Research into potential physiological and therapeutic ways of suppressing PAF activity demonstrated that endogenous or ingested PAF inhibitors could inhibit the actions of PAF [
10,
93]. Endogenous inhibitors of PAF have been identified in humans [
133], many of which were identified as cardiolipins [
134,
135]. As a consequence of discovering that the body circulated PAF antagonists, it was thought that the absence of circulating antagonists could result in increased PAF activity [
5]. Therefore, the potential role of PAF inhibitors in disease prevention and treatment has been of significant interest over the last three decades. Initial indications in the early 1980s demonstrated that PAF release from leukocytes could be modulated pharmacologically [
136]. This was followed by studies using pharmacological compounds such as ticlopidine and calmodulin to study PAF-induced platelet aggregation [
137,
138]. At that time it was also shown that methanolic extracts of garlic bulbs exhibited inhibition of various platelet agonists including PAF [
139]. This seems to be the first time in the literature that compounds originating from food were reported to have inhibited PAF-induced platelet aggregation. This was a significant finding as it demonstrated the existence of not only pharmacological therapeutics, but potentially dietary sources of PAF inhibitors also.
Around this period of PAF research there was a large increase in the number of published research relating to the discovery of PAF antagonists of natural and synthetic origin for which we now know of several hundred natural and synthetic PAF inhibitor molecules in existence [
14]. In particular, researchers were investigating the potential use of compounds known as ginkgolides isolated from the
Ginkgo biloba tree; a tree native to China, the existence of which dates back over 270 million years [
140].
There are several ways to classify PAF inhibitors including if they are of natural of synthetic origin, they can be classified by their various chemical structures, and they can be classified by their interaction with the PAF-R, e.g., specific and non-specific inhibitors [
141]. In terms of their structures, PAF inhibitors can be PAF analogues such as polar lipids, or there are molecules that are dihydropyridines, nitrogen heterocyclic compounds, phenolics, and other various natural medicinal compounds [
141,
142,
143].
Along with being classified into compounds of natural or synthetic origin, PAF inhibitors can be characterised into two main classes according to their specificity: non-specific and specific inhibitors. Non-specific PAF inhibitors are compounds that inhibit certain processes in the PAF-induced signal transduction pathways such as calcium channel blockers, G-protein inhibitors, intracellular calcium chelators, etc. [
14]. Various non-specific PAF inhibitors were crucial to identifying the individual steps of PAF-related signal transduction pathways. However, their pharmacological value is limited due to their low specificity [
144,
145,
146,
147]. By contrast, specific PAF inhibitors competitively or noncompetitively bind with the PAF-R. These types of inhibitors may have potential therapeutic value [
5,
14].
4.1. PAF Inhibitors of Synthetic Origin
The initial synthetic PAF inhibitor compounds such as CV-3988 [
148,
149], CV-6209 [
150], RO 19-3704 [
151], and ONO-6240 [
152] were structurally similar to PAF. In fact CV-3988 a thiazolium derivative was a zwitterionic species that was the first synthetic antagonist of the PAF-R [
148]. Later inhibitors replaced the glycerol backbone with cyclic structures such as SRI 63-441 [
153], SRI 63-073 [
154], UR-11353 [
155], and CL-184,005 [
156]. Subsequently, other PAF antagonists were developed that had no structural similarity to PAF. These antagonists were composed of heterocyclic structures that were characterised by sp
2 nitrogen atom that interacted with the PAF-R as a hydrogen bond acceptor [
141]. Many of these were derivatives of imidazolyl that lead to the development of lexipafant [
157] and modipafant [
158], thiazolidine derivatives such as SM-10661 [
159], pyrrolothiazole-related antagonists such as tulopafant [
160], and hetrazepine derivatives like WEB-2086 and WEB-2170 [
161]. There are a plethora of synthetic PAF-R antagonists including psychotropic triazolobenzodiazepines [
162], L-652,731 [
163], and various examples of inorganic metal complexes [
143,
164]. However, it was later discovered that some of these antagonists were not orally active and some had toxicity issues [
165,
166], thus they had limited therapeutic value [
167].
Clinical trials were conducted for several of these inhibitors, which demonstrated their tolerability and safety, but there were issues with their efficacy; juxtaposed, there were several trials that indicated positive outcomes following PAF-R antagonism. The inhibitors and their target diseases or disorders are outlined in Table 2.
Table 2. A list of some of the major synthetic PAF antagonists assessed against several conditions in clinical trials.
|
PAF-R Antagonist
|
Target Disease or Disorder
|
Outcome
|
Reference
|
|
Lexipafant
|
Cognitive impairment complications as a result of coronary artery bypass graft
|
No significant reduction in cognitive impairment
|
[168]
|
|
Myocardial infarction
|
No significant effect on streptokinase-induced hypotension in myocardial infarction patients
|
[169]
|
|
Sepsis
|
No significant affect in patients with severe sepsis
|
[170]
|
|
Organ failure related to pancreatitis
|
No significant amelioration of systemic inflammatory response syndrome in pancreatitis-induced organ failure
|
[171]
|
|
Modipafant
|
Asthma
|
No significant effect against chronic asthma
|
[158]
|
| |
Asthma
|
No significant effect in early or late responses to allergens
|
[172]
|
|
Responses to inhaled PAF
|
Potent inhibition of airway and neutrophil responses to PAF with a duration of up to 24 h and a reduction of secondary eicosanoid production in response to inhaled PAF
|
[173]
|
|
SR27417A
SR27417A
|
Asthma
|
Modest inhibitory effects against asthma
|
[174,175],
|
|
Ulcerative colitis
|
No evidence of efficacy in the treatment of acute ulcerative colitis
|
[176]
|
|
WEB 2086
|
Asthma
|
No attenuation of early of late allergen-induced responses or airway hyperresponsiveness
|
[177]
|
|
UVB-induced dermatitis
|
Significant inhibition of UVB light-induced erythema
|
[178]
|
|
BN 50730
|
Rheumatoid arthritis
|
Ineffective in the treatment of rheumatoid arthritis
|
[179]
|
|
BN 52021
|
Pulmonary function in the early post ischaemic graft function in clinical lung transplantation
|
Improvement of alveoloarterial oxygen difference and a reduction of PAF levels
|
[180]
|
|
Ro 24-238
|
Psoriasis
|
No significant effects reported
|
[181]
|
|
TCV-309
|
Septic shock
|
No significant difference in adverse events or mortality. A substantial reduction of organ dysfunction and morbidity associated with septic shock was reported
|
[182]
|
|
Levocetirizine
|
Chronic idiopathic urticaria
|
Reduction of urticarial activity score
|
[183]
|
|
Rupatadine
|
Chronic idiopathic urticaria
|
Reduction of urticarial activity score but not as effective as levocetirizine
|
[183,184]
|
| |
Allergic rhinitis and allergies
|
Significant effects against both conditions as demonstrated in the comprehensive review by Mullol et al.
|
[185]
|
|
Y-24180
|
Asthma
|
Improvement of bronchial hyperresponsiveness in patients with asthma
|
[186]
|
Notably, some molecules exhibit dual antagonistic properties towards PAF and other inflammatory mediators. For instance, rupatadine is both an antagonist of the PAF-R and the histamine H(1) receptor [
187], whereas LDP-392 can target both PAF and 5-lipoxygenase [
188]. Likewise, common statins targeting CVD [
189,
190] and antiretrovirals targeting human immunodeficiency virus (HIV) [
191,
192] also exhibit anti-PAF pleiotropic effects. Indeed, various other molecules can inhibit both PAF and inducible nitric oxide synthase induction (iNOS) [
193] or thromboxane synthases [
194].
Finally, apart from the various compounds presented in
Table 2, research has investigated the use of various inorganic metal complexes including other structurally related and structurally dissimilar PAF-R antagonists [
141]. The authors recommend the following comprehensive reviews for further information on various synthetic and inorganic metal complexes with PAF-R antagonistic properties, their structures, synthesis, and biological effects [
116,
141,
167,
185].
4.2. PAF Inhibitors of Natural Origin
Extracts from
Ginkgo biloba were some of the first PAF inhibitors of natural origin to be discovered. Several studies by Pierre Braquet and colleagues demonstrated that one compound in particular, BN 2021, was a highly specific competitive PAF antagonist. Several related ginkgolides also exhibited inhibitory properties against PAF [
195,
196,
197,
198,
199,
200]. Indeed, several other researchers at the time discovered anti-PAF properties in other natural isolates of Chinese medicinal herbs such as phomactin A, kadsurenone, and various xanthones [
201,
202,
203,
204,
205]. In fact, the discovery that compounds from garlic bulbs possess anti-PAF activity stimulated interest in the exploration of natural compounds for anti-PAF activity [
139].
By 1996, several molecules had been discovered with PAF-like activity as reviewed by Demopoulos [
48]. Further experimentation uncovered that a neutral glycerylether lipid without an acetyl group from pine pollen exhibited biological activity against PAF [
206]. Consequently, it was deduced that other lipid extracts could potentially inhibit PAF-induced platelet aggregation. This led to a series of studies investigating food lipid extracts starting around 1993, which initially lead to the discovery of PAF antagonists in the polar lipid fractions of olive oil [
207], honey and wax [
208], milk and yoghurt [
209], mackerel (
Scomber scombrus) [
210], and wine [
211] before the turn of the century. These studies deduced that mainly polar lipids such as glycerophospholipids and glycolipids exhibited potent inhibition against PAF-induced platelet aggregation through competitive binding to the PAF-R. As this research field developed it was noted that many of the compounds discovered that exhibited anti-PAF activity were also constituents of foods of the Mediterranean diet [
5,
212,
213]. Therefore, these constituents may be responsible for the observed beneficial effects of consuming the Mediterranean diet [
5,
212,
213]. Indeed, later research demonstrated that polar lipid extracts of olive oil, olive pomace, and fish could also affect many of the PAF metabolic enzymes both in vitro and in vivo [
54,
214,
215]. These extracts were able to aid in the re-equilibration of PAF levels with beneficial outcomes against models of chronic inflammation.
Research into the effect of lipids on PAF activity and PAF metabolism is still being explored today in the pursuit of finding natural ways to prevent the pro-inflammatory signaling of PAF. It is now known that many foods, beverages, and other natural sources including food industry by-products are rich in PAF antagonists [
142,
216]. However, there have been several critical discoveries in vivo that suggest that PAF inhibitors of natural origin may help prevent diseases such as CVD. In studies in vivo, olive oil, olive oil polar lipids extracts, and olive oil neutral lipids extracts were administered to rabbits consuming an atherogenic diet. It was demonstrated that rabbits consuming olive oil or olive oil polar lipid extracts had more beneficial physiological and biochemical changes as a result of increased plasma levels of PAF-AH, less oxidation in the plasma, a reduction of atherosclerotic lesion thickness, and retention of vessel wall elasticity, thus impeding atherosclerosis development [
217]. These results were corroborated in a subsequent study that found that polar lipid extracts of olive oil and olive pomace can impede early atherosclerosis development through reducing platelet sensitivity to PAF and reducing atherosclerotic lesion thickness [
218]. A later follow-up study in rabbits demonstrated that olive pomace polar lipid extracts were equipotent to simvastatin in preventing the progression of atherogenesis [
219].
It was questioned whether other polar lipid extracts of natural origin could exhibit the same effects. Therefore, two studies of similar design demonstrated anti-atherogenic effects when rabbits consumed polar lipids extracted from fish (seabream,
Sparus aurata) in a model of hypercholesterolaemia. These studies demonstrated that fish polar lipids could also reduce platelet aggregation, reduce atherosclerotic lesion size, and increase HDL levels in rabbits [
220] along with modulating PAF metabolism leading to lower PAF levels and activity in rabbit blood [
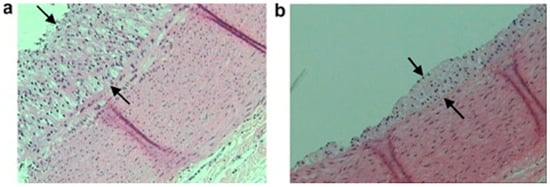
215]. Representative optic micrographs (×100) of the aortic wall of these rabbits are presented in
Figure 2. These images demonstrate that rabbits consuming an atherogenic diet supplemented with fish polar lipids leads to a reduction of atherosclerotic lesion width (b) versus a control group that consumed only an atherogenic diet (a) [
220].
Figure 2. Representative optic micrographs ×100 of aortic wall cross-sections stained with hematoxylin and eosin obtained from the two rabbit experimental groups. Atherosclerotic lesions appear as foam cells between the arrows. Each tissue sample was approximately 5 µm thick. (
a) Group A (atherogenic diet) and (
b) group B (atherogenic diet enriched with seabream polar lipids). Reproduced with permission from Nasopoulou et al. [
220].
However, after discovering that polar lipids could inhibit PAF in vitro and in vivo, the question remained whether these compounds of natural origin could affect human health? It is now known that there have been some promising nutritional trials that indicate that PAF antagonists in wine may affect platelet aggregation and metabolism postprandially in humans [
43,
221]. In people with metabolic syndrome, consumption of meals including wild plants of the Mediterranean diet rich in PAF inhibitors postprandially reduced PAF-induced platelet aggregation [
222]. Other results from dietary intervention studies have shown that the administration of traditional Mediterranean diet meals [
223,
224] to either normal volunteers or individual’s with type II diabetes mellitus (who have a predisposition to CVD) resulted in the characteristic lower PAF activity in blood (measured as PAF-induced platelet aggregability), which correlates with inhibition of atherogenesis according to experiments [
217].
Likewise, dietary supplements can reduce PAF-induced platelet aggregation and increase PAF catabolism in healthy humans [
225]. These studies collectively indicate that consumption of PAF antagonists from foods and nutraceuticals may benefit the consumer by reducing the pro-inflammatory effects of PAF either through inhibition of PAF/PAF-R signaling or by influencing the metabolic enzymes of PAF.
Considering, the potential use of dietary polar lipids for the prevention of CVD, several recent studies have discovered PAF antagonists in various fish species and by-products of the fishing industry including salmon fillet and head, minced boarfish, and herring [
226,
227,
228], and other foods such as sheep and goat meat [
229], milk and fermented dairy products [
230,
231,
232,
233], and beer and brewing by-products [
234,
235]. Future research in this area aims to develop novel functional foods and nutraceuticals that incorporate these bioactive polar lipid extracts for the prevention of CVD and other inflammation-related diseases. For more extensive reviews of the anti-inflammatory and antithrombotic properties of various food polar lipids the authors suggest the following literature [
49,
142].