
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Grace Russell | + 1216 word(s) | 1216 | 2021-12-01 10:04:25 | | | |
| 2 | Nora Tang | Meta information modification | 1216 | 2021-12-02 01:39:20 | | | | |
| 3 | Nora Tang | Meta information modification | 1216 | 2021-12-08 09:30:22 | | | | |
| 4 | Nora Tang | Meta information modification | 1216 | 2022-01-28 09:32:27 | | |
Video Upload Options
Since the late 18th century, molecular hydrogen (H2) has been shown to be well tolerated, firstly in animals, and then in humans. However, although research into the beneficial effects of molecular hydrogen in both plant and mammalian physiology is gaining momentum, the idea of utilising this electrochemically neutral and non-polar diatomic compound for the benefit of health has yet to be widely accepted by regulatory bodies worldwide. Due to the precise mechanisms of H2 activity being as yet undefined, the lack of primary target identification, coupled with difficulties regarding administration methods (e.g., dosage and dosage frequencies, long-term effects of treatment, and the patient’s innate antioxidant profile), there is a requirement for H2 research to evidence how it can reasonably and most effectively be incorporated into medical practice.
1. Mechanisms of Action
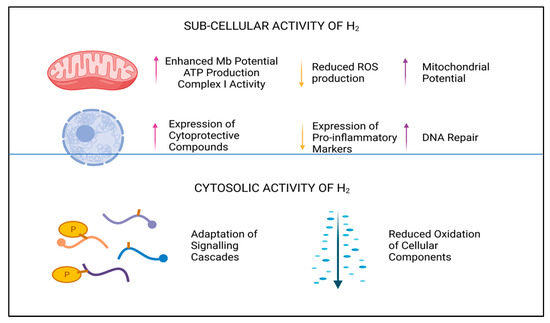
2. Effects on Human Physiology
References
- Shirahata, S.; Kabayama, S.; Nakano, M.; Miura, T.; Kusumoto, K.; Gotoh, M.; Hayashi, H.; Otsubo, K.; Morisawa, S.; Katakura, Y. Electrolyzed–reduced water scavenges active oxygen species and protects DNA from oxidative damage. Biochem. Biophys. Res. Commun. 1997, 234, 269–274.
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.I.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic anti-oxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694.
- Fukuda, K.I.; Asoh, S.; Ishikawa, M.; Yamamoto, Y.; Ohsawa, I.; Ohta, S. Inhalation of hydrogen gas suppresses hepatic injury caused by ischemia/reperfusion through reducing oxidative stress. Biochem. Biophys. Res. Commun. 2007, 361, 670–674.
- Nogueira, J.E.; Branco, L.G. Recent advances in molecular hydrogen research reducing exercise-induced oxidative stress and inflammation. Curr. Pharm. Des. 2021, 27, 731–736.
- Li, Q.; Xie, F.; Yi, Y.; Zhao, P.; Zhang, X.; Zhang, X.; Zhang, X.; Ma, X. Hydroxyl-radical scavenging activity of hydrogen does not significantly contribute to its biological function. bioRxiv 2021.
- Penders, J.; Kissner, R.; Koppenol, W.H. ONOOH does not react with H2: Potential beneficial effects of H2 as an antioxidant by selective reaction with hydroxyl radicals and peroxynitrite. Free Radic. Biol. Med. 2014, 75, 191–194.
- Hancock, J.T.; Russell, G. Downstream Signalling from Molecular Hydrogen. Plants 2021, 10, 367.
- Ghosh, N.; Das, A.; Chaffee, S.; Roy, S.; Sen, C.K. Immunity and Inflammation in Health and Disease: Emerging Roles of Nutraceuticals and Functional Foods in Immune Support; Academic Press: Cambridge, MA, USA, 2018.
- Raghunath, A.; Sundarraj, K.; Nagarajan, R.; Arfuso, F.; Bian, J.; Kumar, A.P.; Sethi, G.; Perumal, E. Antioxidant response elements: Discovery, classes, regulation and potential applications. Redox Biol. 2018, 17, 297–314.
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13.
- Ishihara, G.; Kawamoto, K.; Komori, N.; Ishibashi, T. Molecular hydrogen suppresses superoxide generation in the mitochondrial complex I and reduced mitochondrial membrane potential. Biochem. Biophys. Res. Commun. 2020, 522, 965–970.
- Xie, K.; Wang, Y.; Yin, L.; Wang, Y.; Chen, H.; Mao, X.; Wang, G. Hydrogen gas alleviates sepsis-induced brain injury by improving mitochondrial biogenesis through the activation of PGC-α in mice. Shock 2021, 55, 100–109.
- Iida, A.; Nosaka, N.; Yumoto, T.; Knaup, E.; Naito, H.; Nishiyama, C.; Yamakawa, Y.; Tsukahara, K.; Terado, M.; Sato, K.; et al. The clinical application of hydrogen as a medical treatment. Acta Med. Okayama 2016, 70, 331–337.
- Ge, L.; Yang, M.; Yang, N.N.; Yin, X.X.; Song, W.G. Molecular hydrogen: A preventive and therapeutic medical gas for various diseases. Oncotarget 2017, 8, 102653.
- Nogueira, J.E.; Amorim, M.R.; Pinto, A.P.; da Rocha, A.L.; da Silva, A.S.; Branco, L.G. Molecular hydrogen downregulates acute exhaustive exercise-induced skeletal muscle damage. Can. J. Physiol. Pharmacol. 2021, 99, 812–820.
- Russell, G.; Nenov, A.; Hancock, J.T. Oxy-hydrogen Gas: The Rationale behind Its Use as a Novel and Sustainable Treatment for COVID-19 and Other Respiratory Diseases. Eur. Med. J. 2021, 21-00027.
- Dole, M.; Wilson, F.R.; Fife, W.P. Hyperbaric hydrogen therapy: A possible treatment for cancer. Science 1975, 190, 152–154.
- Ostojic, S.M. Hydrogen-rich water as a modulator of gut microbiota? J. Funct. Foods 2021, 78, 104360.
- Xia, C.; Liu, W.; Zeng, D.; Zhu, L.; Sun, X.; Sun, X. Effect of hydrogen-rich water on oxidative stress, liver function, and viral load in patients with chronic Hepatitis B. Clin. Transl. Sci. 2013, 6, 372–375.
- Korovljev, D.; Stajer, V.; Ostojic, J.; LeBaron, T.W.; Ostojic, S.M. Hydrogen-rich water reduces liver fat accumulation and improves liver enzyme profiles in patients with non-alcoholic fatty liver disease: A randomized controlled pilot trial. Clin. Res. Hepatol. Gastroenterol. 2019, 43, 688–693.
- Mizuno, K.; Sasaki, A.T.; Ebisu, K.; Tajima, K.; Kajimoto, O.; Nojima, J.; Kuratsune, H.; Hori, H.; Watanabe, Y. Hydrogen-rich water for improvements of mood, anxiety, and autonomic nerve function in daily life. Med. Gas Res. 2017, 7, 247.
- Todorovic, N.; Zanini, D.; Stajer, V.; Korovljev, D.; Ostojic, J.; Ostojic, S.M. Hydrogen-rich water and caffeine for alertness and brain metabolism in sleep-deprived habitual coffee drinkers. Food Sci. Nutr. 2021, 9, 5139–5145.
- Wilson, H.R.; Veal, D.; Whiteman, M.; Hancock, J.T. Hydrogen gas and its role in cell signalling. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2017, 12, 1–3.
- Slezak, J.; Kura, B.; LeBaron, T.W.; Singal, P.K.; Buday, J.; Barancik, M. Oxidative stress and pathways of molecular hydrogen effects in medicine. Curr. Pharm. Des. 2021, 27, 610–625.
- Sha, J.B.; Zhang, S.S.; Lu, Y.M.; Gong, W.J.; Jiang, X.P.; Wang, J.J.; Qiao, T.L.; Zhang, H.H.; Zhao, M.Q.; Wang, D.P.; et al. Effects of the long-term consumption of hydrogen-rich water on the anti-oxidant activity and the gut flora in female juvenile soccer players from Suzhou, China. Med Gas Res. 2018, 8, 135.
- Ji, X.; Zheng, W.; Yao, W. Protective role of hydrogen gas on oxidative damage and apoptosis in intestinal porcine epithelial cells (IPEC-J2) induced by deoxynivalenol: A preliminary study. Toxins 2020, 12, 5.
- Murakami, Y.; Ito, M.; Ohsawa, I. Molecular hydrogen protects against oxidative stress-induced SH-SY5Y neuroblastoma cell death through the process of mitohormesis. PLoS ONE 2017, 12, 0176992.
- LeBaron, T.W.; Kura, B.; Kalocayova, B.; Tribulova, N.; Slezak, J. A new approach for the prevention and treatment of cardiovascular disorders. Molecular hydrogen significantly reduces the effects of oxidative stress. Molecules 2019, 24, 2076.
- Safety of Inhaled Hydrogen Gas Mixtures in Healthy Volunteers. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=molecular+hydrogen&cntry=&state=&city=&dist (accessed on 5 July 2021).





