
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Avice O'Connell | + 1355 word(s) | 1355 | 2021-11-08 03:55:54 | | | |
| 2 | Dean Liu | Meta information modification | 1355 | 2021-12-02 01:33:38 | | |
Video Upload Options
Cone-beam breast computed tomography (CBBCT) is a revolutionary modality that will assist in overcoming the limitations of current imaging for dense breast tissue and overlapping structures.
1. Introduction
2. Advantages of Cone-beam Breast Computed Tomography (CBBCT)
The patient positions herself prone in the machine one breast at a time, inserting her own breast into the opening in the table, thereby placing the breast in the image field. No compression is used which vastly improves comfort.
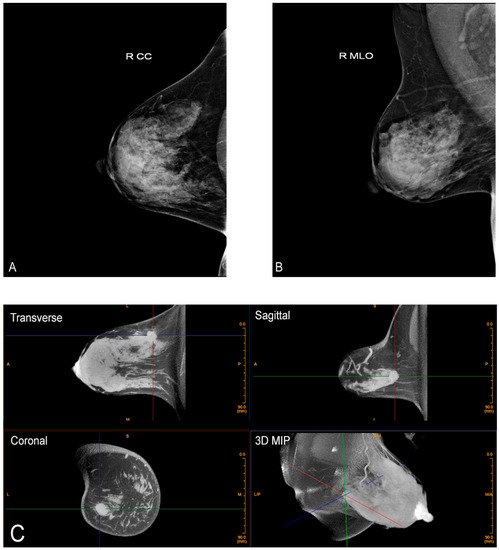
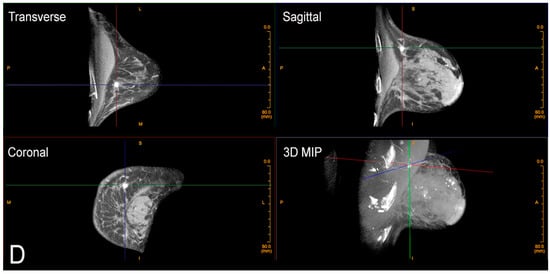
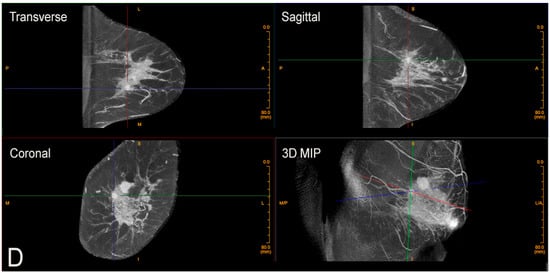
There is also no intrusive handling of the breasts, which is a completely new consideration of privacy and cultural reservations not previously addressed with current technologies. The technologist checks positioning, making any necessary minor adjustments and then takes one 360-degree image in a period of 10 s. The patient holds still, without a breath hold required. The second breast is imaged in the same way. Efficiency or speed of imaging is another advantage of CBBCT. Standard MRI takes at least 30 min, but with CBBCT, breasts (including the chest wall and axilla) are imaged one side at a time using a single 10 s 360-degree sweep.


References
- O’Connell, A. Cone-Beam Breast CT-Essentials; Imaging Science Today LLC: Vikram Dogra, MD, USA, 2017.
- Boone, J.M.; Nelson, T.R.; Lindfors, K.K.; Seibert, J.A. Dedicated Breast CT: Radiation Dose and Image Quality Evaluation. Radiology 2001, 221, 657–667.
- Lindfors, K.K.; Boone, J.M.; Newell, M.S.; D’Orsi, C.J. Dedicated Breast Computed Tomography: The Optimal Cross-Sectional Imaging Solution? Radiol. Clin. N. Am. 2010, 48, 1043–1054.
- O’Connell, A.; Conover, D.L.; Zhang, Y.; Seifert, P.; Logan-Young, W.; Lin, C.-F.L.; Sahler, L.; Ning, R. Cone-Beam CT for Breast Imaging: Radiation Dose, Breast Coverage, and Image Quality. Am. J. Roentgenol. 2010, 195, 496–509.
- O’Connell, A.M.; Karellas, A.; Vedantham, S.; Kawakyu-O’Connor, D.T. Newer Technologies in Breast Cancer Imaging: Dedicated Cone-Beam Breast Computed Tomography. Semin. Ultrasound CT MRI 2018, 39, 106–113.
- Prionas, N.D.; Lindfors, K.K.; Ray, S.; Huang, S.-Y.; Beckett, L.; Monsky, W.L.; Boone, J.M. Contrast-enhanced Dedicated Breast CT: Initial Clinical Experience. Radiology 2010, 256, 714–723.
- CPT Editorial Summary of Panel Action May 2020: American Medical Association. 2021. Available online: https://www.ama-assn.org/system/files/2020-07/may-2020-summary-panelactions.pdf (accessed on 17 September 2021).
- Vedantham, S.; Shi, L.; Karellas, A.; O’Connell, A.M.; Conover, D.L. Personalized estimates of radiation dose from dedicated breast CT in a diagnostic population and comparison with diagnostic mammography. Phys. Med. Biol. 2013, 58, 7921–7936.
- Rahbar, H.; Partridge, S.C.; DeMartini, W.B.; Thursten, B.; Lehman, C.D. Clinical and technical considerations for high quality breast MRI at 3 tesla. J. Magn. Reson. Imaging 2013, 37, 778–790.
- Ali, M.A.; Czene, K.; Hall, P.; Humphreys, K. Association of Microcalcification Clusters with Short-term Invasive Breast Cancer Risk and Breast Cancer Risk Factors. Sci. Rep. 2019, 9, 14604.
- Wilkinson, L.; Thomas, V.; Sharma, N. Microcalcification on mammography: Approaches to interpretation and biopsy. Br. J. Radiol. 2017, 90, 20160594.
- Bennani-Baiti, B.; Baltzer, P.A. MR Imaging for Diagnosis of Malignancy in Mammographic Microcalcifications: A Systematic Review and Meta-Analysis. Radiology 2017, 283, 692–701.
- Ma, Y.; Liu, A.; O’Connell, A.M.; Zhu, Y.; Li, H.; Han, P.; Yin, L.; Lu, H.; Ye, Z. Contrast-enhanced cone beam breast CT features of breast cancers: Correlation with immunohistochemical receptors and molecular subtypes. Eur. Radiol. 2021, 31, 2580–2589.
- Ghaderi, K.F.; Phillips, J.; Perry, H.; Lotfi, P.; Mehta, T.S. Contrast-enhanced Mammography: Current Applications and Future Directions. RadioGraphics 2019, 39, 1907–1920.
- Jochelson, M.S.; Lobbes, M.B.I. Contrast-enhanced Mammography: State of the Art. Radiology 2021, 299, 36–48.
- Malayeri, A.A.; Brooks, K.; Bryant, L.H.; Evers, R.; Kumar, P.; Reich, D.S.; Bluemke, D. National Institutes of Health Perspective on Reports of Gadolinium Deposition in the Brain. J. Am. Coll. Radiol. 2016, 13, 237–241.
- The Swedish Organised Service Screening Evaluation Group. Reduction in Breast Cancer Mortality from Organized Service Screening with Mammography: 1. Further Confirmation with Extended Data. Cancer Epidemiol. Biomark. Prev. 2006, 15, 45–51.
- Duffy, S.W.; Tabár, L.; Yen, A.M.; Dean, P.B.; Smith, R.A.; Jonsson, H.; Törnberg, S.; Chen, S.L.; Chiu, S.Y.; Fann, J.C.; et al. Mammography screening reduces rates of advanced and fatal breast cancers: Results in 549,091 women. Cancer 2020, 126, 2971–2979.
- Tabár, L.; Vitak, B.; Chen, T.H.-H.; Yen, A.M.-F.; Cohen, A.; Tot, T.; Chiu, S.Y.-H.; Chen, S.L.-S.; Fann, J.C.-Y.; Rosell, J.; et al. Swedish Two-County Trial: Impact of Mammographic Screening on Breast Cancer Mortality during 3 Decades. Radiology 2011, 260, 658–663.





