
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Katarzyna Bogunia-Kubik | + 2360 word(s) | 2360 | 2021-11-16 07:08:16 | | | |
| 2 | Dean Liu | Meta information modification | 2360 | 2021-12-07 01:59:11 | | |
Video Upload Options
Interleukin (IL)-4 and IL-13 belong to the T helper 2 (Th2) cytokine family, along with IL-3, IL-5, and IL-9. These cytokines are key mediators of allergic inflammation.
1. Introduction
Interleukin (IL)-4 and IL-13 are members of the T helper (Th) 2 family of cytokines. This group also includes IL-3, IL-5, and IL-9. Genes encoding the IL-4 and IL-13 in humans are located on chromosome 5q31 within a cluster of Th2-related cytokine genes including IL-3, IL-5, and IL-9 [1]. IL-13 was first described in 1983 as a protein secreted by activated mouse Th2 cells, and a human IL-13 was cloned in 1993 [2][3][4]. IL-13 represents a four-helix-bundle cytokine with two disulfide bridges [5][6]. IL-13 has a single open-reading frame of 132 amino acids with a 20-amino-acid sequence that is cleaved and secreted as a 10-kd unglycosylated mature protein [7]. The gene encoding IL-13 has four exons and three introns and is located 12 kb upstream of the IL-4 encoding gene [8]. IL-4 was discovered in 1982 as a factor with the capacity to induce proliferation of murine B lymphocytes [9]. Cloning of a gene encoding both mouse and human IL-4 was performed in 1986 by Noma et al. and Yokota et al. [10][11]. IL-4 and IL-13 share 25% similarity at amino acid level and have overlapping functions [12]. The IL-4 structure is also based on a four-helix bundle and contains three disulfide bridges [13]. The human IL-4’s cDNA encodes 153 amino acids protein with a 129-amino-acid sequence which is cleaved from the pre-protein and secreted as 15.4-kD unglycosylated mature protein [12]. The gene encoding IL-4 comprises four exons and three introns. Th2 cells are the main source of IL-4 and IL-13; however, lower levels of these cytokines might be also secreted by a wide range of other cells, including NK cells, Th1 cells, CD8+ T cells, innate lymphoid type 2 cells, B lymphocytes, mast cells, macrophages, basophils, and eosinophils [12][14][15][16][17][18].
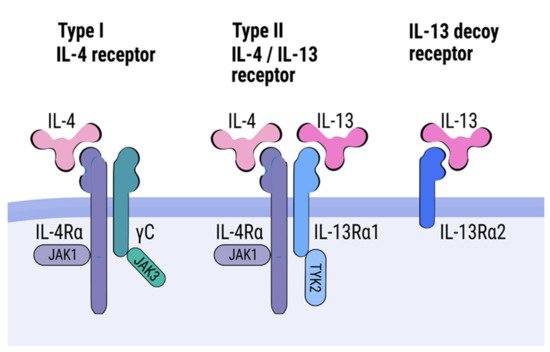
2. Regulation of IL-4/IL-13 Genes Expression
3. IL-4 and IL-13 Polymorphism in Inflammatory Arthritis
4. IL-4 and IL-13 Serum Concentrations in Inflammatory Arthritis
References
- Frazer, K.A.; Ueda, Y.; Zhu, Y.; Gifford, V.R.; Garofalo, M.R.; Mohandas, N.; Martin, C.H.; Palazzolo, M.J.; Cheng, J.F.; Rubin, E.M. Computational and biological analysis of 680 kb of DNA sequence from the human 5q31 cytokine gene cluster region. Genome Res. 1997, 7, 495–512.
- Brown, K.D.; Zurawski, S.M.; Mosmann, T.R.; Zurawski, G. A family of small inducible proteins secreted by leukocytes are members of a new superfamily that includes leukocyte and fibroblast-derived inflammatory agents, growth factors, and indicators of various activation processes. J. Immunol. 1989, 142, 679–687.
- McKenzie, A.N.; Li, X.; Largaespada, D.A.; Sato, A.; Kaneda, A.; Zurawski, S.M.; Doyle, E.L.; Milatovich, A.; Francke, U.; Copeland, N.G. Structural comparison and chromosomal localization of the human and mouse IL-13 genes. J. Immunol. 1993, 150, 5436–5444.
- Minty, A.; Chalon, P.; Derocq, J.M.; Dumont, X.; Guillemot, J.C.; Kaghad, M.; Labit, C.; Leplatoic, P.; Liauzun, P.; Miloux, B.; et al. Interleukin-13 is a new human lymphokine regulating inflammatory and immune responses. Nature 1993, 362, 248–250.
- Eisenmesser, E.Z.; Horita, D.A.; Altieri, A.S.; Byrd, R.A. Solution structure of interleukin-13 and insights into receptor engagement. J. Mol. Biol. 2001, 310, 231–241.
- Moy, F.J.; Diblasio, E.; Wilhelm, J.; Powers, R. Solution structure of human IL-13 and implication for receptorbinding. J. Mol. Biol. 2001, 310, 219–230.
- Mueller, T.D.; Zhang, J.L.; Sebald, W.; Duschl, A. Structure, binding, and antagonists in the IL-4/IL-13 receptorsystem. Biochim. Biophys. Acta 2002, 1592, 237–250.
- Seyfizadeh, N.; Seyfizadeh, N.; Gharibi, T.; Babaloo, Z. Interleukin-13 as an important cytokine: A review on its roles in some human diseases. Acta Microbiol. Immunol. Hung. 2015, 62, 341–378.
- Howard, M.; Farrar, J.; Hilfiker, M.; Johnson, B.; Takatsu, K.; Hamaoka, T.; Paul, W.E. Identification of a T-cell derived B cell growth factor distinct from interleukin-2. J. Exp. Med. 1982, 155, 914–921.
- Noma, Y.; Sideras, P.; Naito, T.; Bergstedt-Lindqvist, S.; Azuma, C.; Severinson, E.; Tanabe, T.; Kinashi, T.; Matsuda, F.; Yaoita, Y. Cloning of cDNA encoding the murine IgG1 induction factor by a novel strategy using SP6 promoter. Nature 1986, 319, 640–646.
- Yokota, T.; Otsuka, T.; Mosmann, T.; Banchereau, J.; DeFrance, T.; Blanchard, D.; De Vries, J.E.; Lee, F.; Arai, K. Isolation and characterization of a human interleukin cDNA clone, homologous to mouse B-cell stimulatory factor 1, that expresses B-cell- and T-cell-stimulating activities. Proc. Natl. Acad. Sci. USA 1986, 83, 5894–5898.
- Chomarat, P.; Banchereau, J. Interleukin-4 and interleukin-13: Their similarities and discrepancies. Int. Rev. Immunol. 1998, 17, 1–52.
- Walter, M.R.; Cook, W.J.; Zhao, B.G.; Cameron, R.P., Jr.; Ealick, S.E.; Walter, R.L., Jr.; Reichert, P.; Nagabhushan, T.L.; Trotta, P.P.; Bugg, C.E. Crystal structure of recombinant human interleukin-4. J. Biol. Chem. 1992, 267, 20371–20376.
- Gallo, E.; Katzman, S.; Villarino, A.V. IL-13-producing Th1 and Th17 cells characterize adaptive responses to both self and foreign antigens. Eur. J. Immunol. 2012, 42, 2322–2328.
- Liang, H.E.; Reinhardt, R.L.; Bando, J.K.; Sullivan, B.M.; Ho, I.-C.; Locksley, R.M. Divergent expression patterns of IL-4 and IL-13 define unique functions in allergic immunity. Nat. Immunol. 2011, 13, 58–66.
- Mosmann, T.R.; Coffman, R.L. TH1 and TH2 Cells: Different Patterns of Lymphokine Secretion Lead to Different Functional Properties. Annu. Rev. Immunol. 1989, 7, 145–173.
- Paul, W.E. History of interleukin-4. Cytokine 2015, 75, 3–7.
- Wills-Karp, M.; Finkelman, F.D. Untangling the complex web of IL-4- and IL-13-mediated signaling pathways. Sci. Signal. 2008, 1, 55.
- Walker, J.A.; McKenzie, A.N.J. TH2 cell development and function. Nat. Rev. Immunol. 2018, 18, 121–133.
- Bao, K.; Reinhardt, R.L. The Differential Expression of IL-4 and IL-13 and Its Impact on Type-2 Immunity. Cytokine 2015, 75, 25–37.
- Dong, C.; Fu, T.; Ji, J.; Li, Z.; Gu, Z. The role of interleukin-4 in rheumatic diseases. Clin. Exp. Pharmacol. Physiol. 2018, 45, 747–754.
- Zhu, J. T helper 2 (Th2) cell differentiation, type 2 innate lymphoid cell (ILC2) development and regulation of interleukin-4 (IL-4) and IL-13 production. Cytokine 2015, 75, 14–24.
- Duffin, K.C.; Freeny, I.C.; Schrodi, S.J.; Wong, B.; Feng, B.J.; Soltani-Arabshahi, R.; Rakkhit, T.; Goldgar, D.; Krueger, G.G. Association between IL13 polymorphisms and psoriatic arthritis is modified by smoking. J. Invest. Dermatol. 2009, 129, 2777–2783.
- Marinou, I.; Till, S.H.; Moore, D.J.; Wilson, A.G. Lack of association or interactions between the IL-4, IL-4Rα and IL-13 genes, and rheumatoid arthritis. Arthritis Res. Ther. 2008, 10, R80.
- Pavkova Goldbergova, M.; Nemec, P.; Lipkova, J.; Gatterova, J.; Ambrozkova, D.; Vasku, A.; Soucek, M.; Pavek, N. Relation of IL-6, IL-13 and IL-15 gene polymorphisms to the rheumatoid factors, anti-CCP and other measures of rheumatoid arthritis activity. Int. J. Immunogenet. 2014, 41, 34–40.
- Wang, M.J.; Zhou, X.D.; Zhang, H.; Liu, R.P. Correlation between IL-3 and IL-13 gene polymorphisms in Chinese patients and rheumatoid arthritis. Genet. Mol. Res. 2016, 23, 15.
- Eder, L.; Chandran, V.; Pellett, F.; Pollock, R.; Shanmugarajah, S.; Rosen, C.F.; Rahman, P.; Gladman, D.D. IL13 gene polymorphism is a marker for psoriatic arthritis among psoriasis patients. Ann. Rheum. Dis. 2011, 70, 1594–1598.
- Bowes, J.; Eyre, S.; Flynn, E.; Ho, P.; Salah, S.; Warren, R.B.; Marzo-Ortega, H.; Coates, L.; McManus, R.; Ryan, A.W.; et al. Evidence to support IL-13 as a risk locus for psoriatic arthritis but not psoriasis vulgaris. Ann. Rheum. Dis. 2011, 70, 1016–1019.
- Li, X.; Chai, W.; Ni, M.; Xu, M.; Lian, Z.; Shi, L.; Bai, Y.; Wang, Y. The effects of gene polymorphisms in interleukin-4 and interleukin-6 on the susceptibility of rheumatoid arthritis in a Chinese population. Biomed. Res. Int. 2014, 2014, 265435.
- Moreno, O.; González, C.I.; Saaibi, D.L.; Otero, W.; Badillo, R.; Martín, J.; Ramírez, G. Polymorphisms in the IL4 and IL4RA genes in Colombian patients with rheumatoid arthritis. J. Rheumatol. 2007, 34, 36–42.
- Pawlik, A.; Wrzesniewska, A.; Florczak, M.; Gawronska-Szklarz, B.; Herczynska, M. The -590 IL-4 promoter polymorphism in patients with rheumatoid arthritis. Rheumatol. Int. 2005, 26, 48–51.
- Hussein, Y.M.; El-Shal, A.S.; Rezk, N.A.; Abdel Galil, S.M.; Alzahrani, S.S. Influence of interleukin-4 gene polymorphisms and interleukin-4 serum level on susceptibility and severity of rheumatoid arthritis in Egyptian population. Cytokine 2013, 61, 849–855.
- Cantagrel, A.; Navaux, F.; Loubet-Lescoulié, P.; Nourhashemi, F.; Enault, G.; Abbal, M.; Constantin, A.; Laroche, M.; Mazières, B. Interleukin-1beta, interleukin-1 receptor antagonist, interleukin-4, and interleukin-10 gene polymorphisms: Relationship to occurrence and severity of rheumatoid arthritis. Arthritis Rheum. 1999, 42, 1093–1100.
- Nunez, C.; Santiago, J.L.; Varadé, J.; de la Calle, H.; Figueredo, M.A.; Fernandez-Gutierrez, B.; de la Concha, E.G.; Urcelay, E.; Martınez, A. IL4 in the 5q31 context: Association studies of type 1 diabetes and rheumatoid arthritis in the Spanish population. Immunogenetics 2008, 60, 19–23.
- Canet, L.M.; Cáliz, R.; Lupiañez, C.B.; Canhão, H.; Martinez, M.; Escudero, A.; Filipescu, I.; Segura-Catena, J.; Soto-Pino, M.J.; Ferrer, M.A.; et al. Genetic variants within immune-modulating genes influence the risk of developing rheumatoid arthritis and anti-TNF drug response: A two-stage case-control study. Pharmacogenet. Genom. 2015, 25, 432–443.
- Park, H.K.; Kim, S.K.; Kweon, H.Y.; Lee, K.G.; Arasu, M.V.; Kim, Y.O. Promoter polymorphism (-590, T/C) of interleukin 4 (IL4) gene is associated with rheumatoid arthritis: An updated meta-analysis. Saudi J. Biol. Sci. 2017, 24, 444–449.
- Krabben, A.; Wilson, A.G.; de Rooy, D.P.C.; Zhernakova, A.; Brouwer, E.; Lindqvist, E.; Saxne, T.; Stoeken, G.; van Nies, J.A.B.; Knevel, R.; et al. Association of genetic variants in the IL4 and IL4R genes with the severity of joint damage in rheumatoid arthritis: A study in seven cohorts. Arthritis Rheum. 2013, 65, 3051–3057.
- Genevay, S.; Di Giovine, F.S.; Perneger, T.V.; Silvestri, T.; Stingelin, S.; Duff, G.; Guerne, P.-A. Association of interleukin-4 and interleukin-1B gene variants with Larsen score progression in rheumatoid arthritis. Arthritis Rheum. 2002, 47, 303–309.
- Buchs, N.; Silvestri, T.; di Giovine, F.S.; Chabaud, M.; Vannier, E.; Duff, G.W.; Miossec, P. IL-4 VNTR gene polymorphism in chronic polyarthritis. The rare allele is associated with protection against destruction. Rheumatology 2000, 39, 1126–1131.
- Prots, I.; Skapenko, A.; Wendler, J.; Mattyasovszky, S.; Yoné, C.L.; Spriewald, B.; Burkhardt, H.; Rau, R.; Kalden, J.R.; Lipsky, P.E.; et al. Association of the IL4R single-nucleotide polymorphism I50V with rapidly erosive rheumatoid arthritis. Arthritis Rheum. 2006, 54, 1491–1500.
- Peng, H.; Wang, W.; Zhou, M.; Liu, C.-Y.; Li, R.; Wen, P.-F.; Qiu, L.-J.; Pan, H.-F.; Ye, D.-Q. Associations of interleukin-4 receptor gene polymorphisms (Q551R, I50V) with rheumatoid arthritis: Evidence from a meta-analysis. Genet. Test. Mol. Biomark. 2013, 17, 768–774.
- Wallis, S.K.; Cooney, L.A.; Endres, J.L.; Lee, M.J.; Ryu, J.; Somers, M.C.; Fox, D.A. A polymorphism in the interleukin-4 receptor affects the ability of interleukin-4 to regulate Th17 cells: A possible immunoregulatory mechanism for genetic control of the severity of rheumatoid arthritis. Arthritis Res. Ther. 2011, 13, R15.
- Lascorz, J.; Hüffmeier, U.; Schulze-Koops, H.; Skapenko, A.; Reis, A.; Lohmann, J.; Wendler, J.; Traupe, H.; Küster, W.; Burkhardt, H. Lack of genetic association of the interleukin-4 receptor single-nucleotide polymorphisms I50V and Q551R with erosive disease in psoriatic arthritis. Arthritis Rheum. 2006, 54, 4023–4024.
- Isomäki, P.; Luukkainen, R.; Toivanen, P.; Punnonen, J. The presence of interleukin-v13 in rheumatoid synovium and its antiinflammatory effects on synovial fluid macrophages from patients with rheumatoid arthritis. Arthritis Rheum. 1996, 39, 1693–1702.
- Spadaro, A.; Rinaldi, T.; Riccieri, V.; Valesini, G.; Taccari, E. Interleukin 13 in synovial fluid and serum of patients with psoriatic arthritis. Ann. Rheum. Dis. 2002, 61, 174–176.
- Szodoray, P.; Alex, P.; Chappell-Woodward, C.M.; Madland, T.M.; Knowlton, N.; Dozmorov, I.; Zeher, M.; Jarvis, J.N.; Nakken, B.; Brun, J.G.; et al. Circulating cytokines in Norwegian patients with psoriatic arthritis determined by a multiplex cytokine array system. Rheumatology 2007, 46, 417–425.
- Silosi, I.; Boldeanu, M.V.; Cojocaru, M.; Biciusca, V.; Padureanu, V.; Bogdan, M.; Badea, R.G.; Avramescu, C.; Petrescu, I.O.; Petrescu, F.; et al. The relationship of cytokines IL-13 and IL-17 with autoantibodies profile in early rheumatoid arthritis. J. Immunol. Res. 2016, 3109135.
- Takei, S.; Hoshino, T.; Matsunaga, K.; Sakazaki, Y.; Sawada, M.; Oda, H.; Takenaka, S.; Imaoka, H.; Kinoshita, T.; Honda, S.; et al. Soluble interleukin-18 receptor complex is a novel biomarker in rheumatoid arthritis. Arthritis Res. Ther. 2011, 13, R52.
- Tokayer, A.; Carsons, S.E.; Chokshi, B.; Santiago-Schwarz, F. High levels of interleukin 13 in rheumatoid arthritis sera are modulated by tumor necrosis factor antagonist therapy: Association with dendritic cell growth activity. J. Rheumatol. 2002, 29, 454–461.
- Raza, K.; Falciani, F.; Curnow, S.J.; Ross, E.; Lee, C.Y.; Akbar, A.N.; Lord, J.M.; Gordon, C.; Buckley, C.D.; Salomon, M. Early rheumatoid arthritis is characterized by a distinct and transient synovial fluid cytokine profile of T cell and stromal cell origin. Arthritis Res. Ther. 2005, 7, R784–R795.
- Azizieh, F.Y.; Al Jarallah, K.; Shehab, D.; Gupta, R.; Dingle, K.; Raghupathy, R. Patterns of circulatory and peripheral blood mononuclear cytokines in rheumatoid arthritis. Rheumatol. Int. 2017, 37, 1727–1734.
- Woods, J.M.; Haines, G.K.; Shah, M.R.; Rayan, G.; Koch, A.E. Low-level production of interleukin-13 in synovial fluid and tissue from patients with arthritis. Clin. Immunol. Immunopathol. 1997, 85, 210–220.
- Barra, L.; Summers, K.; Bell, D.; Cairns, E. Serum cytokine profile of unaffected first-degree relatives of patients with rheumatoid arthritis. J. Rheumatol. 2014, 41, 80–85.
- Constantin, A.; Loubet-Lescoulie, P.; Lambert, N.; Yassine-Diab, B.; Abbal, M.; Mazieres, B.; de Preval, C.; Cantagrel, A. Antiinflammatory and Immunoregulatory Action of Methotrexate in the Treatment of Rheumatoid Arthritis: Evidence of Increased Interleukin-4 and interleukin-10 Gene Expression Demonstrated In Vitro by Competitive Reverse Transcriptase-Polymerase Chain Reaction. Arthritis Rheum. 1998, 41, 48–57.
- Miossec, P.; Naviliat, M.; Dupuy d’Angeac, A.; Sany, J.; Banchereau, J. Low levels of interleukin-4 and high levels of transforming growth factor p in rheumatoid synovitis. Arthritis Rheum. 1990, 33, 1180–1187.
- Simon, A.K.; Seipelt, H.; Sieper, J. Divergent T-cell cytokine patterns in inflammatory arthritis. Proc. Natl. Acad. Sci. USA 1994, 91, 8562–8566.
- Kawashima, M.; Miossec, P. mRNA quantification of T-bet, GATA-3, IFN-gamma, and IL-4 shows a defective Th1 immune response in the peripheral blood from rheumatoid arthritis patients: Link with disease activity. J. Clin. Immunol. 2005, 25, 209–214.
- Haddad, A.; Bienvenu, J.; Miossec, P. Increased production of a Th2 cytokine profile by activated whole blood cells from rheumatoid arthritis patients. J. Clin. Immunol. 1998, 18, 399–403.
- Van Roon, J.A.; Verhoef, C.M.; van Roy, J.L.; Gmelig-Meyling, F.H.; Huber-Bruning, O.; Lafeber, F.P.; Bijlsma, J.W. Decrease in peripheral type 1over type 2 T cell cytokine production in patients with rheumatoid arthritis correlates with an increase in severity of disease. Ann. Rheum. Dis. 1997, 56, 656–660.
- Bahlas, S.; Damiati, L.; Dandachi, N.; Sait, H.; Alsefri, M.; Pushparaj, P.N. Rapid immunoprofiling of cytokines, chemokines and growth factors in patients with active rheumatoid arthritis using Luminex Multiple Analyte Profiling technology for precision medicine. Clin. Exp. Rheumatol. 2019, 37, 112–119.
- Schlaak, J.F.; Pfers, I.; Meyer Zum Büschenfelde, K.H.; Märker-Hermann, E. Different cytokine profiles in the synovial fluid of patients with osteoarthritis, rheumatoid arthritis and seronegative spondylarthropathies. Clin. Exp. Rheumatol. 1996, 14, 155–162.
- Kokkonen, H.; Söderström, I.; Rocklöv, J.; Hallmans, G.; Lejon, K.; Rantapää Dahlqvist, S. Up-regulation of cytokines and chemokines predates the onset of rheumatoid arthritis. Arthritis Rheum. 2010, 62, 383–391.
- Kremer, J.M.; Lawrence, D.A. Correlation of immune parameters with clinical response to methotrexate in patients with rheumatoid a prospective study . Arthritis Rheum. 1991, 3 (Suppl. 9), S159.
- Isomäki, P.; Luukkainen, R.; Lassila, O.; Toivanen, P.; Punnonen, J. Synovial fluid T cells from patients with rheumatoid arthritis are refractory to the Th2 differentiation inducing effects of IL-4. Immunology 1999, 96, 358–364.
- Nissinen, R.; Leirisalo-Repo, M.; Tiittanen, M.; Julkunen, H.; Hirvonen, H.; Palosuo, T.; Vaarala, O. CCR3, CCR5, interleukin 4, and interferon-γ expression on synovial and peripheral T cells and monocytes in patients with rheumatoid arthritis. J. Rheumatol. 2003, 30, 1928–1934.





