
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ronan Lordan | + 3812 word(s) | 3812 | 2021-12-01 10:26:31 | | | |
| 2 | Peter Tang | Meta information modification | 3812 | 2021-12-02 02:55:40 | | |
Video Upload Options
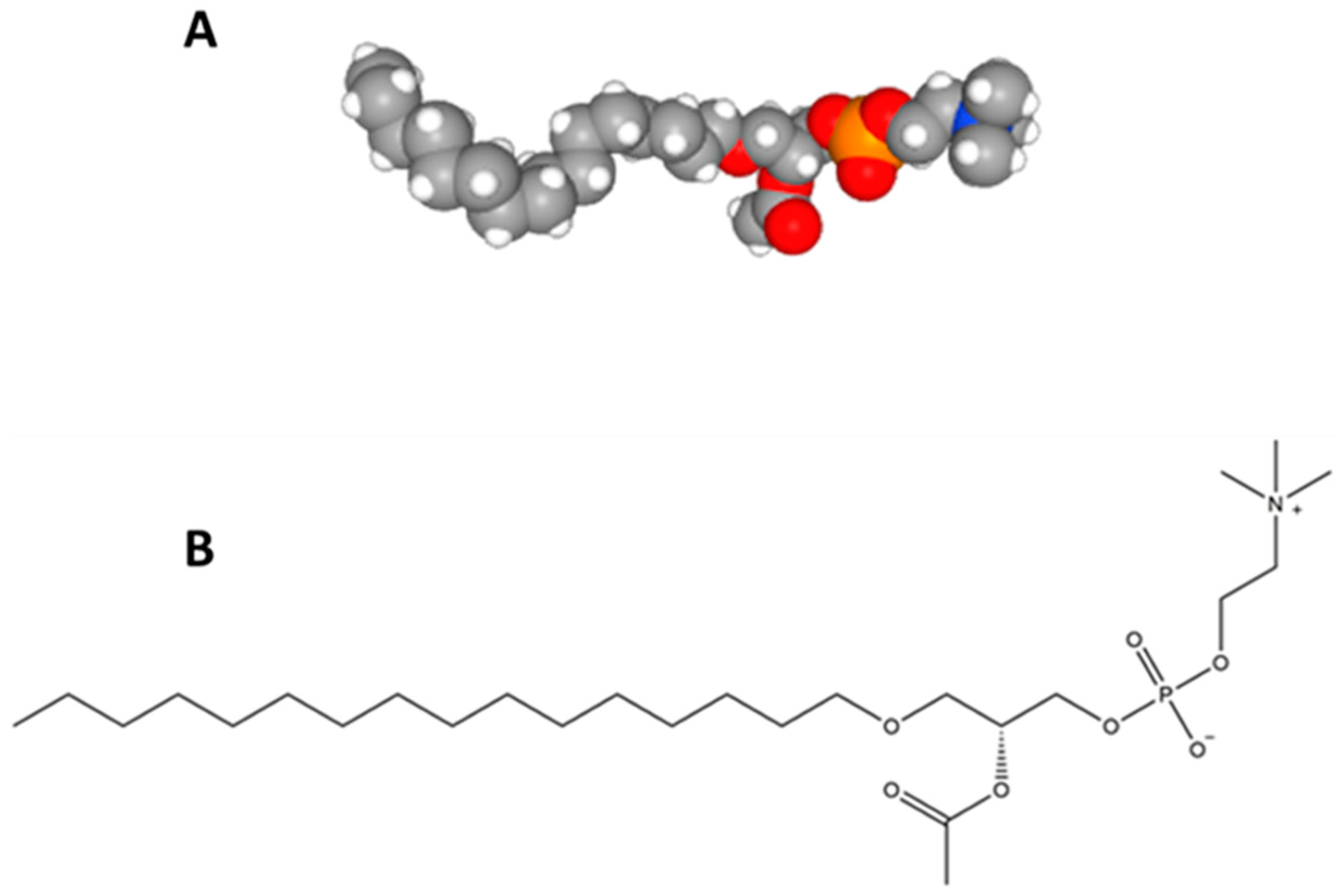
Platelet-activating factor (PAF) refers to the classical structure reported in 1979, which is a pro-inflammatory phospholipid mediator. PAF mediates a wide variety of cellular functions and cell–cell interactions.
1. Platelet-Activating Factor
2. The Discovery and Structural Elucidation of the Platelet-Activating Factor
2.1. The Discovery of the Platelet-Activating Factor
2.2. Structural Elucidation of the Platelet-Activating Factor
3. The Importance of Platelet-Activating Factor Research
|
Title |
Date |
Location |
|---|---|---|
|
1st International Symposium on Platelet-Activating Factor and Structurally Related Ether-Lipids |
26–29 June 1983 |
Paris, France |
|
2nd International Conference on Platelet-Activating Factor and Structurally Related Ether-Lipids |
26–29 October 1986 |
Gatlinburg, Tennessee, USA |
|
3rd International Conference on Platelet-Activating Factor and Structurally Related Ether-Lipids |
8–12 May 1989 |
Tokyo, Japan |
|
4th International Congress on Platelet-Activating Factor and Related Lipid Mediators |
22–25 September 1992 |
Snowbird, Utah, USA |
|
5th International Congress on Platelet-Activating Factor and Related Lipid Mediators |
12–16 September 1995 |
Berlin, Germany |
|
6th International Congress on Platelet-Activating Factor and Related Lipid Mediators |
21–24 September 1998 |
New Orleans, Louisiana, USA |
|
7th International Congress on Platelet-Activating Factor and Related Lipid Mediators |
24–27 September 2001 |
Tokyo, Japan |
|
8th International Congress on Platelet-Activating Factor and Related Lipid Mediators |
6–9 October 2004 |
Berlin, Germany |
|
6th International Conference on Phospholipase A2 and Lipid Mediators |
10–12 February 2015 |
Tokyo, Japan |
4. The Potential Use of Platelet-Activating Factor Inhibitors as Therapeutics and Preventatives of Disease
4.1. PAF Inhibitors of Synthetic Origin
|
PAF-R Antagonist |
Target Disease or Disorder |
Outcome |
Reference |
|---|---|---|---|
|
Lexipafant |
Cognitive impairment complications as a result of coronary artery bypass graft |
No significant reduction in cognitive impairment |
[84] |
|
Myocardial infarction |
No significant effect on streptokinase-induced hypotension in myocardial infarction patients |
[85] |
|
|
Sepsis |
No significant affect in patients with severe sepsis |
[86] |
|
|
Organ failure related to pancreatitis |
No significant amelioration of systemic inflammatory response syndrome in pancreatitis-induced organ failure |
[87] |
|
|
Modipafant |
Asthma |
No significant effect against chronic asthma |
[74] |
|
Asthma |
No significant effect in early or late responses to allergens |
[88] |
|
|
Responses to inhaled PAF |
Potent inhibition of airway and neutrophil responses to PAF with a duration of up to 24 h and a reduction of secondary eicosanoid production in response to inhaled PAF |
[89] |
|
|
SR27417A SR27417A |
Asthma |
Modest inhibitory effects against asthma |
|
|
Ulcerative colitis |
No evidence of efficacy in the treatment of acute ulcerative colitis |
[92] |
|
|
WEB 2086 |
Asthma |
No attenuation of early of late allergen-induced responses or airway hyperresponsiveness |
[93] |
|
UVB-induced dermatitis |
Significant inhibition of UVB light-induced erythema |
[94] |
|
|
BN 50730 |
Rheumatoid arthritis |
Ineffective in the treatment of rheumatoid arthritis |
[95] |
|
BN 52021 |
Pulmonary function in the early post ischaemic graft function in clinical lung transplantation |
Improvement of alveoloarterial oxygen difference and a reduction of PAF levels |
[96] |
|
Ro 24-238 |
Psoriasis |
No significant effects reported |
[97] |
|
TCV-309 |
Septic shock |
No significant difference in adverse events or mortality. A substantial reduction of organ dysfunction and morbidity associated with septic shock was reported |
[98] |
|
Levocetirizine |
Chronic idiopathic urticaria |
Reduction of urticarial activity score |
[99] |
|
Rupatadine |
Chronic idiopathic urticaria |
Reduction of urticarial activity score but not as effective as levocetirizine |
|
|
Allergic rhinitis and allergies |
Significant effects against both conditions as demonstrated in the comprehensive review by Mullol et al. |
[101] |
|
|
Y-24180 |
Asthma |
Improvement of bronchial hyperresponsiveness in patients with asthma |
[102] |
4.2. PAF Inhibitors of Natural Origin
References
- Demopoulos, C.A. State of lipid research in greece. Euro. J. Lipid Sci. Technol. 2000, 102, 665–666.
- Demopoulos, C.A. Biological activity of lipids of pine pollen on platelet aggregation in correlation with the platelet activating factor. In Proceedings of the second international conference on platelet-activating factor and structurally related alkyl ether lipids, Gatlinburg, TN, USA, 26–29 October 1986.
- Prescott, S.M.; Zimmerman, G.A.; Stafforini, D.M.; McIntyre, T.M. Platelet-activating factor and related lipid mediators. Annu. Rev. Biochem. 2000, 69, 419–445.
- Maclennan, K.M.; Smith, P.F.; Darlington, C.L. Platelet-activating factor in the cns. Prog. Neurobiol. 1996, 50, 585–596.
- Tsoupras, A.; Lordan, R.; Zabetakis, I. Inflammation, not cholesterol, is a cause of chronic disease. Nutrients 2018, 10, 604.
- Suvarna, Y.; Maity, N.; Shivamurthy, M.C. Emerging trends in retrograde signaling. Mol. Neurobiol. 2016, 53, 2572–2578.
- Birkl, D.; Quiros, M.; García-Hernández, V.; Zhou, D.W.; Brazil, J.C.; Hilgarth, R.; Keeney, J.; Yulis, M.; Bruewer, M.; García, A.J.; et al. TNF-α promotes mucosal wound repair through enhanced platelet activating factor receptor signaling in the epithelium. Mucosal Immunol. 2019, 12, 909–918.
- Lordan, R.; Tsoupras, A.; Zabetakis, I. The potential role of dietary platelet-activating factor inhibitors in cancer prevention and treatment. Adv. Nutr. 2019, 10, 148–164.
- da Silva-Jr, I.; Chammas, R.; Lepique, A.; Jancar, S. Platelet-activating factor (PAF) receptor as a promising target for cancer cell repopulation after radiotherapy. Oncogenesis 2017, 6, e296.
- Demopoulos, C.A.; Karantonis, H.C.; Antonopoulou, S. Platelet-activating factor — a molecular link between atherosclerosis theories. Eur. J. Lipid Sci. Technol. 2003, 105, 705–716.
- Palur Ramakrishnan, A.V.K.; Varghese, T.P.; Vanapalli, S.; Nair, N.K.; Mingate, M.D. Platelet activating factor: A potential biomarker in acute coronary syndrome? Cardiovasc. Ther. 2017, 35, 64–70.
- Yost, C.C.; Weyrich, A.S.; Zimmerman, G.A. The platelet activating factor (PAF) signaling cascade in systemic inflammatory responses. Biochimie 2010, 92, 692–697.
- Tsoupras, A.B.; Chini, M.; Tsogas, N.; Fragopoulou, E.; Nomikos, T.; Lioni, A.; Mangafas, N.; Demopoulos, C.A.; Antonopoulou, S.; Lazanas, M.C. Anti-platelet-activating factor effects of highly active antiretroviral therapy (HAART): A new insight in the drug therapy of hiv infection? Aids Res. Hum. Retrovir. 2008, 24, 1079–1086.
- Antonopoulou, S.; Nomikos, T.; Karantonis, H.; Fragopoulou, E.; Demopoulos, C.A. PAF, a potent lipid mediator. In Bioactive phospholipids: Role in inflammation and atherosclerosis; Tselepis, A.D., Ed.; Transworld Research Network: Kerala, India, 2008; pp. 85–134.
- Kulikov, V.; Muzya, G. Ether lipids and platelet-activating factor: Evolution and cellular function. Biochem. Biokhimiia 1997, 62, 1103–1108.
- Barbaro, J.F.; Zvaifler, N.J. Antigen induced histamine release from platelets of rabbits producing homologous PGA antibody. Proc. Soc. Exp. Biol. Med. 1966, 122, 1245–1247.
- Siraganian, R.P.; Osler, A.G. Destruction of rabbit platelets in the allergic response of sensitized leukocytes: I. Demonstration of a fluid phase intermediate. J. Immunol. 1971, 106, 1244–1251.
- Benveniste, J.; Henson, P.M.; Cochrane, C.G. Leukocyte-dependent histamine release from rabbit platelets: The role of IGE, basophils, and a platelet-activating factor. J. Exp. Med. 1972, 136, 1356–1377.
- Benveniste, J. Platelet-activating factor, a new mediator of anaphylaxis and immune complex deposition from rabbit and human basophils. Nature 1974, 249, 581–582.
- Chap, H. Forty five years with membrane phospholipids, phospholipases and lipid mediators: A historical perspective. Biochimie 2016, 125, 234–249.
- Slotboom, A.J.; de Haas, G.H.; Bonsen, P.P.M.; Burbach-Westerhuis, G.J.; van Deenen, L.L.M. Hydrolysis of phosphoglycerides by purified lipase preparations i. Substrate-, positional- and stereo-specificity. Chem. Phys. Lipids 1970, 4, 15–29.
- Henson, P.M.; Pinckard, R.N. Basophil-derived platelet-activating factor (PAF) as an in vivo mediator of acute allergic reactions: Demonstration of specific desensitization of platelets to PAF during IGE-induced anaphylaxis in the rabbit. J. Immunol. 1977, 119, 2179–2184.
- Pinckard, R.N.; Farr, R.S.; Hanahan, D.J. Physicochemical and functional identity of rabbit platelet-activating factor (PAF) released in vivo during IGE anaphylaxis with PAF released in vitro from IGE sensitized basophils. J. Immunol. 1979, 123, 1847–1857.
- Chignard, M.; Le Couedic, J.; Tence, M.; Vargaftig, B.; Benveniste, J. The role of platelet-activating factor in platelet aggregation. Nature 1979, 279, 799–800.
- Benveniste, J.; Le Couedic, J.P.; Polonsky, J.; Tence, M. Structural analysis of purified platelet-activating factor by lipases. Nature 1977, 269, 170–171.
- De Haas, G.H.; Van Deenen, L.L.M. Structural identification of isomeric lysolecithins. Biochim. Biophys. Lipids Lipid Metab. 1965, 106, 315–325.
- Demopoulos, C.; Pinckard, R.; Hanahan, D.J. Platelet-activating factor. Evidence for 1-O-alkyl-2-acetyl-sn-glyceryl-3-phosphorylcholine as the active component (a new class of lipid chemical mediators). J. Biol. Chem. 1979, 254, 9355–9358.
- Blank, M.L.; Snyder, F.; Byers, L.W.; Brooks, B.; Muirhead, E.E. Antihypertensive activity of an alkyl ether analog of phosphatidylcholine. Biochem. Biophys. Res. Commun. 1979, 90, 1194–1200.
- Benveniste, J.; Tence, M.; Varenne, P.; Bidault, J.; Boullet, C.; Polonsky, J. Semi-synthese et structure proposde du facteur activant les plaquettes (PAF): Paf-acether, un alkyl ether analogue de la lysophosphatidylcholine. C R. Acad. Sci. Paris 1979, 289D, 1037–1040.
- Mcmanus, L.M.; Hanahan, D.; Demopoulos, C.; Pinckard, R. Pathobiology of the intravenous infusion of acetyl glyceryl ether phosphorylcholine (AGEPC), a synthetic platelet-activating factor (PAF), in the rabbit. J. Immunol. 1980, 124, 2919–2924.
- Halonen, M.; Palmer, J.D.; Lohman, I.C.; McManus, L.M.; Pinckard, R.N. Respiratory and circulatory alterations induced by acetyl glyceryl ether phosphorylcholine, a mediator of ige anaphylaxis in the rabbit. Am. Rev. Respir. Dis. 1980, 122, 915–924.
- Pinckard, R.N.; O’Rourke, R.A.; Crawford, M.H.; Grover, F.S.; McManus, L.M.; Ghidoni, J.J.; Storrs, S.B.; Olson, M.S. Complement localization and mediation of ischemic injury in baboon myocardium. J. Clin. Investig. 1980, 66, 1050–1056.
- Halonen, M.; Palmer, J.D.; Lohman, I.C.; McManus, L.M.; Pinckard, R.N. Differential effects of platelet depletion on the physiologic alterations of ige anaphylaxis and acetyl glyceryl ether phosphorylcholine infusion in the rabbit. Am. Rev. Resp. Dis. 1981, 124, 416–421.
- National Center for Biotechnology Information. Pubchem Database, Platelet-Activating Factor, cid = 108156. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Platelet-activating-factor (accessed on 17 October 2019).
- Hanahan, D.J.; Demopoulos, C.; Liehr, J.; Pinckard, R. Identification of platelet activating factor isolated from rabbit basophils as acetyl glyceryl ether phosphorylcholine. J. Biol. Chem. 1980, 255, 5514–5516.
- Polonsky, J.; Tencé, M.; Varenne, P.; Das, B.C.; Lunel, J.; Benveniste, J. Release of 1-O-alkylglyceryl 3-phosphorylcholine, O-deacetyl platelet-activating factor, from leukocytes: Chemical ionization mass spectrometry of phospholipids. Proc. Natl. Acad. Sci. USA 1980, 77, 7019–7023.
- Valone, F. Quantifying platelet-activating factor in biologic systems. J. Allergy Clin. Immunol. 1993, 91, 551–552.
- Chap, H.; Mauco, G.; Simon, M.F.; Benveniste, J.; Douste-Blazy, L. Biosynthetic labelling of platelet activating factor from radioactive acetate by stimulated platelets. Nature 1981, 289, 312–314.
- Ribbes, G.; Ninio, E.; Fontan, P.; Record, M.; Chap, H.; Benveniste, J.; Douste-Blazy, L. Evidence that biosynthesis of platelet-activating factor (PAF-acether) by human neutrophils occurs in an intracellular membrane. Febs Lett. 1985, 191, 195–199.
- Pincock, S. Jacques benveniste. Lancet 2004, 364, 1660.
- Watts, G. Jacques benveniste. BMJ 2004, 329, 1290.
- Cusack, N.J. Platelet-activating factor. Nature 1980, 285, 193.
- Argyrou, C.; Vlachogianni, I.; Stamatakis, G.; Demopoulos, C.A.; Antonopoulou, S.; Fragopoulou, E. Postprandial effects of wine consumption on platelet-activating factor metabolic enzymes. Prostaglandins Other Lipid Mediat. 2017, 130, 23–29.
- Marathe, G.K.; Johnson, C.; Billings, S.D.; Southall, M.D.; Pei, Y.; Spandau, D.; Murphy, R.C.; Zimmerman, G.A.; McIntyre, T.M.; Travers, J.B. Ultraviolet B radiation generates platelet-activating factor-like phospholipids underlying cutaneous damage. J. Biol. Chem. 2005, 280, 35448–35457.
- Zabetakis, I.; Lordan, R.; Tsoupras, A. The Impact of Nutrition and Statins on Cardiovascular Diseases, 1st ed.; Elsevier: London, UK, 2019; 348p.
- Platelet-Activating Factor as an Effector for Environmental Stressors. Available online: https://link.springer.com/chapter/10.1007/164_2019_218. (accessed on 17 October 2019).
- Proceeding of the PAF communications, 6th International Conference on Phospholipase A2 and Lipid Mediators, Tokyo, Japan, 10–12 February 2015; Available online: https://www.bioweb.ne.jp/mid_meeting/stg_info/id_16774/ (accessed on 2 December 2019).
- Woodard, D.S.; Ostrom, K.K.; McManus, L.M. Lipid inhibitors of platelet-activating factor (PAF) in normal human plasma. J. Lipid Mediat. Cell Signal. 1995, 12, 11–28.
- Macpherson, J.L.; Kemp, A.; Rogers, M.; Mallet, A.I.; Toia, R.F.; Spur, B.; Earl, J.W.; Chesterman, C.N.; Krilis, S.A. Occurrence of platelet-activating factor (PAF) and an endogenous inhibitor of platelet aggregation in diffuse cutaneous mastocytosis. Clin. Exp. Immunol. 1989, 77, 391–396.
- Antonopoulou, S.; Demopoulos, C.A.; Iatrou, C. Blood cardiolipin in haemodialysis patients. Its implication in the biological action of platelet-activating factor. Int. J. Biochem. Cell Biol. 1996, 28, 43–51.
- Tsoukatos, D.; Demopoulos, C.A.; Tselepis, A.D.; Moschidis, M.C.; Donos, A.; Evangelou, A.; Benveniste, J. Inhibition by cardiolipins of platelet-activating factor-induced rabbit platelet activation. Lipids 1993, 28, 1119–1124.
- Bussolino, F.; Benveniste, J. Pharmacological modulation of platelet-activating factor (paf) release from rabbit leucocytes. I. Role of camp. Immunology 1980, 40, 367–376.
- Lecrubier, C.; Conard, J.; Horellou, M.H.; Samama, M. Study of platelet aggregation induced by platelet activating factor (PAF) after administration of ticlopidine or aspirin. Agents Actions 1983, 13, 77–80.
- Levy, J.V. Calmodulin antagonist w-7 inhibits aggregation of human platelets induced by platelet activating factor. Proc. Soc. Exp. Biol. Med. 1983, 172, 393–395.
- Apitz-Castro, R.; Cabrera, S.; Cruz, M.R.; Ledezma, E.; Jain, M.K. Effects of garlic extract and of three pure components isolated from it on human platelet aggregation, arachidonate metabolism, release reaction and platelet ultrastructure. Thromb. Res. 1983, 32, 155–169.
- Isah, T. Rethinking ginkgo biloba l.: Medicinal uses and conservation. Pharm. Rev. 2015, 9, 140–148.
- Papakonstantinou, V.D.; Lagopati, N.; Tsilibary, E.C.; Demopoulos, C.A.; Philippopoulos, A.I. A review on platelet activating factor inhibitors: Could a new class of potent metal-based anti-inflammatory drugs induce anticancer properties? Bioinorg. Chem. Appl. 2017, 2017.
- Lordan, R.; Nasopoulou, C.; Tsoupras, A.; Zabetakis, I. The anti-inflammatory properties of food polar lipids. In Bioactive Molecules in Food; Mérillon, J.M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–34.
- Sioriki, E.; Lordan, R.; Nahra, F.; van hecke, K.; Zabetakis, I.; Nolan, S.P. In vitro anti-atherogenic properties of n-heterocyclic carbene aurate(I) compounds. ChemMedChem 2018, 13.
- Honda, Z.-I.; Ishii, S.; Shimizu, T. Platelet-activating factor receptor. J. Biochem. 2002, 131, 773–779.
- Ishii, S.; Shimizu, T. Platelet-activating factor (PAF) receptor and genetically engineered paf receptor mutant mice. Prog. Lipid Res. 2000, 39, 41–82.
- Chao, W.; Olson, M.S. Platelet-activating factor: Receptors and signal transduction. Biochem. J. 1993, 292 Pt 3, 617–629.
- Hwang, S.B. Specific receptors of platelet-activating factor, receptor heterogeneity, and signal transduction mechanisms. J. Lipid Mediat. 1990, 2, 123–158.
- Terashita, Z.-I.; Tsushima, S.; Yoshioka, Y.; Nomura, H.; Inada, Y.; Nishikawa, K. Cv-3988 - a specific antagonist of platelet activating factor (PAF). Life Sci. 1983, 32, 1975–1982.
- Valone, F.H. Inhibition of binding of the platelet-activating factor agepc to platelets by the AGEPC analog rac-3-(n-n-octadecylcarbamoyloxy)-2-methoxypropyl 2-thiazolioethyl phosphate (CV-3988). Biochem. Biophys. Res. Commun. 1985, 126, 502–508.
- Terashita, Z.; Imura, Y.; Takatani, M.; Tsushima, S.; Nishikawa, K. CV-6209, a highly potent antagonist of platelet activating factor in vitro and in vivo. J. Pharm. Exp. 1987, 242, 263–268.
- D’Humières, S.; Russo-Marie, F.; Boris Vargaftig, B. PAF-acether-induced synthesis of prostacyclin by human endothelial cells. Eur. J. Pharm. 1986, 131, 13–19.
- Toyofuku, T.; Kubo, K.; Kobayashi, T.; Kusama, S. Effects of ONO-6240, a platelet-activating factor antagonist, on endotoxin shock in unanesthetized sheep. Prostaglandins 1986, 31, 271–281.
- Handley, D.A.; Tomesch, J.C.; Saunders, R.N. Inhibition of PAF-induced systemic responses in the rat, guinea pig, dog and primate by the receptor antagonist SRI 63–441. Thromb. Haemost. 1986, 55, 040–044.
- Saunders, R.N.; Handley, D.A. Platelet-activating factor antagonists. Annu. Rev. Pharm. Toxicol. 1987, 27, 237–255.
- Merlos, M.; Gómez, L.A.; Giral, M.; Vericat, M.L.; García-Rafanell, J.; Forn, J. Effects of paf-antagonists in mouse ear oedema induced by several inflammatory agents. Br. J. Pharm. 1991, 104, 990–994.
- Wissner, A.; Carroll, M.L.; Green, K.E.; Kerwar, S.S.; Pickett, W.C.; Schaub, R.E.; Torley, L.W.; Wrenn, S.; Kohler, C.A. Analogues of platelet activating factor. 6. Mono-and bis-aryl phosphate antagonists of platelet activating factor. J. Med. Chem. 1992, 35, 1650–1662.
- Kingsnorth, A.N.; Galloway, S.W.; Formela, L.J. Randomized, double-blind phase II trial of lexipafant, a platelet-activating factor antagonist, in human acute pancreatitis. Br. J. Surg. 1995, 82, 1414–1420.
- Kuitert, L.M.; Angus, R.M.; Barnes, N.C.; Barnes, P.J.; Bone, M.F.; Chung, K.F.; Fairfax, A.J.; Higenbotham, T.W.; O’Connor, B.J.; Piotrowska, B. Effect of a novel potent platelet-activating factor antagonist, modipafant, in clinical asthma. Am. J. Respir. Crit. Care Med. 1995, 151, 1331–1335.
- Komuro, Y.; Imanishi, N.; Uchida, M.; Morooka, S. Biological effect of orally active platelet-activating factor receptor antagonist SM-10661. Mol. Pharm. 1990, 38, 378–384.
- O’Hair, D.P.; Roza, A.M.; Komorowski, R.; Moore, G.; McManus, R.P.; Johnson, C.P.; Adams, M.B.; Pieper, G.M. Tulopafant, a paf receptor antagonist, increases capillary patency and prolongs survival in discordant cardiac xenotransplants. J. Lipid Mediat. 1993, 7, 79–84.
- Casals-Stenzel, J.; Heuer, H.O. Use of WEB 2086 and WEB 2170 as platelet-activating factor antagonists. In Methods Enzymol; Murphy, R.C., Fitzpatrick, F.A., Eds.; Academic Press: Cambridge, MA, USA, 1990; Volume 187, pp. 455–465.
- Kornecki, E.; Ehrlich, Y.; Lenox, R. Platelet-activating factor-induced aggregation of human platelets specifically inhibited by triazolobenzodiazepines. Science 1984, 226, 1454–1456.
- Hwang, S.B.; Lam, M.H.; Biftu, T.; Beattie, T.R.; Shen, T.Y. Trans-2,5-bis-(3,4,5-trimethoxyphenyl) tetrahydrofuran. An orally active specific and competitive receptor antagonist of platelet activating factor. J. Biol. Chem. 1985, 260, 15639–15645.
- Tsoupras, A.B.; Papakyriakou, A.; Demopoulos, C.A.; Philippopoulos, A.I. Synthesis, biochemical evaluation and molecular modeling studies of novel rhodium complexes with nanomolar activity against platelet activating factor. J. Inorg. Biochem. 2013, 120, 63–73.
- Arnout, J.; van Hecken, A.; De Lepeleire, I.; Miyamoto, Y.; Holmes, I.; De Schepper, P.; Vermylen, J. Effectiveness and tolerability of CV-3988, a selective paf antagonist, after intravenous administration to man. Br. J. Clin. Pharm. 1988, 25, 445–451.
- Hsieh, K.H. Effects of PAF antagonist, bn52021, on the PAF-, methacholine-, and allergen-induced bronchoconstriction in asthmatic children. Chest 1991, 99, 877–882.
- Gómez, F.P.; Rodriguez-Roisin, R. Platelet-activating factor antagonists. Biodrugs 2000, 14, 21–30.
- Taggart, D.P.; Browne, S.M.; Wade, D.T.; Halligan, P.W. Neuroprotection during cardiac surgery: A randomised trial of a platelet activating factor antagonist. Heart 2003, 89, 897–900.
- Taylor, R.; Fatovich, D.; Hitchcock, T.; Morrison, C.; Curtis, L. Platelet-activating factor antagonism and streptokinase-induced hypotension in clinical acute myocardial infarction. Clin. Sci. 2001, 100, 601–607.
- Suputtamongkol, Y.; Intaranongpai, S.; Smith, M.D.; Angus, B.; Chaowagul, W.; Permpikul, C.; Simpson, J.A.; Leelarasamee, A.; Curtis, L.; White, N.J. A double-blind placebo-controlled study of an infusion of lexipafant (platelet-activating factor receptor antagonist) in patients with severe sepsis. Antimicrob. Agents Chemother. 2000, 44, 693–696.
- Johnson, C.D.; Kingsnorth, A.N.; Imrie, C.W.; McMahon, M.J.; Neoptolemos, J.P.; McKay, C.; Toh, S.K.C.; Skaife, P.; Leeder, P.C.; Wilson, P.; et al. Double blind, randomised, placebo controlled study of a platelet activating factor antagonist, lexipafant, in the treatment and prevention of organ failure in predicted severe acute pancreatitis. Gut 2001, 48, 62–69.
- Kuitert, L.M.; Hui, K.P.; Uthayarkumar, S.; Burke, W.; Newland, A.C.; Uden, S.; Barnes, N.S. Effect of the platelet-activating factor antagonist UK-74,505 on the early and late response to allergen. Am. Rev. Respir. Dis. 1993, 147, 82–86.
- O’Connor, B.J.; SUden, S.; Carty, T.J.; Eskra, J.D.; Barnes, P.J.; Chung, K.J. Inhibitory effect of uk,74505, a potent and specific oral platelet activating factor (PAF) receptor antagonist, on airway and systemic responses to inhaled paf in humans. Am. J. Respir. Crit. Care Med. 1994, 150, 35–40.
- Evans, D.J.; Barnes, P.J.; Cluzel, M.; O’Connor, B.J. Effects of a potent platelet-activating factor antagonist, SR27417a, on allergen-induced asthmatic responses. Am. J. Respir. Crit. Care Med. 1997, 156, 11–16.
- Gomez, F.; Roca, J.; Barbera, J.; Chung, K.; Peinado, V.; Rodriguez-Roisin, R. Effect of a platelet-activating factor (PAF) antagonist, SR27417a, on paf-induced gas exchange abnormalities in mild asthma. Eur. Respir. J. 1998, 11, 835–839.
- Stack, W.A.; Jenkins‡, D.; Vivet§, P.; Hawkey*, C.J. Lack of effectiveness of the platelet-activating factor antagonist SR27417a in patients with active ulcerative colitis: A randomized controlled trial. Gastroenterology 1998, 115, 1340–1345.
- Freitag, A.; Watson, R.M.; Matsos, G.; Eastwood, C.; O’Byrne, P.M. Effect of a platelet activating factor antagonist, WEB 2086, on allergen induced asthmatic responses. Thorax 1993, 48, 594–598.
- Baltás, E.; Trach, V.; Dobozy, A.; Kemény, L. Platelet-activating factor antagonist WEB 2086 inhibits ultraviolet-B radiation-induced dermatitis in the human skin. Ski. Pharm. Physiol. 2003, 16, 259–262.
- Hilliquin, P.; Chermat-Izard, V.; Menkes, C.J. A double blind, placebo controlled study of a platelet activating factor antagonist in patients with rheumatoid arthritis. J. Rheumatol. 1998, 25, 1502–1507.
- Wittwer, T.; Grote, M.; Oppelt, P.; Franke, U.; Schaefers, H.-J.; Wahlers, T. Impact of PAF antagonist BN 52021 (ginkolide b) on post-ischemic graft function in clinical lung transplantation. J. Heart Lung Transplant. 2001, 20, 358–363.
- Elders, M.E.; Gerritsen, M.J.P.; Van De Kerkhof, P.C.M. The effect of topical application of the platelet-activating factor-antagonist, RO 24–0238, in psoriasis vulgaris—a clinical and immunohistochemical study. Clin. Exp. Derm. 1994, 19, 453–457.
- Poeze, M.; Froon, A.H.; Ramsay, G.; Buurman, W.A.; Greve, J.W. Decreased organ failure in patients with severe sirs and septic shock treated with the platelet-activating factor antagonist TCV-309: A prospective, multicenter, double-blind, randomized phase II trial. TCV-309 septic shock study group. Shock 2000, 14, 421–428.
- Johnson, M.; Kwatra, G.; Badyal, D.K.; Thomas, E.A. Levocetirizine and rupatadine in chronic idiopathic urticaria. Int. J. Derm. 2015, 54, 1199–1204.
- Mullol, J.; Bousquet, J.; Bachert, C.; Canonica, W.G.; Gimenez-Arnau, A.; Kowalski, M.L.; Marti-Guadano, E.; Maurer, M.; Picado, C.; Scadding, G.; et al. Rupatadine in allergic rhinitis and chronic urticaria. Allergy 2008, 63 (Suppl. 87), 5–28.
- Mullol, J.; Bousquet, J.; Bachert, C.; Canonica, G.W.; Giménez-Arnau, A.; Kowalski, M.L.; Simons, F.E.R.; Maurer, M.; Ryan, D.; Scadding, G. Update on rupatadine in the management of allergic disorders. Allergy 2015, 70, 1–24.
- Hozawa, S.; Haruta, Y.; Ishioka, S.; Yamakido, M. Effects of a PAF antagonist, Y-24180, on bronchial hyperresponsiveness in patients with asthma. Am. J. Respir. Crit. Care Med. 1995, 152, 1198–1202.
- Saint-Martin, F.; Dumur, J.P.; Pérez, I.; Izquierdo, I.; French Rupatadine-Rhinitis Study Group. A randomized, double-blind, parallel-group study, comparing the efficacy and safety of rupatadine (20 and 10 mg), a new PAF and H1 receptor-specific histamine antagonist, to loratadine 10 mg in the treatment of seasonal allergic rhinitis. J. Investig. Allergol. Clin. Immunol. 2004, 14, 34–40.
- Qian, C.; Hwang, S.B.; Libertine-Garahan, L.; Eckman, J.B.; Cai, X.; Scannell, R.T.; Yeh, C.G. Anti-inflammatory activities of LDP-392, a dual paf receptor antagonist and 5-lipoxygenase inhibitor. Pharm. Res. 2001, 44, 213–220.
- Sultan, S.; D’Souza, A.; Zabetakis, I.; Lordan, R.; Tsoupras, A.; Kavanagh, E.P.; Hynes, N. Chapter 6 - statins: Rationale, mode of action, and side effects. In The Impact of Nutrition and Statins on Cardiovascular Diseases; Zabetakis, I., Lordan, R., Tsoupras, A., Eds.; Academic Press: London, UK, 2019; pp. 171–200.
- Tsantila, N.; Tsoupras, A.B.; Fragopoulou, E.; Antonopoulou, S.; Iatrou, C.; Demopoulos, C.A. In vitro and in vivo effects of statins on platelet-activating factor and its metabolism. Angiology 2011, 62, 209–218.
- Chini, M.; Tsoupras, A.B.; Mangafas, N.; Tsogas, N.; Papakonstantinou, V.D.; Fragopoulou, E.; Antonopoulou, S.; Gargalianos, P.; Demopoulos, C.A.; Lazanas, M.C. Effects of haart on platelet-activating factor metabolism in naive HIV-infected patients I: Study of the tenofovir-df/emtricitabine/efavirenz haart regimen. Aids Res. Hum. Retrovir. 2012, 28, 766–775.
- Chini, M.; Tsoupras, A.B.; Mangafas, N.; Tsogas, N.; Papakonstantinou, V.D.; Fragopoulou, E.; Antonopoulou, S.; Gargalianos, P.; Demopoulos, C.A.; Lazanas, M.C. Effects of highly active antiretroviral therapy on platelet activating factor metabolism in naive HIV-infected patients: II) study of the abacavir/lamivudine/efavirenz haart regimen. Int. J. Immunopathol. Pharm. 2012, 25, 247–258.
- Hussaini, I.M.; Yi-Hua, Z.; Lysiak, J.J.; Shen, T.Y. Dithiolane analogs of lignans inhibit interferon-γ and lipopolysaccharide-induced nitric oxide production in macrophages. Acta Pharm. Sin. 2000, 21, 897–904.
- Fujita, M.; Seki, T.; Inada, H.; Shimizu, K.; Takahama, A.; Sano, T. Novel agents combining platelet activating factor (PAF) receptor antagonist with thromboxane synthase inhibitor (TXSI). Bioorg. Med. Chem. Lett. 2002, 12, 771–774.
- Hyland, I.K.; O’Toole, R.F.; Smith, J.A.; Bissember, A.C. Progress in the development of platelet-activating factor receptor (PAFR) antagonists and applications in the treatment of inflammatory diseases. Chem. Med. Chem. 2018, 13, 1873–1884.
- Braquet, P. Treatment and prevention of PAF-acether-induced sickness by a new series of highly specific inhibitors. Gb Pat. 1984, 8, 418–424.
- Touvay, C.; Etienne, A.; Braquet, P. Inhibition of antigen-induced lung anaphylaxis in the guinea-pig by BN 52021 a new specific PAF-acether receptor antagonist isolated from ginkgo biloba. Agents Actions 1986, 17, 371–372.
- Braquet, P.G.; Spinnewyn, B.; Braquet, M.; Bourgain, R.; Taylor, J.E.; Etienne, A.; Drieu, K. BN 52021 and related compounds: A new series of highly specific PAF-acether receptor antagonists isolated from Ginkgo biloba L. Blood Vessel 1985, 16, 558–572.
- Papakonstantinou, V.D. Ginkgo biloba and its anti-inflammatory value as a medical tool. Hellenic J. Atherosclerosis 2013, 4, 109–115.
- Braquet, P. Proofs of involvement of PAF-acether in various immune disorders using BN 52021 (ginkgolide b): A powerful PAF-acether antagonist isolated from Ginkgo biloba L. Adv. Prostaglandin. Thromboxane. Leukot. Res. 1986, 16, 179–198.
- Bourgain, R.H.; Maes, L.; Andries, R.; Braquet, P. Thrombus induction by endogenic PAF-acether and its inhibition by Ginkgo biloba extracts in the guinea pig. Prostaglandins 1986, 32, 142–144.
- Shen, T.Y.; Hwang, S.B.; Chang, M.N.; Doebber, T.W.; Lam, M.H.; Wu, M.S.; Wang, X.; Han, G.Q.; Li, R.Z. Characterization of a platelet-activating factor receptor antagonist isolated from haifenteng (piper futokadsura): Specific inhibition of in vitro and in vivo platelet-activating factor-induced effects. Proc. Nat. Acad. Sci. USA 1985, 82, 672–676.
- Koltai, M.; Braquet, P.G. Platelet-activating factor antagonists. Clin. Rev. Allergy 1994, 12, 361–380.
- Lee, I.S.; Jung, K.Y.; Oh, S.R.; Kim, D.S.; Kim, J.H.; Lee, J.J.; Lee, H.-K.; Lee, S.-H.; Kim, E.-H.; Cheong, C. Platelet-activating factor antagonistic activity and 13c NMR assignment of pregomisin and chamigrenal fromschisandra chinensis. Arch. Pharm. Res. 1997, 20, 633–636.
- Zeng, H.-W.; Jiang, Y.-Y.; Cai, D.-G.; Bian, J.; Long, K.; Chen, Z.-L. Piperbetol, methylpiperbetol, piperol a and piperol b: A new series of highly specific PAF receptor agonists from piper betle. Planta Med. 1997, 63, 296–298.
- Jantan, I.; Juriyati, J.; Warif, N.A. Inhibitory effects of xanthones on platelet activating factor receptor binding in vitro. J. Ethnopharmacol. 2001, 75, 287–290.
- Demopoulos, C.A.; Antonopoulou, S. A discovery trip to compounds with PAF-like activity. Adv. Exp. Med. Biol. 1996, 416, 59–63.
- Siafaka-Kapadai, A.; Demopoulos, C.A.; Andrikopoulos, N.K. Biological activity of lipids of pine pollen on platelet aggregation in correlation with the platelet activating factor. Biochem. Int. 1986, 12, 33–41.
- Koussissis, S.G.; Semidalas, C.E.; Antonopoulou, S.; Kapoulas, V.M.; Demopoulos, C.A.; Kalyvas, V. Paf antagonists in foods: Isolation and identification of paf antagonists in virgin olive oil. Rev. Française Des Corps Gras 1993, 40, 323–327.
- Koussissis, S.G.; Semidalas, C.E.; Hadzistavrou, E.C.; Kalyvas, V.G.; Antonopoulou, S.; Demopoulos, C.A. Paf antagonists in foods: Isolation and identification of PAF antagonists in honey and wax. Rev. Française Des Corps Gras 1994, 41, 127–132.
- Antonopoulou, S.; Semidalas, C.E.; Koussissis, S.; Demopoulos, C.A. Platelet-activating factor (PAF) antagonists in foods: A study of lipids with paf or anti-paf-like activity in cow’s milk and yogurt. J. Agric. Food Chem. 1996, 44, 3047–3051.
- Rementzis, J.; Antonopoulou, S.; Argyropoulos, D.; Demopoulos, C.A. Biologically active lipids from S. scombrus. In Platelet-Activating Factor and Related Lipid Mediators 2: Roles in Health and Disease; Nigam, S., Kunkel, G., Prescott, S.M., Eds.; Springer: Boston, MA, USA, 1996; pp. 68–72.
- Fragopoulou, E.; Nomikos, T.; Antonopoulou, S.; Mitsopoulou, C.A.; Demopoulos, C.A. Separation of biologically active lipids from red wine. J. Agric. Food Chem. 2000, 48, 1234–1238.
- Tierney, A.; Lordan, R.; Tsoupras, A.; Zabetakis, I. Chapter 8 - diet and cardiovascular disease: The Mediterranean diet. In The Impact of Nutrition and Statins on Cardiovascular Diseases; Zabetakis, I., Lordan, R., Tsoupras, A., Eds.; Academic Press: London, UK, 2019; pp. 267–288.
- Detopoulou, P.; Demopoulos, C.; Karantonis, H.; Antonopoulou, S. Mediterranean diet and its protective mechanisms against cardiovascular disease: An insight into platelet activating factor (PAF) and diet interplay. Ann. Nutr. Disord. 2015, 2, 1–10.
- Tsoupras, A.B.; Fragopoulou, E.; Nomikos, T.; Iatrou, C.; Antonopoulou, S.; Demopoulos, C.A. Characterization of the de novo biosynthetic enzyme of platelet activating factor, ddt-insensitive cholinephosphotransferase, of human mesangial cells. Mediat. Inflamm. 2007, 2007, 27683.
- Tsoupras, A.B.; Fragopoulou, E.; Iatrou, C.; Demopoulos, C.A. In vitro protective effects of olive pomace polar lipids towards platelet activating factor metabolism in human renal cells. Curr. Top. Nutraceutical Res. 2011, 9, 105.
- Nasopoulou, C.; Tsoupras, A.B.; Karantonis, H.C.; Demopoulos, C.A.; Zabetakis, I. Fish polar lipids retard atherosclerosis in rabbits by down-regulating PAF biosynthesis and up-regulating PAF catabolism. Lipids Health Dis. 2011, 10, 1–18.
- Karantonis, H.C.; Tsantila, N.; Stamatakis, G.; Samiotaki, M.; Panayotou, G.; Antonopoulou, S.; Demopoulos, C.A. Bioactive polar lipids in olive oil, pomace and waste byproducts. J. Food Biochem. 2008, 32, 443–459.
- Karantonis, H.C.; Antonopoulou, S.; Perrea, D.N.; Sokolis, D.P.; Theocharis, S.E.; Kavantzas, N.; Iliopoulos, D.G.; Demopoulos, C.A. In vivo antiatherogenic properties of olive oil and its constituent lipid classes in hyperlipidemic rabbits. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 174–185.
- Tsantila, N.; Karantonis, H.C.; Perrea, D.N.; Theocharis, S.E.; Iliopoulos, D.G.; Antonopoulou, S.; Demopoulos, C.A. Antithrombotic and antiatherosclerotic properties of olive oil and olive pomace polar extracts in rabbits. Mediat. Inflamm. 2007, 2007.
- Tsantila, N.; Karantonis, H.C.; Perrea, D.N.; Theocharis, S.E.; Iliopoulos, D.G.; Iatrou, C.; Antonopoulou, S.; Demopoulos, C.A. Atherosclerosis regression study in rabbits upon olive pomace polar lipid extract administration. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 740–747.
- Nasopoulou, C.; Karantonis, H.C.; Perrea, D.N.; Theocharis, S.E.; Iliopoulos, D.G.; Demopoulos, C.A.; Zabetakis, I. In vivo anti-atherogenic properties of cultured gilthead sea bream (Aparus aurata) polar lipid extracts in hypercholesterolaemic rabbits. Food Chem. 2010, 120, 831–836.
- Xanthopoulou, M.N.; Kalathara, K.; Melachroinou, S.; Arampatzi-Menenakou, K.; Antonopoulou, S.; Yannakoulia, M.; Fragopoulou, E. Wine consumption reduced postprandial platelet sensitivity against platelet activating factor in healthy men. Eur. J. Nutr. 2016, 56, 1485–1492.
- Fragopoulou, E.; Detopoulou, P.; Nomikos, T.; Pliakis, E.; Panagiotakos, D.B.; Antonopoulou, S. Mediterranean wild plants reduce postprandial platelet aggregation in patients with metabolic syndrome. Metabolism 2012, 61, 325–334.
- Antonopoulou, S.; Fragopoulou, E.; Karantonis, H.C.; Mitsou, E.; Sitara, M.; Rementzis, J.; Mourelatos, A.; Ginis, A.; Phenekos, C. Effect of traditional greek Mediterranean meals on platelet aggregation in normal subjects and in patients with type 2 diabetes mellitus. J. Med. Food 2006, 9, 356–362.
- Karantonis, H.C.; Fragopoulou, E.; Antonopoulou, S.; Rementzis, J.; Phenekos, C.; Demopoulos, C.A. Effect of fast-food Mediterranean-type diet on type 2 diabetics and healthy human subjects’ platelet aggregation. Diabetes Res. Clin. Pr. 2006, 72, 33–41.
- Gavriil, L.; Detopoulou, M.; Petsini, F.; Antonopoulou, S.; Fragopoulou, E. Consumption of plant extract supplement reduces platelet activating factor-induced platelet aggregation and increases platelet activating factor catabolism: A randomised, double-blind and placebo-controlled trial. Br. J. Nutr. 2019, 1–10.
- Tsoupras, A.; Lordan, R.; Demuru, M.; Shiels, K.; Saha, S.K.; Nasopoulou, C.; Zabetakis, I. Structural elucidation of irish organic farmed salmon (Salmo salar) polar lipids with antithrombotic activities. Mar. Drugs 2018, 16, 176.
- Tsoupras, A.; O’Keeffe, E.; Lordan, R.; Redfern, S.; Zabetakis, I. Bioprospecting for antithrombotic polar lipids from salmon, herring, and boarfish by-products. Foods 2019, 8, 416.
- Tsoupras, A.; Lordan, R.; Shiels, K.; Saha, S.K.; Nasopoulou, C.; Zabetakis, I. In vitro antithrombotic properties of salmon (Salmo salar) phospholipids in a novel food-grade extract. Mar. Drugs 2019, 17, 62.
- Poutzalis, S.; Lordan, R.; Nasopoulou, C.; Zabetakis, I. Phospholipids of goat and sheep origin: Structural and functional studies. Small Rumin. Res. 2018, 167, 39–47.
- Megalemou, K.; Sioriki, E.; Lordan, R.; Dermiki, M.; Nasopoulou, C.; Zabetakis, I. Evaluation of sensory and in vitro anti-thrombotic properties of traditional greek yogurts derived from different types of milk. Heliyon 2017, 3, e00227.
- Lordan, R.; Walsh, A.M.; Crispie, F.; Finnegan, L.; Demuru, M.; Tsoupras, A.; Cotter, P.D.; Zabetakis, I. Caprine milk fermentation enhances the antithrombotic properties of cheese polar lipids. J. Funct. Foods 2019, 61, 103507.
- Lordan, R.; Walsh, A.M.; Crispie, F.; Finnegan, L.; Cotter, P.D.; Zabetakis, I. The effect of ovine milk fermentation on the antithrombotic properties of polar lipids. J. Funct. Foods 2019, 54, 289–300.
- Lordan, R.; Zabetakis, I. Invited review: The anti-inflammatory properties of dairy lipids. J. Dairy Sci. 2017, 100, 4197–4212.
- Lordan, R.; O’Keeffe, E.; Dowling, D.; Mullally, M.; Heffernan, H.; Tsoupras, A.; Zabetakis, I. The in vitro antithrombotic properties of ale, lager, and stout beers. Food Biosci. 2019, 28, 83–88.
- Lordan, R.; O’Keeffe, E.; Tsoupras, A.; Zabetakis, I. Total, neutral, and polar lipids of brewing ingredients, by-products and beer: Evaluation of antithrombotic activities. Foods 2019, 8, 171.
- Lordan, R.; Tsoupras, A.; Zabetakis, I. Phospholipids of animal and marine origin: Structure, function, and anti-inflammatory properties. Molecules 2017, 22, 1964.




