
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Xiangyang Song | + 3791 word(s) | 3791 | 2021-11-29 09:19:07 | | | |
| 2 | Vivi Li | Meta information modification | 3791 | 2021-12-01 10:40:41 | | |
Video Upload Options
Microbially induced carbonate precipitation (MICP) is a promising technology for solidifying sandy soil, ground improvement, repairing concrete cracks, and remediation of polluted land. By solidifying sand into soil capable of growing shrubs, MICP can facilitate peak and neutralization of CO2 emissions because each square meter of shrub can absorb 253.1 grams of CO2 per year.
1. Introduction
Microbially induced carbonate precipitation (MICP) is a promising technology applied to many civil and environmental engineering scenarios, especially combating desertification [1]. Desertification refers to the process of land degradation in arid, early semi-dry, and arid and subhumid areas under the action of various factors, including climate and human activities. The severe problem of global desertification is caused by natural and human factors, climate change, wind and rain erosion of the soil, the pursuit of economic benefits, destruction of vegetation, and unreasonable use of water resources, all of which aggravate the formation of desertification. Because the emergence of desertification has caused a significant impact on the environment and economical construction, it is highly urgent to control it [2]. Kimura et al. [3] classified and counted the global dry areas in 2017 according to the satellite-based aridity index (SbAI). Figure 1 [3] shows areas of global arid areas in 2017 and their proportion in the total land area. It can be seen from Figure 1 [3] that the total area of global arid areas accounts for 41% of the total land area. Figure 2 [4] shows the global desertification risk level distribution map from 2000 to 2014, estimated based on the global Desertification Vulnerability Index and the ratio of areas with different risk levels. The colors in Figure 1 represent different levels of global desertification risk.
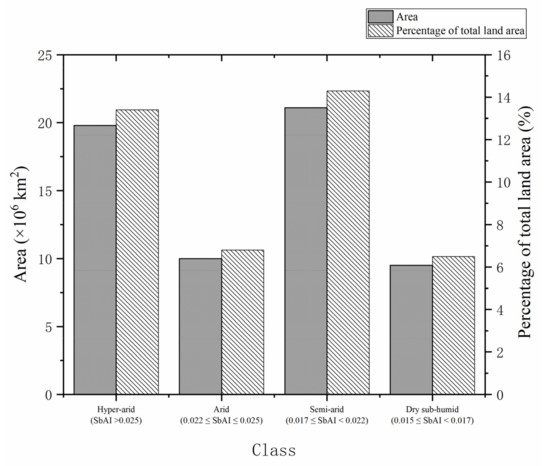
Figure 1. Schematic diagram of arid areas worldwide and their percentage of total land area in 2017 based on the Satellite Drought Index (SbAI) [3].
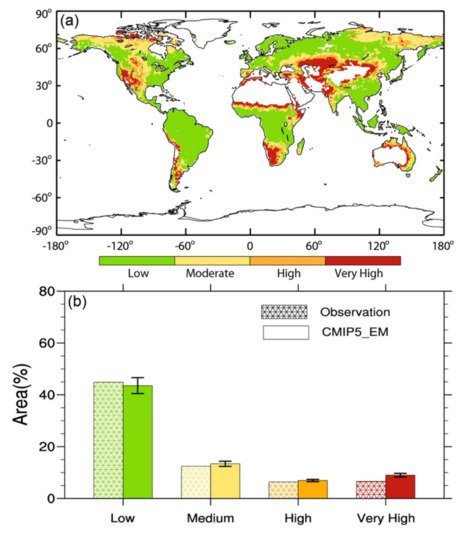
Figure 2. (a) The global desertification risk level distribution from 2000 to 2014, estimated based on the Global Desertification Vulnerability Index; (b) The proportion of areas with different desertification risk levels in the global land area [4].
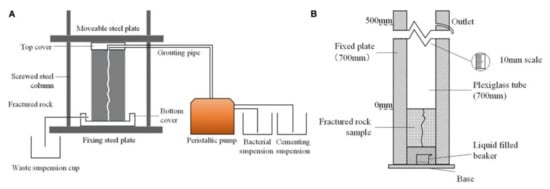
Sand consolidation uses various ways to reduce the sand porosity and fix the sand particles [5]. As one of the common ways of consolidation, grouting consolidation is defined as applying pressurized grouting slurry to infiltrate within the void of sandy soil, followed by the compaction and solidification of sand along with the slurry [6]. The grouting method usually includes the chemical grouting method and the biological grouting method [7][8]. In the early stage, methods of sand consolidation in desertification were limited to chemical grouting with cement, lime, and other chemical materials. However, various sand consolidation methods emerged following extensive research, including sand fixation with the sand barrier, chemicals, and microbial grouting [7][8][9]. The grouting method can only be used for coarse sand with a particle size greater than 4.75 mm, and microbial sand consolidation can be used for fine or medium sand with less than 0.6 mm [10]. Chemical grouting methods cost less, but chemical grouting materials (e.g., cement, lime, or adhesive) are harmful to the environment. The microbial grouting method has a relatively high cost but is friendly to the environment and can effectively improve the properties of sand [7][8]. Figure 3 [6] shows a schematic diagram of the grouting method. Microbial sand fixation refers to adding cementation solution to stimulate bacteria and then forming calcium carbonate crystals in the sand to consolidate the sand. Cementation solution generally refers to a mixture of calcium salts, nutrients and urea. Figure 4 [11] shows a diagram of the experimental setup for MICP. Microbial sand fixation not only has the advantages of environmental protection, low pollution, effective maintenance of soil moisture in sandy deserts, improvement of soil fertility, and improvement of soil thermal conductivity, but can also turn the sandy desert into soil and increase the area of state-owned arable land, which is of practical significance for the curbing of desertification. Countries around the world put forward the strategic goal of carbon peak, and carbon neutrality due to global climate change leads to many extreme climate events. “Peak carbon” refers to when carbon dioxide emissions reach a peak and then stop rising and gradually fall back. Carbon neutrality means achieving zero carbon dioxide emissions by offsetting total greenhouse gas emissions through afforestation, energy conservation, and emission reduction. The United States and the European Union have announced carbon neutrality by 2050 and China by 2060. Microbial sand fixation can also facilitate carbon peaking and carbon neutralization because microbial sand solidification technology can enable the sand to grow shrubs. Li et al. [12] found that each square meter of shrub can absorb 253.1 grams of carbon dioxide per year. All in all, microbial sand fixation has gradually become an important research topic [13][14].
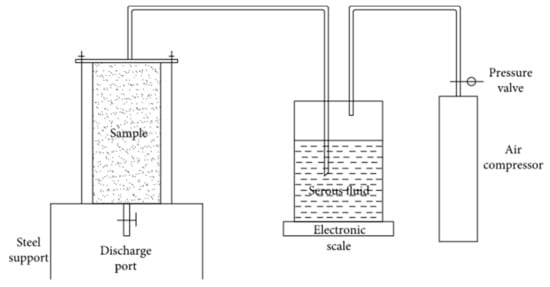
Figure 3. Schematic diagram of grouting consolidation device [6].

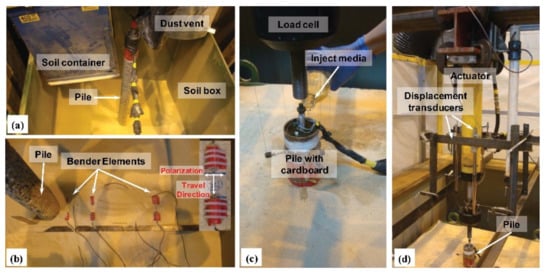
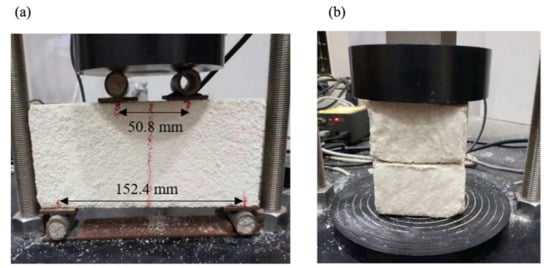
2. Microbial Sources of Solidified Sand
2.1. External Bacteria Solidifying the Sandy Soil
2.2. Solidification of Sandy Soil by Indigenous Bacteria
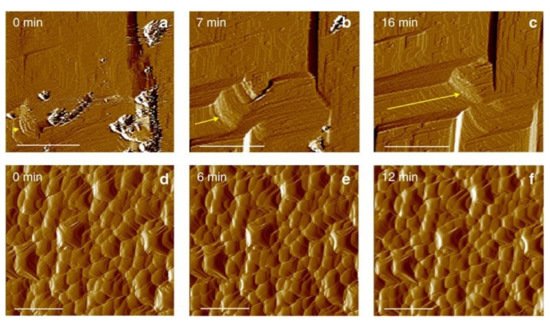
3. Models for Predicting the Curing Process of MICP in the Field
| Model Names | The Role of Models | References, Year |
|---|---|---|
| Aquifer conceptual model | Finding that the sedimentation rate of calcite is closely related to the hydrolysis rate of urea | [[68], 2005 |
| A three-dimensional (3D) discrete element method (DEM)-based numerical model | Simulating the macroscopic mechanical properties of CaCO3 sediment-solidified sandy soil induced by micro-organisms under the condition of no triaxial compression of the drainage system | [[69], 2019 |
| A loose sandstone numerical model based on a one-dimensional advective dispersion model | Predicting the movement of micro-organisms in soil and rock | [[70], 2014 |
| A pore-scale network model | Simulating the CaCO3 precipitation process and the influence of different operations on CaCO3 precipitation | [[71], 2016 |
| Thermal conductivity predictive models | Predicting the thermal conductivities of MICP-treated sands | [[72], 2020 |
| A small repeated five-point treatment model | Predicting solidification treatment in large-scale field experiments | [[73], 2014 |
| The biogrouting foam model | Simulating key solidification processes such as on-site bacterial solution perfusion and adhesion and urea hydrolysis | [[74], 2019 |
References
- Portugal, C.R.M.E.; Fonyo, C.; Machado, C.C.; Meganck, R.; Jarvis, T. Microbiologically Induced Calcite Precipitation bio-cementation, green alternative for roads–is this the breakthrough? A critical review. J. Clean. Prod. 2020, 262, 121372.
- Wang, X.; Chen, F.; Hasi, E.; Li, J. Desertification in China: An assessment. Earth-Sci. Rev. 2008, 88, 188–206.
- Kimura, R.; Moriyama, M. Determination by MODIS satellite-based methods of recent global trends in land surface aridity and degradation. J. Agric. Meteorol. 2019, 75, 153–159.
- Huang, J.; Zhang, G.; Zhang, Y.; Guan, X.; Wei, Y.; Guo, R. Global desertification vulnerability to climate change and human activities. Land Degrad. Dev. 2020, 31, 1380–1391.
- Mariina; Fahriani, F.; Apriyanti, Y. Utilization of palm kernel shell ash as stabilization materials for clay to settlement con-solidation. In IOP Conference Series. Earth and Environmental Science; IOP Publishing Ltd.: Bangka, Indonesia, 2020; p. 599.
- Yang, J.; Cheng, Y.; Chen, W. Experimental Study on Diffusion Law of Post-Grouting Slurry in Sandy Soil. Adv. Civ. Eng. 2019, 2019, 3493942.
- Ma, G.; Ran, F.; Feng, E.; Dong, Z.; Lei, Z. Effectiveness of an Eco-friendly Polymer Composite Sand-Fixing Agent on Sand Fixation. Water Air Soil Pollut. 2015, 226, 221.
- Liu, S.; Wen, K.; Armwood, C.; Bu, C.; Li, C.; Amini, F.; Li, L. Enhancement of MICP-Treated Sandy Soils against Environmental Deterioration. J. Mater. Civ. Eng. 2019, 31, 04019294.
- Qu, J.; Zu, R.; Zhang, K.; Fang, H. Field observations on the protective effect of semi-buried checkerboard sand barriers. Geomorphology 2007, 88, 193–200.
- Pan, X.; Chu, J.; Yang, Y.; Cheng, L. A new biogrouting method for fine to coarse sand. Acta Geotech. 2019, 15, 1–16.
- Peng, S.; Di, H.; Fan, L.; Fan, W.; Qin, L. Factors Affecting Permeability Reduction of MICP for Fractured Rock. Front. Earth Sci. 2020, 8.
- Li, Y.; Sun, X.; Zhao, X.; Zhao, L.; Xu, S.; Gu, S.; Zhang, F.; Yu, G. Seasonal variations and mechanism for environmental con-trol of NEE of CO2 concerning the Potentilla fruticosa in alpine shrub meadow of Qinghai-Tibet Plateau. Sci. China Ser. D Earth Sci. 2006, 49, 174–185.
- Sharaky, A.M.; Mohamed, N.; Elmashad, M.E.; Shredah, N.M. Application of microbial biocementation to improve the physico-mechanical properties of sandy soil. Constr. Build. Mater. 2018, 190, 861–869.
- Wang, Y.; Li, C.; Wang, C.; Gao, Y. Improving the Erosion Resistance Performance of Pisha Sandstone Weathered Soil Using MICP Technology. Crystals 2021, 11, 1112.
- Dejong, J.T.; Soga, K.; Kavazanjian, E.; Burns, S.; Van Paassen, L.A.; Al Qabany, A.; Aydilek, A.; Bang, S.S.; Burbank, M.; Caslake, L.F.; et al. Biogeochemical processes and geotechnical applications: Progress, opportunities and challenges. Géotechnique 2013, 63, 287–301.
- Mitchell, J.K.; Santamarina, J.C. Biological Considerations in Geotechnical Engineering. J. Geotech. Geoenviron. Eng. 2005, 131, 1222–1233.
- Mujah, D.; Shahin, M.A.; Cheng, L. State-of-the-Art Review of Biocementation by Microbially Induced Calcite Precipitation (MICP) for Soil Stabilization. Geomicrobiol. J. 2016, 34, 524–537.
- Han, Z.; Cheng, X.; Ma, Q. An experimental study on dynamic response for MICP strengthening liquefiable sands. Earthq. Eng. Eng. Vib. 2016, 15, 673–679.
- Eryürük, K.; Yang, S.; Suzuki, D.; Sakaguchi, I.; Akatsuka, T.; Tsuchiya, T.; Katayama, A. Reducing hydraulic conductivity of porous media using CaCO3 precipitation induced by Sporosarcina pasteurii. J. Biosci. Bioeng. 2015, 119, 331–336.
- Sasaki, T.; Kuwano, R. Undrained cyclic triaxial testing on sand with non-plastic fines content cemented with microbially induced CaCO3. Soils Found. 2016, 56, 485–495.
- Liu, B.; Zhu, C.; Tang, C.-S.; Xie, Y.-H.; Yin, L.-Y.; Cheng, Q.; Shi, B. Bio-remediation of desiccation cracking in clayey soils through microbially induced calcite precipitation (MICP). Eng. Geol. 2019, 264, 105389.
- Sun, X.; Miao, L.; Chen, R. Effects of different clay’s percentages on improvement of Sand-Clay mixtures with microbially in-duced calcite precipitation. Geomicrobiol. J. 2019, 36, 810–818.
- Nassar, M.K.; Gurung, D.; Bastani, M.; Ginn, T.R.; Shafei, B.; Gomez, M.G.; Graddy, C.M.R.; Nelson, D.C.; Dejong, J.T. Large-scale experiments in microbially induced calcite precipitation (MICP): Reactive transport model development and predic-tion. Water Resour. Res. 2018, 54, 480–500.
- Lin, H.; Suleiman, M.T.; Jabbour, H.M.; Brown, D.G. Bio-grouting to enhance axial pull-out response of pervious concrete ground improvement piles. Can. Geotech. J. 2018, 55, 119–130.
- Liu, S.; Du, K.; Huang, W.; Wen, K.; Amini, F.; Li, L. Improvement of erosion-resistance of bio-bricks through fiber and mul-tiple MICP treatments. Constr. Build. Mater. 2021, 271, 121573.
- Meng, H.; Gao, Y.; He, J.; Qi, Y.; Hang, L. Microbially induced carbonate precipitation for wind erosion control of desert soil: Field-scale tests. Geoderma 2020, 383, 114723.
- Cunningham, A.B.; Class, H.; Ebigbo, A.; Gerlach, R.; Phillips, A.; Hommel, J. Field-scale modeling of microbially induced calcite precipitation. Comput. Geosci. 2018, 23, 399–414.
- Kadhim, F.J.; Zheng, J. Review of the factors that influence on the microbial induced calcite precipitation. J. Civ. Environ. Res. 2016, 8, 69–76.
- Fang, C.; Achal, V. Biostimulation of calcite precipitation process by bacterial community in improving cement stabilized rammed earth as sustainable material. Appl. Microbiol. Biotechnol. 2019, 103, 7719–7727.
- Montoya, B.; DeJong, J.T. Stress-Strain Behavior of Sands Cemented by Microbially Induced Calcite Precipitation. J. Geotech. Geoenviron. Eng. 2015, 141, 04015019.
- Wangjie, L.; Chunxiang, Q.; Ruixing, W. Study on soil solidification based on microbiological precipitation of CaCO3. Sci. China Technol. Sci. 2010, 53, 2372–2377.
- Sun, X.; Miao, L.; Chen, R.; Wang, H.; Xia, J. Surface rainfall erosion resistance and freeze-thaw durability of bio-cemented and polymer-modified loess slopes. J. Environ. Manag. 2021, 301, 113883.
- Gebru, K.A.; Kidanemariam, T.G.; Gebretinsae, H.K. Bio-cement production using microbially induced calcite precipitation (MICP) method: A review. Chem. Eng. Sci. 2021, 238, 116610.
- Gomez, M.G.; Graddy, C.M.R.; DeJong, J.T.; Nelson, D.C. Biogeochemical Changes During Bio-cementation Mediated by Stimulated and Augmented Ureolytic Microorganisms. Sci. Rep. 2019, 9, 11517.
- Bernardi, D.; DeJong, J.; Montoya, B.; Martinez, B. Bio-bricks: Biologically cemented sandstone bricks. Constr. Build. Mater. 2014, 55, 462–469.
- Nafisi, A.; Safavizadeh, S.; Montoya, B.M. Influence of Microbe and Enzyme-Induced Treatments on Cemented Sand Shear Response. J. Geotech. Geoenviron. Eng. 2019, 145, 06019008.
- Ahenkorah, I.; Rahman, M.M.; Karim, M.R.; Teasdale, P.R. A comparison of mechanical responses for microbial- and en-zyme-induced cemented sand. Géotechnique Lett. 2020, 10, 559–567.
- Cheng, L.; Shahin, M.A.; Chu, J. Soil bio-cementation using a new one-phase low-pH injection method. Acta Geotech. 2019, 14, 615–626.
- Cheng, L.; Shahin, M.A.; Cord-Ruwisch, R. Bio-cementation of sandy soil using microbial-induced carbonate precipitation (MICP) for marine environments. Géotechnique 2014, 64, 1010–1013.
- Xu, H.; Zheng, H.; Wang, J.; Ding, X.; Chen, P. Laboratory method of microbial induced solidification/stabilization for mu-nicipal solid waste incineration fly ash. MethodsX 2019, 6, 1036–1043.
- Wang, Z.; Zhang, N.; Ding, J.; Lu, C.; Jin, Y. Experimental Study on Wind Erosion Resistance and Strength of Sands Treated with Microbial-Induced Calcium Carbonate Precipitation. Adv. Mater. Sci. Eng. 2018, 2018, 1–10.
- Jroundi, F.; Schiro, M.; Ruiz-Agudo, E.; Elert, K.; Martín-Sánchez, I.; González-Muñoz, M.T.; Rodriguez-Navarro, C. Protection and consolidation of stone heritage by self-inoculation with indigenous carbonatogenic bacterial communities. Nat. Commun. 2017, 8, 279.
- Burbank, M.B.; Weaver, T.J.; Green, T.L.; Williams, B.C.; Crawford, R.L. Precipitation of calcite by indigenous microorganisms to strengthen liquefiable soils. Geomicrobiol. J. 2011, 28, 301–312.
- Cheng, L.; Shahin, M.A.; Cord-Ruwisch, R. Surface Percolation for Soil Improvement by Biocementation Utilizing In Situ Enriched Indigenous Aerobic and Anaerobic Ureolytic Soil Microorganisms. Geomicrobiol. J. 2016, 34, 546–556.
- Burbank, M.B.; Weaver, T.J.; Williams, B.C.; Crawford, R.L. Urease Activity of Ureolytic Bacteria Isolated from Six Soils in which Calcite was Precipitated by Indigenous Bacteria. Geomicrobiol. J. 2012, 29, 389–395.
- Kumari, D.; Pan, X.; Lee, D.-J.; Achal, V. Immobilization of cadmium in soil by microbially induced carbonate precipitation with Exiguobacterium undae at low temperature. Int. Biodeterior. Biodegrad. 2014, 94, 98–102.
- Burbank, M.; Weaver, T.; Lewis, R.; Williams, T.; Williams, B.; Crawford, R. Geotechnical tests of sands following bioinduced calcite precipitation catalyzed by indigenous bacteria. J. Geotech. Geoenviron. Eng. 2013, 139, 928–936.
- Chahal, N.; Siddique, R. Permeation properties of concrete made with fly ash and silica fume: Influence of ureolytic bacteria. Constr. Build. Mater. 2013, 49, 161–174.
- Gowthaman, S.; Iki, T.; Nakashima, K.; Ebina, K.; Kawasaki, S. Feasibility study for slope soil stabilization by microbial induced carbonate precipitation (MICP) using indigenous bacteria isolated from cold subarctic region. SN Appl. Sci. 2019, 1, 1480.
- Wang, Y.-J.; Han, X.-L.; Jiang, N.-J.; Wang, J.; Feng, J. The effect of enrichment media on the stimulation of native ureolytic bacteria in calcareous sand. Int. J. Environ. Sci. Technol. 2019, 17, 1795–1808.
- Khan, M.N.H.; Amarakoon, G.G.N.N.; Shimazaki, S.; Kawasaki, S. Coral sand solidification test based on microbially induced carbonate precipitation using ureolytic bacteria. Mater. Trans. 2015, 56, 1725–1732.
- Oualha, M.; Bibi, S.; Sulaiman, M.; Zouari, N. Microbially induced calcite precipitation in calcareous soils by endogenous Bacillus cereus, at high pH and harsh weather. J. Environ. Manag. 2019, 257, 109965.
- Song, W.; Yang, Y.; Qi, R.; Li, J.; Pan, X. Suppression of coal dust by microbially induced carbonate precipitation usingStaphylococcus succinus. Environ. Sci. Pollut. Res. 2019, 26, 35968–35977.
- Imran, M.A.; Nakashima, K.; Evelpidou, N.; Kawasaki, S. Factors affecting the urease activity of native ureolytic bacteria isolated from coastal areas. Geomech. Eng. 2019, 17, 421–427.
- Chu, J.; Stabnikov, V.; Ivanov, V. Microbially Induced Calcium Carbonate Precipitation on Surface or in the Bulk of Soil. Geomicrobiol. J. 2012, 29, 544–549.
- Phillips, A.J.; Lauchnor, E.; Eldring, J.; Esposito, R.; Mitchell, A.C.; Gerlach, R.; Cunningham, A.B.; Spangler, L.H. Potential CO2 Leakage Reduction through Biofilm-Induced Calcium Carbonate Precipitation. Environ. Sci. Technol. 2012, 47, 142–149.
- Cheng, L.; Cord-Ruwisch, R. Upscaling Effects of Soil Improvement by Microbially Induced Calcite Precipitation by Surface Percolation. Geomicrobiol. J. 2014, 31, 396–406.
- Cuthbert, M.O.; McMillan, L.A.; Handley-Sidhu, S.; Riley, M.S.; Tobler, D.J.; Phoenix, V.R. A Field and Modeling Study of Fractured Rock Permeability Reduction Using Microbially Induced Calcite Precipitation. Environ. Sci. Technol. 2013, 47, 13637–13643.
- van Paassen, L.A. Bio-Mediated Ground Improvement: From Laboratory Experiment to Pilot Applications. In Geo-Frontiers 2011: Advances in Geotechnical Engineering; ASCE: Reston, VA, USA, 2011.
- Harkes, M.P.; van Paassen, L.A.; Booster, J.L.; Whiffin, V.S.; van Loosdrecht, M.C.M. Fixation and distribution of bacterial activity in sand to induce carbonate precipitation for ground reinforcement. Ecol. Eng. 2010, 36, 112–117.
- Ohan, J.A.; Saneiyan, S.; Lee, J.; Bartlow, A.W.; Ntarlagiannis, D.; Burns, S.E.; Colwell, F.S. Microbial and geochemical dy-namics of an aquifer stimulated for microbial induced calcite precipitation (MICP). Front. Microbiol. 2020, 11, 1327.
- Feng, K.; Montoya, B.; Evans, T. Discrete element method simulations of bio-cemented sands. Comput. Geotech. 2017, 85, 139–150.
- Fauriel, S.; Laloui, L. A bio-chemo-hydro-mechanical model for microbially induced calcite precipitation in soils. Comput. Geotech. 2012, 46, 104–120.
- Connolly, J.; Kaufman, M.; Rothman, A.; Gupta, R.; Redden, G.; Schuster, M.; Colwell, F.; Gerlach, R. Construction of two ureolytic model organisms for the study of microbially induced calcium carbonate precipitation. J. Microbiol. Methods 2013, 94, 290–299.
- Gai, X.; Sánchez, M. An elastoplastic mechanical constitutive model for microbially mediated cemented soils. Acta Geotech. 2018, 14, 709–726.
- Wang, X.; Nackenhorst, U. A coupled bio-chemo-hydraulic model to predict porosity and permeability reduction during microbially induced calcite precipitation. Adv. Water Resour. 2020, 140, 103563.
- Martinez, B.C.; Dejong, J.T.; Ginn, T.R. Bio-geochemical reactive transport modeling of microbial induced calcite precipitation to predict the treatment of sand in one-dimensional flow. Comput. Geotech. 2014, 58, 1–13.
- Colwell, F.S.; Smith, R.W.; Ferris, F.G.; Reysenbach, A.; Fujita, Y.; Tyler, T.L.; Taylor, J.L.; Banta, A.; Delwiche, M.E.; Mcling, T.L.; et al. Microbially mediated subsurface calcite precipitation for removal of hazardous divalent cations: Microbial activity, molecular biology, and modeling. Subsurf. Contam. Remediat. 2005, 904, 117–137.
- Yang, P.; Kavazanjian, E.; Neithalath, N. Particle-Scale Mechanisms in Undrained Triaxial Compression of Biocemented Sands: Insights from 3D DEM Simulations with Flexible Boundary. Int. J. Géoméch. 2019, 19, 04019009.
- Tobler, D.J.; Cuthbert, M.O.; Phoenix, V.R. Transport of Sporosarcina pasteurii in sandstone and its significance for subsurface engineering technologies. Appl. Geochem. 2014, 42, 38–44.
- Qin, C.; Hassanizadeh, S.M.; Ebigbo, A. Pore-scale network modeling of microbially induced calcium carbonate precipita-tion: Insight into scale dependence of biogeochemical reaction rates. Water Resour. Res. 2016, 52, 8794–8810.
- Wang, Z.; Zhang, N.; Ding, J.; Li, Q.; Xu, J. Thermal conductivity of sands treated with microbially induced calcite precipita-tion (MICP) and model prediction. Int. J. Heat Mass Tran. 2020, 147, 118899.
- DeJong, J.; Martinez, B.C.; Ginn, T.R.; Hunt, C.; Major, D.; Tanyu, B. Development of a Scaled Repeated Five-Spot Treatment Model for Examining Microbial Induced Calcite Precipitation Feasibility in Field Applications. Geotech. Test. J. 2014, 37.
- Minto, J.M.; Lunn, R.J.; El Mountassir, G. Development of a Reactive Transport Model for Field-Scale Simulation of Microbially Induced Carbonate Precipitation. Water Resour. Res. 2019, 55, 7229–7245.
- Ebigbo, A.; Phillips, A.; Gerlach, R.; Helmig, R.; Cunningham, A.B.; Class, H.; Spangler, L.H. Darcy-scale modeling of mi-crobially induced carbonate mineral precipitation in sand columns. Water Resour. Res. 2012, 48, 1–17.




