitating antibiotic penetration. Int. J. Mol. Sci. 2014, 15, 22296–22308.
- Cheung, R.C.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar. Drugs 2015, 13, 5156–5186.
- Bellich, B.; D’Agostino, I.; Semeraro, S.; Gamini, A.; Cesàro, A. “The Good, the Bad and the Ugly” of Chitosans. Mar. Drugs 2016, 14, 99.
- Jamil, B.; Habib, H.; Abbasi, S.A.; Ihsan, A.; Nasir, H.; Imran, M. Development of Cefotaxime Impregnated Chitosan as Nano-antibiotics: De Novo Strategy to Combat Biofilm Forming Multi-drug Resistant Pathogens. Front. Microbiol. 2016, 7, 330.
- Jiang, F.; Deng, Y.; Yeh, C.K.; Sun, Y. Quaternized chitosans bind onto preexisting biofilms and eradicate pre-attached microorganisms. J. Mater. Chem. B 2014, 2, 8518–8527.
- Su, Y.; Tian, L.; Yu, M.; Gao, Q.; Wang, D.; Xi, Y.; Yang, P.; Lei, B.; Ma, P.X.; Li, P. Cationic peptidopolysaccharides synthesized by ‘click’chemistry with enhanced broad-spectrum antimicrobial activities. Polym. Chem. 2017, 8, 3788–3800.
- Li, P.; Zhou, C.; Rayatpisheh, S.; Ye, K.; Poon, Y.F.; Hammond, P.T.; Duan, H.; Chan-Park, M.B. Cationic peptidopolysaccharides show excellent broad-spectrum antimicrobial activities and high selectivity. Adv. Mater. 2012, 24, 4130–4137.
- Rubini, D.; Farisa Banu, S.; Veda Hari, B.N.; Ramya Devi, D.; Gowrishankar, S.; Karutha Pandian, S.; Nithyanand, P. Chitosan extracted from marine biowaste mitigates staphyloxanthin production and biofilms of Methicillin- resistant Staphylococcus aureus. Food Chem. Toxicol. 2018, 118, 733–744.
- Costa, E.M.; Silva, S.; Veiga, M.; Vicente, S.; Tavaria, F.K.; Pintado, M.E. Investigation of chitosan’s antibacterial activity against vancomycin resistant microorganisms and their biofilms. Carbohydr. Polym. 2017, 174, 369–376.
- Kim, G.; Dasagrandhi, C.; Kang, E.H.; Eom, S.H.; Kim, Y.M. In vitro antibacterial and early stage biofilm inhibitory potential of an edible chitosan and its phenolic conjugates against Pseudomonas aeruginosa and Listeria monocytogenes. 3 Biotech 2018, 8, 439.
- Mu, H.; Zhang, A.; Zhang, L.; Niu, H.; Duan, J. Inhibitory effects of chitosan in combination with antibiotics on Listeria monocytogenes biofilm. Food Control 2014, 38, 215–220.
- Sender, R.; Fuchs, S.; Milo, R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016, 14, e1002533.
- Savage, D.C. Microbial ecology of the gastrointestinal tract. Annu. Rev. Microbiol. 1977, 31, 107–133.
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65.
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 2015, 17, 690–703.
- Lynch, S.V.; Pedersen, O. The human intestinal microbiome in health and disease. N. Engl. J. Med. 2016, 375, 2369–2379.
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638.
- Reyes, A.; Haynes, M.; Hanson, N.; Angly, F.E.; Heath, A.C.; Rohwer, F.; Gordon, J.I. Viruses in the faecal microbiota of monozygotic twins and their mothers. Nature 2010, 466, 334–338.
- Goodrich, J.K.; Waters, J.L.; Poole, A.C.; Sutter, J.L.; Koren, O.; Blekhman, R.; Beaumont, M.; Van Treuren, W.; Knight, R.; Bell, J.T.; et al. Human genetics shape the gut microbiome. Cell 2014, 159, 789–799.
- Laursen, M.F.; Bahl, M.I.; Licht, T.R. Settlers of our inner surface-Factors shaping the gut microbiota from birth to toddlerhood. FEMS Microbiol. Rev. 2021, 45, fuab001.
- Wang, S.; Charbonnier, L.M.; Noval Rivas, M.; Georgiev, P.; Li, N.; Gerber, G.; Bry, L.; Chatila, T.A. MyD88 adaptor-dependent microbial sensing by regulatory t cells promotes mucosal tolerance and enforces commensalism. Immunity 2015, 43, 289–303.
- Ferretti, P.; Pasolli, E.; Tett, A.; Asnicar, F.; Gorfer, V.; Fedi, S.; Armanini, F.; Truong, D.T.; Manara, S.; Zolfo, M.; et al. Mother-to-infant microbial transmission from different body sites shapes the developing infant gut microbiome. Cell Host Microbe 2018, 24, 133–145.e135.
- Galazzo, G.; van Best, N.; Bervoets, L.; Dapaah, I.O.; Savelkoul, P.H.; Hornef, M.W.; Lau, S.; Hamelmann, E.; Penders, J. Development of the microbiota and associations with birth mode, diet, and atopic disorders in a longitudinal analysis of stool samples, collected from infancy through early childhood. Gastroenterology 2020, 158, 1584–1596.
- Brooks, B.; Olm, M.R.; Firek, B.A.; Baker, R.; Thomas, B.C.; Morowitz, M.J.; Banfield, J.F. Strain-resolved analysis of hospital rooms and infants reveals overlap between the human and room microbiome. Nat. Commun. 2017, 8, 1814.
- Rodríguez, J.M.; Murphy, K.; Stanton, C.; Ross, R.P.; Kober, O.I.; Juge, N.; Avershina, E.; Rudi, K.; Narbad, A.; Jenmalm, M.C.; et al. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb. Ecol. Health Dis. 2015, 26, 26050.
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56.
- Patterson, E.; Ryan, P.M.; Cryan, J.F.; Dinan, T.G.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Gut microbiota, obesity and diabetes. Postgrad. Med. J. 2016, 92, 286–300.
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031.
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60.
- Ni, J.; Wu, G.D.; Albenberg, L.; Tomov, V.T. Gut microbiota and IBD: Causation or correlation? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 573–584.
- Vollaard, E.J.; Clasener, H.A. Colonization resistance. Antimicrob. Agents Chemother. 1994, 38, 409–414.
- Ducarmon, Q.R.; Zwittink, R.D.; Hornung, B.V.H.; van Schaik, W.; Young, V.B.; Kuijper, E.J. Gut Microbiota and Colonization Resistance against Bacterial Enteric Infection. Microbiol. Mol. Biol. Rev. 2019, 83, e00007-19.
- Win, P.P.; Shin-Ya, Y.; Hong, K.-J.; Kajiuchi, T. Formulation and characterization of pH sensitive drug carrier based on phosphorylated chitosan (PCS). Carbohydr. Polym. 2003, 53, 305–310.
- Niaz, T.; Sarkar, A.; Mackie, A.; Imran, M. Impact of albumin corona on mucoadhesion and antimicrobial activity of carvacrol loaded chitosan nano-delivery systems under simulated gastro-intestinal conditions. Int. J. Biol. Macromol. 2021, 169, 171–182.
- Mura, C.; Valenti, D.; Floris, C.; Sanna, R.; De Luca, M.A.; Fadda, A.M.; Loy, G. Metronidazole prodrugs: Synthesis, physicochemical properties, stability, and ex vivo release studies. Eur. J. Med. Chem. 2011, 46, 4142–4150.
- Zaki, N.M.; Hafez, M.M. Enhanced antibacterial effect of ceftriaxone sodium-loaded chitosan nanoparticles against intracellular Salmonella typhimurium. AAPS PharmSciTech 2012, 13, 411–421.
- Braim, S.; Śpiewak, K.; Brindell, M.; Heeg, D.; Alexander, C.; Monaghan, T. Lactoferrin-loaded alginate microparticles to target Clostridioides difficile infection. J. Pharm. Sci. 2019, 108, 2438–2446.
- Liu, Y.; Wang, X.Q.; Ren, W.X.; Chen, Y.L.; Yu, Y.; Zhang, J.K.; Bawudong, D.; Gu, J.P.; Xu, X.D.; Zhang, X.N. Novel albendazole-chitosan nanoparticles for intestinal absorption enhancement and hepatic targeting improvement in rats. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 998–1005.
- Asadpoor, M.; Peeters, C.; Henricks, P.A.J.; Varasteh, S.; Pieters, R.J.; Folkerts, G.; Braber, S. Anti-pathogenic functions of non-digestible oligosaccharides in vitro. Nutrients 2020, 12, 1789.
- Chabra, A.; Rahimi-Esboei, B.; Habibi, E.; Monadi, T.; Azadbakht, M.; Elmi, T.; Valian, H.K.; Akhtari, J.; Fakhar, M.; Naghshvar, F. Effects of some natural products from fungal and herbal sources on Giardia lamblia in vivo. Parasitology 2019, 146, 1188–1198.
- Madureira, A.R.; Pereira, A.; Pintado, M. Current state on the development of nanoparticles for use against bacterial gastrointestinal pathogens. Focus on chitosan nanoparticles loaded with phenolic compounds. Carbohydr. Polym. 2015, 130, 429–439.
- Su, X.; Zivanovic, S.; D’Souza, D.H. Effect of chitosan on the infectivity of murine norovirus, feline calicivirus, and bacteriophage MS2. J. Food Prot. 2009, 72, 2623–2628.
- Abdel-Latif, M.; El-Shahawi, G.; Aboelhadid, S.M.; Abdel-Tawab, H. Immunoprotective effect of chitosan particles on hymenolepis nana-infected mice. Scand. J. Immunol. 2017, 86, 83–90.
- Milewska, A.; Chi, Y.; Szczepanski, A.; Barreto-Duran, E.; Dabrowska, A.; Botwina, P.; Obloza, M.; Liu, K.; Liu, D.; Guo, X.; et al. HTCC as a polymeric inhibitor of SARS-CoV-2 and MERS-CoV. J. Virol. 2021, 95, e01622-20.
- Ma, Z.; Kang, M.; Meng, S.; Tong, Z.; Yoon, S.D.; Jang, Y.; Jeong, K.C. Selective killing of Shiga Toxin-Producing Escherichia coli with antibody-conjugated chitosan nanoparticles in the gastrointestinal tract. ACS Appl. Mater. Interfaces 2020, 12, 18332–18341.
- Alfaro-Viquez, E.; Esquivel-Alvarado, D.; Madrigal-Carballo, S.; Krueger, C.G.; Reed, J.D. Proanthocyanidin-chitosan composite nanoparticles prevent bacterial invasion and colonization of gut epithelial cells by extra-intestinal pathogenic Escherichia coli. Int. J. Biol. Macromol. 2019, 135, 630–636.
- Cui, Z.; Han, D.; Sun, X.; Zhang, M.; Feng, X.; Sun, C.; Gu, J.; Tong, C.; Lei, L.; Han, W. Mannose-modified chitosan microspheres enhance OprF-OprI-mediated protection of mice against Pseudomonas aeruginosa infection via induction of mucosal immunity. Appl. Microbiol. Biotechnol. 2015, 99, 667–680.
- Nagarwal, R.C.; Singh, P.N.; Kant, S.; Maiti, P.; Pandit, J.K. Chitosan coated PLA nanoparticles for ophthalmic delivery: Characterization, in-vitro and in-vivo study in rabbit eye. J. Biomed. Nanotechnol. 2010, 6, 648–657.
- Abu-Elala, N.M.; AbuBakr, H.O.; Khattab, M.S.; Mohamed, S.H.; El-Hady, M.A.; Ghandour, R.A.; Morsi, R.E. Aquatic environmental risk assessment of chitosan/silver, copper and carbon nanotube nanocomposites as antimicrobial agents. Int. J. Biol. Macromol. 2018, 113, 1105–1115.
- Kim, J.H.; Yu, D.; Eom, S.H.; Kim, S.H.; Oh, J.; Jung, W.K.; Kim, Y.M. Synergistic Antibacterial Effects of Chitosan-Caffeic Acid Conjugate against Antibiotic-Resistant Acne-Related Bacteria. Mar. Drugs 2017, 15, 167.
- Prajapati, B.; Rajput, P.; Jena, P.K.; Seshadri, S. Investigation of chitosan for prevention of diabetic progression through gut microbiota alteration in sugar rich diet induced diabetic rats. Curr. Pharm. Biotechnol. 2015, 17, 173–184.
- Wang, J.; Zhang, C.; Guo, C.; Li, X. Chitosan ameliorates DSS-Induced ulcerative colitis mice by enhancing intestinal barrier function and improving microflora. Int. J. Mol. Sci. 2019, 20, 5751.
- Yuan, X.; Zheng, J.; Jiao, S.; Cheng, G.; Feng, C.; Du, Y.; Liu, H. A review on the preparation of chitosan oligosaccharides and application to human health, animal husbandry and agricultural production. Carbohydr. Polym. 2019, 220, 60–70.
- Nakanishi, N.; Tashiro, K.; Kuhara, S.; Hayashi, T.; Sugimoto, N.; Tobe, T. Regulation of virulence by butyrate sensing in enterohaemorrhagic Escherichia coli. Microbiology 2009, 155, 521–530.
- Xiong, H.; Guo, B.; Gan, Z.; Song, D.; Lu, Z.; Yi, H.; Wu, Y.; Wang, Y.; Du, H. Butyrate upregulates endogenous host defense peptides to enhance disease resistance in piglets via histone deacetylase inhibition. Sci. Rep. 2016, 6, 27070.
- EI-Ziney, M.G.; De Meyer, H.; Debevere, J.M. Growth and survival kinetics of Yersinia enterocolitica IP 383 0:9 as affected by equimolar concentrations of undissociated short-chain organic acids. Int. J. Food Microbiol. 1997, 34, 233–247.
- Hang, S.; Purdy, A.E.; Robins, W.P.; Wang, Z.; Mandal, M.; Chang, S.; Mekalanos, J.J.; Watnick, P.I. The acetate switch of an intestinal pathogen disrupts host insulin signaling and lipid metabolism. Cell Host Microbe 2014, 16, 592–604.




