
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | A. K. M. Aminul Islam | + 5603 word(s) | 5603 | 2021-11-29 07:44:52 | | | |
| 2 | Lindsay Dong | + 346 word(s) | 5949 | 2021-11-30 02:20:57 | | |
Video Upload Options
Grain legumes are important sources of proteins, essential micronutrients and vitamins and for human nutrition. Climate change, including drought, is a severe threat to grain legume production throughout the world. The yield loss of grain legumes varies from species to species, even variety to variety within a species, depending upon the severity of drought stress and several other factors, such as phenology, soil textures and agro-climatic conditions. Closure of stomata leads to an increase in leaf temperature by reducing the transpiration rate, and, so, the legume plant faces another stress under drought stress. The biosynthesis of reactive oxygen species (ROS) is the most detrimental effect of drought stress. Legumes can adapt to the drought stress by changing their morphology, physiology and molecular mechanism. Improved root system architecture (RSA), reduced number and size of leaves, stress-induced phytohormone, stomatal closure, antioxidant defense system, solute accumulation (e.g., proline) and altered gene expression play a crucial role in drought tolerance.
1. Effects of Drought Stress in Grain Legumes
1.1. Morphological Effects
1.1.1. Plant Growth
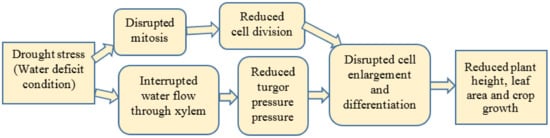
1.1.2. Leaf Area
1.1.3. Pod Number
1.1.4. Nodulation of Grain Legumes
1.2. Physiological Effects
1.2.1. Leaf Temperature
1.2.2. Water-Use Efficiency
1.2.3. Chlorophyll Content
1.2.4. Photosynthesis
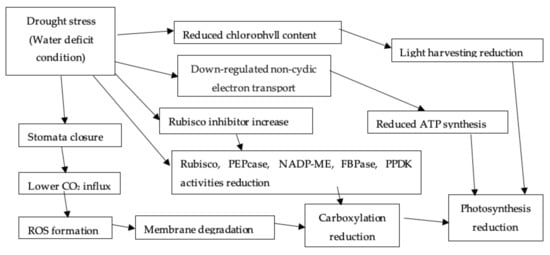
1.2.5. Transpiration and Stomatal Conductance
1.2.6. Plant–Water Relations
1.2.7. Plant Nutrient Relations
1.3. Morpho-Physiological Effects
1.3.1. Growth Stages
1.3.2. Grain Composition
1.3.3. Yield
1.3.4. Physio-Biochemical Level
1.3.5. Molecular Level
2. Tolerance Mechanisms of Grain Legumes against Drought Stress
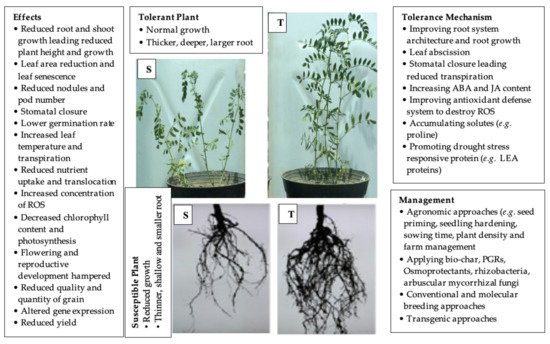
2.1. Morphological Mechanisms
2.2. Phenotypic Plasticity
2.3. Leaf Abscission
3. Physio-Biochemical Mechanisms
3.1. ABA Mediated Stomatal Closure
3.2. Antioxidant
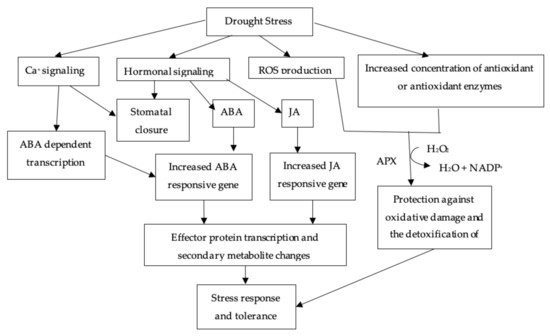
3.3. Solute Accumulation
3.4. Plant Growth Regulators (PGRs)
3.5. Water-Use Efficiency (WUE) in Drought Tolerance
3.6. Molecular Mechanisms
4. Management of Drought Stress in Grain Legumes
4.1. Traditional Agronomic Approaches
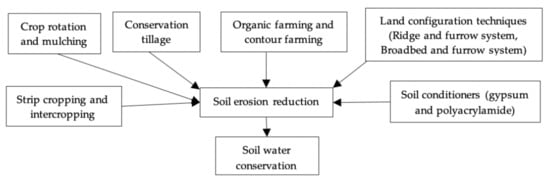
4.2. New Approaches in Agronomy
4.2.1. Biochar Application
4.2.2. Exogenous Application of Plant Growth Regulators (PGRs) and Osmoprotectants
| Phytohormones | Functions | References |
|---|---|---|
| Abscisic acid | • Manages the water status of the plant by regulating the guard cell | Zhu [84] |
| • Transmits signals from the root to the shoot, leading in the closure of leaf stomata and a reduction in transpiration | Wilkinson and Davies [85] | |
| • Induces genes coding for protein and enzymes linked to drought tolerance | Ali et al. [61] | |
| • Limit excessive ethylene production and preserve root and shoot growth | Ober and Sharp [86] | |
| Salicylic acid | • Improved membrane stability index (MSI), photosynthetic parameters, leaf water potential, carbonic anhydrase, activity of nitrate reductase, relative water content and chlorophyll content | Hayat et al. [87] |
| Jasmonic acid | • Play a crucial part in antioxidant responses produced by drought, particularly ascorbate metabolism | Bao et al. [88] |
| Cytokinins | • Late leaf senescence | Peleg and Blumwald [89] |
| • Encouraging root development and more efficient nutrient uptake | Coque and Gallais [90] | |
| Ethylene | • Produces H2O2 in the guard cell, which causes stomatal closure | Desikan et al. [91] |
| • Abscission of the leaves | Salazar et al. [42] | |
| • Reduced root and shoot growth due to plant homoeostasis | Vurukonda et al. [92] | |
| Auxin | • Phenotypic plasticity with developmental changes to root system architecture and root growth | Korver et al. [93] |
| Gibberellin | • Signaling in either growth repression or promotion as a result of stress-induced growth regulation | Colebrook et al. [94] |
4.2.3. Plant-Growth-Promoting Rhizobacteria (PGPR)
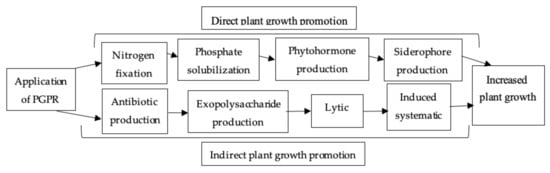
4.2.4. Use of Arbuscular Mycorrhizal Fungi (AMF)
5. Breeding Approaches
5.1. Conventional Breeding
5.2. Genome-Wide Association Studies (GWASs)
5.3. Marker-Assisted Selection (MAS)
Radhika et al. [111], for example, found the QTL Qncl.Sw1 linked to grain yield in chickpea. The improvement of drought tolerance in crop legumes based on MAS involves a variety of breeding procedures. The MAS approach divides QTL by mapping, using molecular markers, and this is a prerequisite for MAS. Markers are frequently used in conjunction with MAS to reduce linkage drag caused by unfavorable alleles associated with target genes. PCR-based markers have mostly substituted previous generation markers, such as restriction fragment length polymorphism (RFLP), making MAS more cost-effective. MAS, which integrates many genes into a single genotype, includes marker assisted pyramiding [112]. Various backcrossing approaches have been developed to lessen linkage drag in gene pools. One such technique is marker assisted backcrossing selection (MABS), which separates QTL with larger phenotypic variance and labels them as significant QTL. They can be introgressed into poor drought-resistant genotypes without conveying the unwanted gene once they have been validated. This method produces superior lines that are more drought resistant (Gupta et al. 2010).
5.4. Genomic Selection (GS)
5.5. Biotechnological Approaches
Through the transfer of targeted genes, transgenic techniques involve changes in both qualitative and quantitative traits [120]. Recent advances in biotechnology have allowed us to find specific genes that are resistant to abiotic stress from any other organism or even distinct species, allowing us to change the genetic makeup of grain legume crops to protect them against drought. Biolistic or agrobacterium-mediated transformation can be used to transform transgenic legumes.
5.6. OMICS Strategy
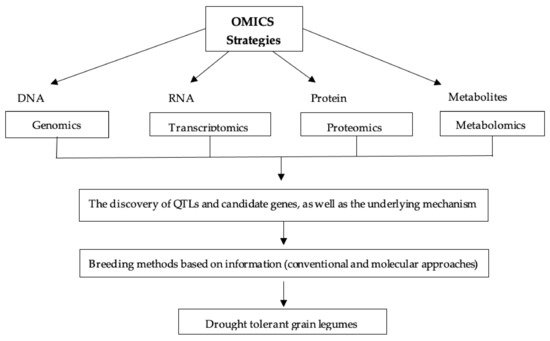
5.7. CRISPR/Cas9: Sophisticated Technology for Genome Editing (GE)
6. Conclusions
References
- Taiz, L.; Zeiger, E. Plant Physiology, 4th ed.; Sinauer Associates Inc. Publishers: Sunderland, MA, USA, 2006.
- Farooq, M.; Aziz, T.; Basra, S.M.A.; Cheema, M.A.; Rehman, H. Chilling tolerance in hybrid maize induced by seed priming with salicylic acid. J. Agron. Crop Sci. 2008, 194, 161–168.
- Farooq, M.; Wahid, A.; Kobayashi, N.S.M.A.; Fujita, D.B.S.M.A.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Sustain. Agric. 2009, 153–188.
- Fathi, A.; Tari, D.B. Effect of drought stress and its mechanism in plants. Int. J. Life Sci. 2016, 10, 1–6.
- Ye, H.; Roorkiwal, M.; Valliyodan, B.; Zhou, L.; Chen, P.; Varshney, R.K.; Nguyen, H.T. Genetic diversity of root system architecture in response to drought stress in grain legumes. J. Exp. Bot. 2018, 69, 3267–3277.
- Hudak, C.M.; Patterson, R.P. Root distribution and soil moisture depletion pattern of a drought-resistant soybean plant introduction. Agron. J. 1996, 88, 478–485.
- Bagheri, A. Effects of drought stress on chlorophyll, proline and rates of photosynthesis and respiration and activity of superoxide dismutase and peroxidase in millet (Panicum milenaceum L.). In Proceedings of the National Conference on Water Scarcity and Drought Management in Agriculture, Arsanjan; 2009; p. 16.
- Kabiri, R.; Farahbakhsh, H.; Nasibi, F. Salicylic acid ameliorates the effects of oxidative stress induced by water deficit in hydroponic culture of Nigella sativa. J. Stress Physiol. Biochem. 2012, 8, 13–22.
- Sarkar, S.; Khatun, M.; Era, F.M.; Islam, A.K.M.M.; Anwar, M.P.; Danish, S.; Datta, R.; Islam, A.K.M.A. Abiotic stresses: Alteration of composition and grain quality in food legumes. Agronomy 2021, 11, 2238.
- De Souza, P.I.; Egli, D.B.; Bruening, W.P. Water stress during seed filling and leaf senescence in soybean. Agron. J. 1997, 89, 807–812.
- Lu, H.; Tabassum, A.; Zhou, G. Plant hydraulic conductivity determines photosynthesis in rice under PEG-induced drought stress. Pak. J. Bot. 2021, 53, 409–417.
- Mafakheri, A.; Siosemardeh, A.F.; Bahramnejad, B.; Struik, P.C.; Sohrabi, Y. Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Aust. J. Crop Sci. 2010, 4, 580–585.
- Busse, M.D.; Bottomley, P.J. Growth and nodulation responses of Rhizobium meliloti to water stress induced by permeating and nonpermeating solutes. Appl. Environ. Microbiol. 1989, 55, 2431–2436.
- Monclus, R.; Dreyer, E.; Villar, M.; Delmotte, F.M.; Delay, D.; Petit, J.M.; Barbaroux, C.; Thiec, D.L.; Bréchet, C.; Brignolas, F. Impact of drought on productivity and water use efficiency in 29 genotypes of Populus deltoides× Populus nigra. New Phytol. 2006, 169, 765–777.
- Anjum, S.A.; Xie, X.Y.; Wang, L.C.; Saleem, M.F.; Man, C.; Lei, W. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 2011, 6, 2026–2032.
- Awasthi, R.; Kaushal, N.; Vadez, V.; Turner, N.C.; Berger, J.; Siddique, K.H.; Nayyar, H. Individual and combined effects of transient drought and heat stress on carbon assimilation and seed filling in chickpea. Funct. Plant Biol. 2014, 41, 1148–1167.
- Sohrawardy, H.; Hossain, M. Response of short duration tropical legumes and maize to water stress: A glasshouse study. Adv. Agric. 2014, 2014, 641319.
- Ullah, A.; Farooq, M. The challenge of drought stress for grain legumes and options for improvement. Arch. Agron. Soil Sci. 2021, 1–18.
- Kaya, M.D.; Okçu, G.; Atak, M.; Cıkılı, Y.; Kolsarıcı, Ö. Seed treatments to overcome salt and drought stress during germination in sunflower (Helianthus annuus L.). Eur. J. Agron. 2006, 24, 291–295.
- Harris, D.; Tripathi, R.S.; Joshi, A. On-farm seed priming to improve crop establishment and yield in dry direct-seeded rice. In Direct Seeding: Research Strategies and Opportunities; International Research Institute: Manila, Philippines, 2002; pp. 231–240.
- Okçu, G.; Kaya, M.D.; Atak, M. Effects of salt and drought stresses on germination and seedling growth of pea (Pisum sativum L.). Turk. J. Agric. For. 2005, 29, 237–242.
- Farooq, M.; Hussain, M.; Siddique, K.H. Drought stress in wheat during flowering and grain-filling periods. Crit. Rev. Plant Sci. 2014, 33, 331–349.
- Fang, X.; Turner, N.C.; Yan, G.; Li, F.; Siddique, K.H. Flower numbers, pod production, pollen viability, and pistil function are reduced and flower and pod abortion increased in chickpea (Cicer arietinum L.) under terminal drought. J. Exp. Bot. 2010, 61, 335–345.
- Al-Ghzawi, A.A.; Zaitoun, S.; Gosheh, H.Z.; Alqudah, A.M. The impacts of drought stress on bee attractively and flower pollination of Trigonella moabitica (fabaceae). Arch. Agron. Soil Sci. 2009, 55, 683–692.
- Singh, S.P. Drought resistance in the race Durango dry bean landraces and cultivars. Agron. J. 2007, 99, 1219–1225.
- Ghanbari, A.A.; Shakiba, M.R.; Toorchi, M.; Choukan, R. Nitrogen changes in the leaves and accumulation of some minerals in the seeds of red, white and chitti beans (Phaseolus vulgaris) under water deficit conditions. Aust. J. Crop Sci. 2013, 7, 706–712.
- Ghanbari, A.A.; Mousavi, S.H.; Gorji, A.M.; Idupulapati, R.A.O. Effects of water stress on leaves and seeds of bean (Phaseolus vulgaris L.). Turk. J. Field Crop. 2013, 18, 73–77.
- Bellaloui, N.; Mengistu, A.; Kassem, M.A. Effects of genetics and environment on fatty acid stability in soybean seed. Food Nutr. Sci. 2013, 4, 165–175.
- Estrada-Campuzano, G.; Miralles, D.J.; Slafer, G.A. Genotypic variability and response to water stress of pre-and post-anthesis phases in triticale. Eur. J. Agron. 2008, 28, 171–177.
- Samarah, N.H. Effects of drought stress on growth and yield of barley. Agron. Sustain. Dev. 2005, 25, 145–149.
- Tomer, A.; Singh, S.K. Drought Stress Tolerance in Legume Crops. In Agronomic Crops; Springer: Singapore, 2020; pp. 149–155.
- Thankamani, C.K.; Chempakam, B.; Ashokan, P.K. Water stress induced changes in enzyme activities and lipid peroxidation in black pepper (Piper nigrum). J. Med. Aromat. Plant Sci. 2003, 25, 646–650.
- Møller, I.M.; Jensen, P.E.; Hansson, A. Oxidative modifications to cellular components in plants. Annu. Rev. Plant Biol. 2007, 58, 459–481.
- Moran, J.F.; Becana, M.; Iturbe-Ormaetxe, I.; Frechilla, S.; Klucas, R.V.; Aparicio-Tejo, P. Drought induces oxidative stress in pea plants. Planta 1994, 194, 346–352.
- Ingram, J.; Bartels, D. The molecular basis of dehydration tolerance in plants. Annu. Rev. Plant Biol. 1996, 47, 377–403.
- Gao, W.R.; Wang, X.S.; Liu, Q.Y.; Peng, H.; Chen, C.; Li, J.G.; Zhang, J.S.; Song-Nian Hub, S.N.; Ma, H. Comparative analysis of ESTs in response to drought stress in chickpea (Cicer arietinum L.). Biochem. Biophys. Res. Commun. 2008, 376, 578–583.
- Varshney, R.K.; Tuberosa, R.; Tardieu, F. Progress in understanding drought tolerance: From alleles to cropping systems. J. Exp. Bot. 2018, 69, 3175–3179.
- Kavar, T.; Maras, M.; Kidrič, M.; Šuštar-Vozlič, J.; Meglič, V. Identification of genes involved in the response of leaves of Phaseolus vulgaris to drought stress. Mol. Breed. 2008, 21, 159–172.
- Kramer, P.J. Plant and soil water relationships: A modern synthesis. In Plant and Soil Water Relationships: A Modern Synthesis; McGraw-Hill Book Company: New York, NY, USA, 1969.
- Prince, S.J.; Murphy, M.; Mutava, R.N.; Durnell, L.A.; Valliyodan, B.; Shannon, J.G.; Nguyen, H.T. Root xylem plasticity to improve water use and yield in water-stressed soybean. J. Exp. Bot. 2017, 68, 2027–2036.
- Jaganathan, D.; Thudi, M.; Kale, S.; Azam, S.; Roorkiwal, M.; Gaur, P.M.; Kishor, P.B.; Nguyen, H.; Sutton, T.; Varshney, R.K. Genotyping-by-sequencing based intra-specific genetic map refines a ‘‘QTL-hotspot” region for drought tolerance in chickpea. Mol. Genet. Genom. 2015, 290, 559–571.
- Salazar, C.; Hernández, C.; Pino, M.T. Plant water stress: Associations between ethylene and abscisic acid response. Chil. J. Agric. Res. 2015, 75, 71–79.
- Kobraee, S.; Shamsi, K.; Rasekhi, B. Soybean production under water deficit conditions. Ann. Biol. Res. 2011, 2, 423–434.
- Shekari, F. Effect of Drought Stress on Phenology, Water Relations, Growth, Yield and Quality Canola. Ph.D. Thesis, University of Tabriz, Tabriz, Iran, 2000.
- Kafi, M.; Damghany Mahdavi, A. Mechanism of Resistance of Plants to Environmental Stresses; University of Mashhad: Mashhad, Iran, 1999.
- Daryanto, S.; Wang, L.; Jacinthe, P.A. Global synthesis of drought effects on food legume production. PLoS ONE 2015, 10, e0127401.
- Schachtman, D.P.; Goodger, J.Q. Chemical root to shoot signaling under drought. Trends Plant Sci. 2008, 13, 281–287.
- Matysik, J.; Alia; Bhalu, B.; Mohanty, P. Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. Curr. Sci. 2002, 82, 525–532.
- Nayyar, H.; Walia, D.P. Water stress induced proline accumulation in contrasting wheat genotypes as affected by calcium and abscisic acid. Biol. Plant. 2003, 46, 275–279.
- Anjum, S.A.; Wang, L.; Farooq, M.; Khan, I.; Xue, L. Methyl jasmonate-induced alteration in lipid peroxidation, antioxidative defence system and yield in soybean under drought. J. Agron. Crop Sci. 2011, 197, 296–301.
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97.
- Nadeem, M.; Li, J.; Yahya, M.; Sher, A.; Ma, C.; Wang, X.; Qiu, L. Research progress and perspective on drought stress in legumes: A review. Int. J. Mol. Sci. 2019, 20, 2541.
- Farooq, M.; Irfan, M.; Aziz, T.; Ahmad, I.; Cheema, S.A. Seed priming with ascorbic acid improves drought resistance of wheat. J. Agron. Crop Sci. 2013, 199, 12–22.
- Desoky, E.S.M.; Mansour, E.; El-Sobky, E.S.E.; Abdul-Hamid, M.I.; Taha, T.F.; Elakkad, H.A.; Arnaout, S.M.; Eid, R.S.; El-Tarabily, K.A.; Yasin, M.A. Physio-biochemical and agronomic responses of faba beans to exogenously applied nano-silicon under drought stress conditions. Front. Plant Sci. 2021, 12, 637783.
- Chen, T.H.; Murata, N. Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes. Curr. Opin. Plant Biol. 2002, 5, 250–257.
- Verbruggen, N.; Hermans, C. Proline accumulation in plants: A review. Amino Acids 2008, 35, 753–759.
- Fahad, S.; Hussain, S.; Matloob, A.; Khan, F.A.; Khaliq, A.; Saud, S.; Hassan, S.; Shan, D.; Khan, F.; Ullah, N.; et al. Phytohormones and plant responses to salinity stress: A review. Plant Growth Regul. 2015, 75, 391–404.
- Abobatta, W.F. Drought adaptive mechanisms of plants—A review. Adv. Agric. Environ. Sci. 2019, 2, 62–65.
- Saradadevi, R.; Palta, J.A.; Siddique, K.H. ABA-mediated stomatal response in regulating water use during the development of terminal drought in wheat. Front. Plant Sci. 2017, 8, 1251.
- Rani, A.; Devi, P.; Jha, U.C.; Sharma, K.D.; Siddique, K.H.; Nayyar, H. Developing climate-resilient chickpea involving physiological and molecular approaches with a focus on temperature and drought stresses. Front. Plant Sci. 2020, 10, 1759.
- Ali, F.; Bano, A.; Fazal, A. Recent methods of drought stress tolerance in plants. Plant Growth Regul. 2017, 82, 363–375.
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223.
- Chinnusamy, V.; Schumaker, K.; Zhu, J.K. Molecular genetic perspectives on cross-talk and specificity in abiotic stress signalling in plants. J. Exp. Bot. 2004, 55, 225–236.
- Bray, E.A. Classification of genes differentially expressed during water-deficit stress in Arabidopsis thaliana: An analysis using microarray and differential expression data. Ann. Bot. 2002, 89, 803–811.
- Xiong, L.; Schumaker, K.S.; Zhu, J.K. Cell signaling during cold, drought, and salt stress. Plant Cell 2002, 14 Suppl. S1, S165–S183.
- Wrzaczek, M.; Hirt, H. Plant MAP kinase pathways: How many and what for? Biol. Cell 2001, 93, 81–87.
- Abe, H.; Urao, T.; Ito, T.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell 2003, 15, 63–78.
- Bartels, D. Desiccation tolerance studied in the resurrection plant Craterostigma plantagineum. Integr. Comp. Biol. 2005, 45, 696–701.
- Bajwa, A.A.; Farooq, M. Seed priming with sorghum water extract and benzyl amino purine along with surfactant improves germination metabolism and early seedling growth of wheat. Arch. Agron. Soil Sci. 2017, 63, 319–329.
- Villar-Salvador, P.; Heredia, N.; Millard, P. Remobilization of acorn nitrogen for seedling growth in holm oak (Quercus ilex), cultivated with contrasting nutrient availability. Tree Physiol. 2010, 30, 257–263.
- Kumawat, A.; Yadav, D.; Samadharmam, K.; Rashmi, I. Soil and water conservation measures for agricultural sustainability. In Soil Moisture Importance; IntechOpen: London, UK, 2020.
- Fazal, A.; Bano, A. Role of plant growth-promoting rhizobacteria (pgpr), biochar, and chemical fertilizer under salinity stress. Commun. Soil Sci. Plant Anal. 2016, 47, 1985–1993.
- Thomas, S.C.; Frye, S.; Gale, N.; Garmon, M.; Launchbury, R.; Machado, N.; Sarah Melamed, S.; Murray, J.; Petroff, A.; Winsborough, C. Biochar mitigates negative effects of salt additions on two herbaceous plant species. J. Environ. Manag. 2013, 129, 62–68.
- Wang, Y.; Pan, F.; Wang, G.; Zhang, G.; Wang, Y.; Chen, X.; Mao, Z. Effects of biochar on photosynthesis and antioxidative system of Malus hupehensis Rehd. seedlings under replant conditions. Sci. Hortic. 2014, 175, 9–15.
- Atkinson, C.J.; Fitzgerald, J.D.; Hipps, N.A. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: A review. Plant Soil 2010, 337, 1–18.
- Zhang, M.; Duan, L.; Zhai, Z.; Li, J.; Tian, X.; Wang, B.; He, Z.; Li, Z. Effects of plant growth regulators on water deficit-induced yield loss in soybean. In Proceedings of the 4th International Crop Science Congress, Brisbane, QLD, Australia, 26 September–1 October 2004; pp. 252–256.
- Upreti, K.K.; Sharma, M. Role of plant growth regulators in abiotic stress tolerance. In Abiotic Stress Physiology of Horticultural Crops; Springer: New Delhi, India, 2016; pp. 19–46.
- Miyashita, K.; Tanakamaru, S.; Maitani, T.; Kimura, K. Recovery responses of photosynthesis, transpiration, and stomatal conductance in kidney bean following drought stress. Environ. Exp. Bot. 2005, 53, 205–214.
- Sharma, P.; Dubey, R.S. Drought induces oxidative stress and enhances the activities of antioxidant enzymes in growing rice seedlings. Plant Growth Regul. 2005, 46, 209–221.
- Rathinasabapathi, B. Metabolic engineering for stress tolerance: Installing osmoprotectant synthesis pathways. Ann. Bot. 2000, 86, 709–716.
- Ashraf, M.F.M.R.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216.
- Hussain, M.; Malik, M.A.; Farooq, M.; Ashraf, M.Y.; Cheema, M.A. Improving drought tolerance by exogenous application of glycinebetaine and salicylic acid in sunflower. J. Agron. Crop Sci. 2008, 194, 193–199.
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147.
- Zhu, J.K. Cell signaling under salt, water and cold stresses. Curr. Opin. Plant Biol. 2001, 4, 401–406.
- Wilkinson, S.; Davies, W.J. ABA-based chemical signalling: The co-ordination of responses to stress in plants. Plant Cell Environ. 2002, 25, 195–210.
- Ober, E.S.; Sharp, R.E. Electrophysiological responses of maize roots to low water potentials: Relationship to growth and ABA accumulation. J. Exp. Bot. 2003, 54, 813–824.
- Hayat, S.; Ali, B.; Ahmad, A. Salicylic acid: Biosynthesis, metabolism and physiological role in plants. In Salicylic Acid: A Plant Hormone; Springer: Dordrecht, The Netherlands, 2007; pp. 1–14.
- Bao, A.K.; Wang, S.M.; Wu, G.Q.; Xi, J.J.; Zhang, J.L.; Wang, C.M. Overexpression of the Arabidopsis H+-PPase enhanced resistance to salt and drought stress in transgenic alfalfa (Medicago sativa L.). Plant Sci. 2009, 176, 232–240.
- Peleg, Z.; Blumwald, E. Hormone balance and abiotic stress tolerance in crop plants. Curr. Opin. Plant Biol. 2011, 14, 290–295.
- Coque, M.; Gallais, A. Genomic regions involved in response to grain yield selection at high and low nitrogen fertilization in maize. Theor. Appl. Genet. 2006, 112, 1205–1220.
- Desikan, R.; Last, K.; Harrett-Williams, R.; Tagliavia, C.; Harter, K.; Hooley, R.; Hancock, J.T.; Neill, S.J. Ethylene-induced stomatal closure in Arabidopsis occurs via AtrbohF-mediated hydrogen peroxide synthesis. Plant J. 2006, 47, 907–916.
- Vurukonda, S.S.K.P.; Vardharajula, S.; Shrivastava, M.; Skz, A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 2016, 184, 13–24.
- Korver, R.A.; Koevoets, I.T.; Testerink, C. Out of shape during stress: A key role for auxin. Trends Plant Sci. 2018, 23, 783–793.
- Colebrook, E.H.; Thomas, S.G.; Phillips, A.L.; Hedden, P. The role of gibberellin signalling in plant responses to abiotic stress. J. Exp. Biol. 2014, 217, 67–75.
- Singh, J.S. Plant growth promoting rhizobacteria. Resonance 2013, 18, 275–281.
- Marulanda, A.; Barea, J.M.; Azcón, R. Stimulation of plant growth and drought tolerance by native microorganisms (AM fungi and bacteria) from dry environments: Mechanisms related to bacterial effectiveness. J. Plant Growth Regul. 2009, 28, 115–124.
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012, 2012, 963401.
- Saharan, B.S.; Nehra, V. Plant growth promoting rhizobacteria: A critical review. Life Sci. Med. Res. 2011, 21, 30.
- Sarma, R.K.; Saikia, R. Alleviation of drought stress in mung bean by strain Pseudomonas aeruginosa GGRJ21. Plant Soil 2014, 377, 111–126.
- Priyanka, J.P.; Goral, R.T.; Rupal, K.S.; Saraf, M. Rhizospheric microflora: A natural alleviator of drought stress in agricultural crops. In Plant Growth Promoting Rhizobacteria for Sustainable Stress Management; Springer: Singapore, 2019; pp. 103–115.
- Augé, R.M. Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 2001, 11, 3–42.
- Smith, S.E.; Facelli, E.; Pope, S.; Smith, F.A. Plant performance in stressful environments: Interpreting new and established knowledge of the roles of arbuscular mycorrhizas. Plant Soil 2010, 326, 3–20.
- Habibzadeh, Y.; Evazi, A.R.; Abedi, M. Alleviation drought stress of mungbean (Vigna radiata L.) plants by using arbuscular mycorrhizal fungi. J. Agric. Nat. Res. 2014, 1, 1–6.
- Beebe, S.E.; Rao, I.M.; Cajiao, C.; Grajales, M. Selection for drought resistance in common bean also improves yield in phosphorus limited and favorable environments. Crop Sci. 2008, 48, 582–592.
- Mir, R.R.; Zaman-Allah, M.; Sreenivasulu, N.; Trethowan, R.; Varshney, R.K. Integrated genomics, physiology and breeding approaches for improving drought tolerance in crops. Theor. Appl. Genet. 2012, 125, 625–645.
- Brachi, B.; Morris, G.P.; Borevitz, J.O. Genome-wide association studies in plants: The missing heritability is in the field. Genome Biol. 2011, 12, 1–8.
- Jha, U.C.; Bohra, A.; Nayyar, H. Advances in “omics” approaches to tackle drought stress in grain legumes. Plant Breed. 2020, 139, 1–27.
- Dhanapal, A.P.; Ray, J.D.; Singh, S.K.; Hoyos-Villegas, V.; Smith, J.R.; Purcell, L.C.; King, C.A.; Cregan, P.C.; Song, Q.; Fritschi, F.B. Genome-wide association study (GWAS) of carbon isotope ratio (δ13C) in diverse soybean genotypes. Theor. Appl. Genet. 2015, 128, 73–91.
- Kaler, A.S.; Ray, J.D.; Schapaugh, W.T.; Asebedo, A.R.; King, C.A.; Gbur, E.E.; Purcell, L.C. Association mapping identifies loci for canopy temperature under drought in diverse soybean genotypes. Euphytica 2018, 214, 1–18.
- Hoyos-Villegas, V.; Song, Q.; Kelly, J.D. Genome-wide association analysis for drought tolerance and associated traits in common bean. Plant Genome 2017, 10, 1–12.
- Radhika, P.; Gowda, S.J.M.; Kadoo, N.Y.; Mhase, L.B.; Jamadagni, B.M.; Sainani, M.N.; Chandra, S.; Gupta, V.S. Development of an integrated intraspecific map of chickpea (Cicer arietinum L.) using two recombinant inbred line populations. Theor. Appl. Genet. 2007, 115, 209–216.
- Witcombe, J.R.; Hollington, P.A.; Howarth, C.J.; Reader, S.; Steele, K.A. Breeding for abiotic stresses for sustainable agriculture. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 703–716.
- Varshney, R.K.; Pandey, M.K.; Bohra, A.; Singh, V.K.; Thudi, M.; Saxena, R.K. Toward the sequence-based breeding in legumes in the post-genome sequencing era. Theor. Appl. Genet. 2019, 132, 797–816.
- Collins, N.C.; Tardieu, F.; Tuberosa, R. QTL approaches for improving crop performance under abiotic stress conditions: Where do we stand. Plant Physiol. 2008, 147, 469–486.
- Singh, B.; Bohra, A.; Mishra, S.; Joshi, R.; Pandey, S. Embracing new-generation ‘omics’ tools to improve drought tolerance in cereal and food-legume crops. Biol. Plant. 2015, 59, 413–428.
- Goddard, M.E.; Hayes, B.J. Genomic selection. J. Anim. Breed. Genet. 2007, 124, 323–330.
- Meuwissen, T.H.; Hayes, B.J.; Goddard, M.E. Prediction of total genetic value using genome-wide dense marker maps. Genetics 2001, 157, 1819–1829.
- Hickey, L.T.; Hafeez, A.N.; Robinson, H.; Jackson, S.A.; Leal-Bertioli, S.C.; Tester, M.; Gao, C.; Godwin, I.D.; Hayes, B.J.; Wulff, B.B.H. Breeding crops to feed 10 billion. Nat. Biotechnol. 2019, 37, 744–754.
- Crossa, J.; Pérez-Rodríguez, P.; Cuevas, J.; Montesinos-López, O.; Jarquín, D.; de los Campos, G.; Burgueño, J.; Camacho-González, J.M.; Pérez-Elizalde, S.; Beyene, Y.; et al. Genomic selection in plant breeding: Methods, models, and perspectives. Trends Plant Sci. 2017, 22, 961–975.
- Ashraf, M. Inducing drought tolerance in plants: Recent advances. Biotechnol. Adv. 2010, 28, 169–183.
- Song, Q.X.; Liu, Y.F.; Hu, X.Y.; Zhang, W.K.; Ma, B.; Chen, S.Y.; Zhang, J.S. Identification of miRNAs and their target genes in developing soybean seeds by deep sequencing. BMC Plant Biol. 2011, 11, 5.
- Joshi, T.; Yan, Z.; Libault, M.; Jeong, D.H.; Park, S.; Green, P.J.; Sherrier, D.J.; Farmer, A.; May, G.; Meyers, B.C.; et al. Prediction of novel miRNAs and associated target genes in Glycine max. BMC Bioinform. 2010, 11, S14.
- Wang, L.; Dong, S.; Liu, L.; Ma, Y.; Li, S.; Zu, W. Transcriptome profiling reveals PEG-simulated drought, heat and combined stress response mechanisms in soybean. Comput. Biol. Chem. 2018, 77, 413–419.
- Prince, S.J.; Joshi, T.; Mutava, R.N.; Syed, N.; Vitor, M.D.S.J.; Patil, G.; Song, L.; Wang, J.J.; Lin, L.; Chen, W.; et al. Comparative analysis of the drought-responsive transcriptome in soybean lines contrasting for canopy wilting. Plant Sci. 2015, 240, 65–78.




