Oxidative stress, metabolic dysregulation, and inflammation are known to underpin numerous disease pathologies, including acute and chronic respiratory disorders (e.g., COVID-19, COPD, respectively)
[16][5], cancer, metabolic diseases, and neurodegeneration
[17][18][19][20][21][22][8,9,10,11,12,13]. It is generally accepted that H
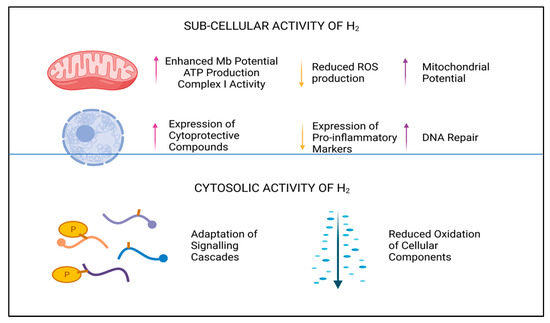
2 selectively reduces oxidative and nitrosative stress in biological systems and reduces both the apoptotic and inflammatory responses in cells, although which system has the cardinal influence here is currently unknown. Due to the physical non-polarity, electrochemical neutrality, and low molecular weight of this diatomic molecule, it is unlikely to be perceived as a typical signaling molecule
[23][40], relying on receptor binding mechanisms or electrochemical attraction for modulating cellular responses. However, what is clear is that H
2 has remarkable potential as an antioxidant and cellular protective compound, not only reducing accumulative damage caused by reactive signaling molecules
[14][24][3,41], but also by upregulating endogenous production of redox-balancing proteins, including catalase (CAT), glutathione peroxidase (GPx), and superoxide dismutase (SOD)
[25][42]. In addition to—or perhaps as a result of—the antioxidant influence of H
2, a marked reduction in the expression of intrinsic pro-apoptotic and pro-inflammatory proteins, peptides, and transcription factors is also described
[24][25][41,42]. Downregulation of pro-apoptotic biomarkers are noted to include Bax and caspase proteins
[26][43], whilst inhibition of pro-inflammatory markers interleukins (e.g., IL-6), NF-kB, and tumor necrosis factor alpha (TNFα), and others, have also been observed
[25][26][42,43]. By moderating the hyper-inflammatory response during infection or disease, H
2 can reduce both cellular and tissue damage and subsequent harm associated with aberrant inflammation.
Considering the intricate crosstalk between these intrinsic defense systems, and their role in disease pathology, the modulative effect demonstrated by H
2 in these fundamental pathways
[3][24][25][26][29,41,42,43] may help to both prevent and ameliorate the symptoms of numerous healthcare conditions. Furthermore, to date, there are no reports, clinical or empirical, that suggest H
2 acts as a strong cellular reductive agent, a mechanism typically initiated by prolonged antioxidant signaling or mitochondrial inactivity. Instead, H
2 is likely to act as an hormetic substance, ensuring the redox balance is kept intact
[27][28][44,45].
The effectiveness of H
2 as a novel therapeutic, however, may largely depend on the duration and route of administration, along with the dosage received by the patient. In this regard, both hydrogen inhalation (HRW) and intravenous injection (HRS) can provide monitorable and quantifiable means of treatment, which is favorable when considering future clinical applications of H
2 [13][2]. Alternatively, gels, patches, nanotechnologies, and topical applications may only afford semi-quantifiable results due to the rates of H
2 absorption and consequent distribution dynamics, for example. As such, the semi-quantifiable application methods may only be viable as non-clinical interventions until further research is conducted.