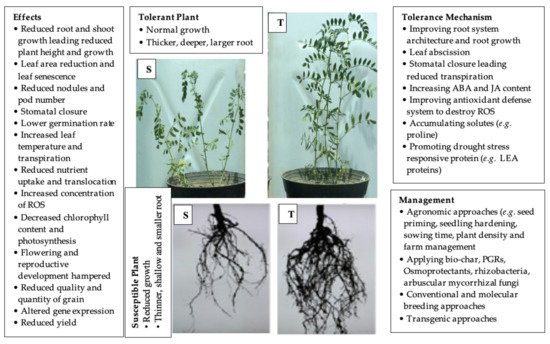
Grain legumes are important sources of proteins, essential micronutrients and vitamins and for human nutrition. Climate change, including drought, is a severe threat to grain legume production throughout the world. The yield loss of grain legumes varies from species to species, even variety to variety within a species, depending upon the severity of drought stress and several other factors, such as phenology, soil textures and agro-climatic conditions. Closure of stomata leads to an increase in leaf temperature by reducing the transpiration rate, and, so, the legume plant faces another stress under drought stress. The biosynthesis of reactive oxygen species (ROS) is the most detrimental effect of drought stress. Legumes can adapt to the drought stress by changing their morphology, physiology and molecular mechanism. Improved root system architecture (RSA), reduced number and size of leaves, stress-induced phytohormone, stomatal closure, antioxidant defense system, solute accumulation (e.g., proline) and altered gene expression play a crucial role in drought tolerance.
- grain legumes
- drought stress
- effects
- tolerance mechanism
1. Effects of Drought Stress in Grain Legumes
1.1. Morphological Effects
1.1.1. Plant Growth
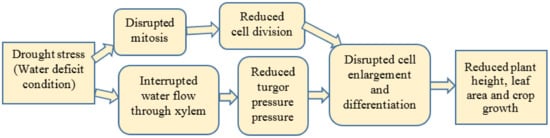
1.1.2. Leaf Area
1.1.3. Pod Number
1.1.4. Nodulation of Grain Legumes
1.2. Physiological Effects
1.2.1. Leaf Temperature
1.2.2. Water-Use Efficiency
1.2.3. Chlorophyll Content
1.2.4. Photosynthesis
Drought has an impact on the photosynthetic apparatus by affecting all of its primary components, including stomatal CO2 supply regulation, electron transport and the carbon-reduction cycle (Figure 3) [16][54]. Drought stress results in a reduction in total chlorophyll concentration, implying a reduced ability for light harvesting and thus reduced photosynthesis [4][39]. During drought, partial stomatal closure or mesophyll cell collapse owing to turgor loss demonstrates variation in leaf photosynthesis [3][38]. The carboxylation process and ribulose-1,6-bisphosphate (RuBP) regeneration are suppressed as a result of this condition, and photorespiration increases [11]. Rubisco binding inhibitors become more active when tissue water content is reduced (Figure 3). Furthermore, non-cyclic electron transport is inhibited to match the lower NADPH production requirements, lowering ATP synthesis [3][38].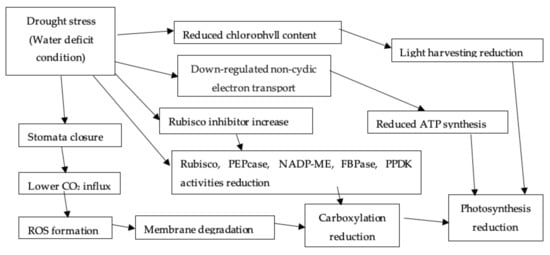
1.2.5. Transpiration and Stomatal Conductance
1.2.6. Plant–Water Relations
1.2.7. Plant Nutrient Relations
1.3. Morpho-Physiological Effects
1.3.1. Growth Stages
1.3.2. Grain Composition
1.3.3. Yield
Water stress affects a variety of yield-determining physiological processes in plants [3][38]. Drought stress decreases crop yields through reduction of photosynthetic active radiation, radiation efficiency and harvest index [4][39]. Plants that were stressed during the vegetative stage but not afterwards yielded much more than those that were stressed during anthesis or both the vegetative and anthesis stages [12][45]. For instance, pre-anthesis moisture stress shortened the duration to anthesis, but post-anthesis stress shortened the grain-filling period [29][78]. Regardless of the intensity of the drought, post-anthesis drought stress was deleterious to grain output [30][79]. During different phenological stages of crop growth, drought stress reduced yields in grain legumes.1.3.4. Physio-Biochemical Level
1.3.5. Molecular Level
2. Tolerance Mechanisms of Grain Legumes against Drought Stress
2.1. Morphological Mechanisms
2.2. Phenotypic Plasticity
2.3. Leaf Abscission
34. Physio-Biochemical Mechanisms
3.1. ABA Mediated Stomatal Closure
4.1. ABA Mediated Stomatal Closure
3.2. Antioxidant
4.2. Antioxidant
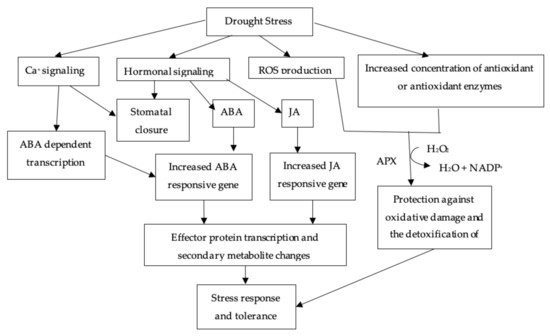
3.3. Solute Accumulation
4.3. Solute Accumulation
3.4. Plant Growth Regulators (PGRs)
4.4. Plant Growth Regulators (PGRs)
3.5. Water-Use Efficiency (WUE) in Drought Tolerance
4.5. Water-Use Efficiency (WUE) in Drought Tolerance
3.6. Molecular Mechanisms
4.6. Molecular Mechanisms
45. Management of Drought Stress in Grain Legumes
4.1. Traditional Agronomic Approaches
5.1. Traditional Agronomic Approaches
Under normal and stressful conditions, seed priming has been shown to improve germination metabolism and early stand establishment of crops [69][133]. Another strategy to adapt to drought-stressed conditions is to change the sowing time, plant density and farm management. Due to the implementation of cell membrane stability, the use of potassium fertilization during drought stress boosted drought resistance [58][121]. Drought resistance was also improved by hardening seedlings, which reduced stomatal regulation and osmotic potential and boosted the capacity of new root growth and stability of cell membrane [70][134]. Soil erosion is one of the most important hazards to soil and water resource degradation. To protect soil and water from degradation, judicious use of natural resources and appropriate management strategies are essential. Various measures used for reducing soil erosion ultimately reduce the water stress condition by conserving soil water or reducing water losses (Figure 5).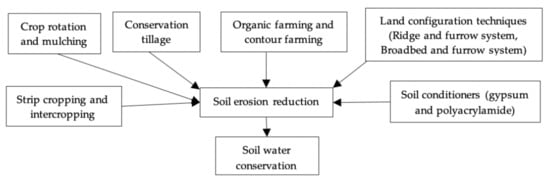
5.2. New Approaches in Agronomy
4.2. New Approaches in Agronomy
45.2.1. Biochar Application
45.2.2. Exogenous Application of Plant Growth Regulators (PGRs) and Osmoprotectants
Exogenous PGR therapy boosted chlorophyll content and increased water potential inside the cell [76][140]. PGRs and osmoprotectants are exogenously applied to legumes. Auxins, gibberellins, ethylene, cytokinins and ABA are the five major groups of plant growth regulators. Functions of some important PGRs under drought stress are presented in Table 13. Furthermore, a large number of compounds with unambiguous growth-regulating effects have been amassed, and a few of them have been shown to have widespread applications in enhancing crop growth, yield and quality [77][141]. Reduced stomatal conductance was linked to a rise in ABA accumulation induced by re-watering in the kidney bean (Phaseolus vulgaris L.) [78][142]. ABA increases root hydraulic conductivity that helps the plant to absorb and transport water more efficiently. ABA also boosted the genesis of O2- and H2O2 radicals, which boosted the activity of antioxidant enzymes, such as GR. As a result, overexpression of the ABA synthesis gene could be a promising approach for dealing with drought [11]. Plants may be able to counteract the harmful effects of ROS by maintaining larger amounts of antioxidants [79][143]. Osmoprotectant protects cell membranes from damage caused by inorganic ions and oxidative damage. Installing osmoprotectant production pathways has been suggested as a possible approach to produce stress-tolerant crops [80][144]. Exogenous osmoprotectant treatment has also been shown to promote drought resistance in plants [81][145]. The use of glycine betaine, for example, can aid crops in boosting their performance in drought settings [82][146]. In plants, it enhances stomatal conductance, proline accumulation and photosynthetic rate [83][48].| Phytohormones | Functions | References |
|---|---|---|
| Abscisic acid | • Manages the water status of the plant by regulating the guard cell | Zhu [84] |
| • Transmits signals from the root to the shoot, leading in the closure of leaf stomata and a reduction in transpiration | Wilkinson and Davies [85] | |
| • Induces genes coding for protein and enzymes linked to drought tolerance | Ali et al. [61] | |
| • Limit excessive ethylene production and preserve root and shoot growth | Ober and Sharp [86] | |
| Salicylic acid | • Improved membrane stability index (MSI), photosynthetic parameters, leaf water potential, carbonic anhydrase, activity of nitrate reductase, relative water content and chlorophyll content | Hayat et al. [87] |
| Jasmonic acid | • Play a crucial part in antioxidant responses produced by drought, particularly ascorbate metabolism | Bao et al. [88] |
| Cytokinins | • Late leaf senescence | Peleg and Blumwald [89] |
| • Encouraging root development and more efficient nutrient uptake | Coque and Gallais [90] | |
| Ethylene | • Produces H2O2 in the guard cell, which causes stomatal closure | Desikan et al. [91] |
| • Abscission of the leaves | Salazar et al. [42] | |
| • Reduced root and shoot growth due to plant homoeostasis | Vurukonda et al. [92] | |
| Auxin | • Phenotypic plasticity with developmental changes to root system architecture and root growth | Korver et al. [93] |
| Gibberellin | • Signaling in either growth repression or promotion as a result of stress-induced growth regulation | Colebrook et al. [94] |
45.2.3. Plant-Growth-Promoting Rhizobacteria (PGPR)
Azotobacter, Azospirillum, Bacillus, Pseudomonas, Rhizobium and other genera of PGPR have plant-growth stimulating properties [95][158]. PGPR are rhizosphere microorganisms that can boost plant development through a range of direct and indirect ways (Figure 6). Drought tolerance is controlled in semiarid and arid areas by inoculating plants with the PGPR [96][159]. Plants’ rhizospheres are colonized by PGPR, which directly or indirectly promotes plant growth [97][160] PGPR can solubilize inorganic P, making it accessible to crop plants and boosting plant growth [98][161]. During drought stress, rhizobacterial activities that promote crop growth have been described in the mung bean [99][162].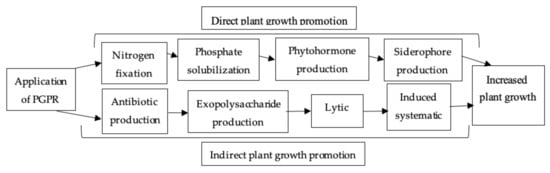
45.2.4. Use of Arbuscular Mycorrhizal Fungi (AMF)
56. Breeding Approaches
5.1. Conventional Breeding
6.1. Conventional Breeding
Traditional breeding is an established strategy for improving drought tolerance in crop species, and it is predictable to remain the primary way for crop improvement [22][66]. To improve drought tolerance in grain legumes, however, the selection and breeding procedure necessitates a large amount of heritable diversity [104][168]. In arid regions, heritability is generally poor due to changes in precipitation timing and amount, as well as significant genotype and environment interactions. Regardless, identifying essential characteristics that confer yield stability and potential in drought stress is crucial. Furthermore, accurate environmental characterization is required to improve the utility of any particular feature of interest [105][26]. Mass selection and screening may be beneficial in obtaining desirable phenotypic features based on variables that are highly connected to yield. However, precisely phenotyping crop plants for the desired characteristic is typically difficult, as most physiological variables with a high connection with drought necessitate advanced methodologies that can only be applied to a small number of genotypes. As a result, the initial tier of selection could be based on a trait that is simple, quick and straightforward to quantify. In the second tier, more precise tests of a smaller number of genotypes may be performed. As a result, mass selection should be based on the heritable trait, making it cost-effective and reasonably straightforward to quantify; moreover, the heritable trait should not result in disadvantages under favorable conditions or have unfavorable pleiotropic effects on other essential agronomic traits [22][66]. Certain traits show promise for drought resistance and could be used to screen grain legume genotypes. Another breeding approach used to obtain a particular characteristic within or between species is wide hybridization. Many grain legumes have undergone interspecific crosses, with varying degrees of success [11]. This technique has a lot of potential for use in breeding programs aiming at improving drought tolerance in grain legumes with some breeding success.5.2. Genome-Wide Association Studies (GWASs)
6.2. Genome-Wide Association Studies (GWASs)
5.3. Marker-Assisted Selection (MAS)
6.3. Marker-Assisted Selection (MAS)
Radhika et al. [111][211], for example, found the QTL Qncl.Sw1 linked to grain yield in chickpea. The improvement of drought tolerance in crop legumes based on MAS involves a variety of breeding procedures. The MAS approach divides QTL by mapping, using molecular markers, and this is a prerequisite for MAS. Markers are frequently used in conjunction with MAS to reduce linkage drag caused by unfavorable alleles associated with target genes. PCR-based markers have mostly substituted previous generation markers, such as restriction fragment length polymorphism (RFLP), making MAS more cost-effective. MAS, which integrates many genes into a single genotype, includes marker assisted pyramiding [112][212]. Various backcrossing approaches have been developed to lessen linkage drag in gene pools. One such technique is marker assisted backcrossing selection (MABS), which separates QTL with larger phenotypic variance and labels them as significant QTL. They can be introgressed into poor drought-resistant genotypes without conveying the unwanted gene once they have been validated. This method produces superior lines that are more drought resistant (Gupta et al. 2010).
5.4. Genomic Selection (GS)
6.4. Genomic Selection (GS)
5.5. Biotechnological Approaches
6.5. Biotechnological Approaches
Through the transfer of targeted genes, transgenic techniques involve changes in both qualitative and quantitative traits [120][233]. Recent advances in biotechnology have allowed us to find specific genes that are resistant to abiotic stress from any other organism or even distinct species, allowing us to change the genetic makeup of grain legume crops to protect them against drought. Biolistic or agrobacterium-mediated transformation can be used to transform transgenic legumes.
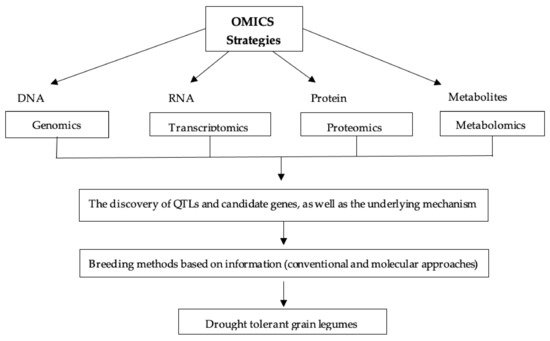
5.6. OMICS Strategy
6.6. OMICS Strategy
Recently OMICS-based technology has been used to discover the desired trait genes and their specific function. This innovative method locates candidate genes by using transcriptome, genome, microme, proteome and metabolome data (Figure 7) to aid in QTL mapping. Series of scientific studies and research have recently been available to elucidate the role of genes, proteins and metabolites in legume drought sensitivity.