| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Timothy Corson | + 1719 word(s) | 1719 | 2020-08-10 08:22:02 | | | |
| 2 | Bruce Ren | Meta information modification | 1719 | 2020-08-17 04:58:03 | | |
Video Upload Options
Multiple natural-source and synthetic small molecules have been tested preclinical for treating corneal neovascularization. Such small molecules include synthetic inhibitors of the vascular endothelial growth factor (VEGF) receptor and other tyrosine kinases, plus repurposed antimicrobials, as well as natural source-derived flavonoid and non-flavonoid phytochemicals, immunosuppressants, vitamins, and histone deacetylase inhibitors.
Under healthy conditions, the cornea is an avascular structure which allows for transparency and optimal visual acuity. Its avascular nature is maintained by a balance of proangiogenic and antiangiogenic factors. An imbalance of these factors can result in abnormal blood vessel proliferation into the cornea. This corneal neovascularization (CoNV) can stem from a variety of insults including hypoxia and ocular surface inflammation caused by trauma, infection, chemical burns, and immunological diseases. CoNV threatens corneal transparency, resulting in permanent vision loss. Mainstay treatments of CoNV have partial efficacy and associated side effects, revealing the need for novel treatments. Numerous natural products and synthetic small molecules have shown potential in preclinical studies in vivo as antiangiogenic therapies for CoNV. Such small molecules include synthetic inhibitors of the vascular endothelial growth factor (VEGF) receptor and other tyrosine kinases, plus repurposed antimicrobials, as well as natural source-derived flavonoid and non-flavonoid phytochemicals, immunosuppressants, vitamins, and histone deacetylase inhibitors. They induce antiangiogenic and anti-inflammatory effects through inhibition of VEGF, NF-κB, and other growth factor receptor pathways.
1. Introduction
As the main refractive surface of the anterior aspect of the eye, the cornea plays a key role in optimal visual acuity (Figure 1). Corneal transparency is fundamental to its optical function and is possible due to its avascular structure. Under healthy conditions, the avascular nature of the cornea is maintained by a balance of proangiogenic and antiangiogenic factors. The cornea releases proangiogenic factors such as vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), basic fibroblast growth factor (b-FGF), and interleukins (ILs) that are sequestered and/or counterbalanced by local antiangiogenic factors that include angiostatin, pigment epithelium-derived factor (PEDF), and soluble vascular endothelial growth factor receptor 1 (sVEGFR-1) that maintain the corneal angiogenic privilege.[1] An imbalance of these factors allows for the abnormal proliferation of preexisting blood vessels (hemangiogenesis) and lymph vessels (lymphangiogenesis) into the corneal stroma, a process referred to as corneal neovascularization (CoNV).
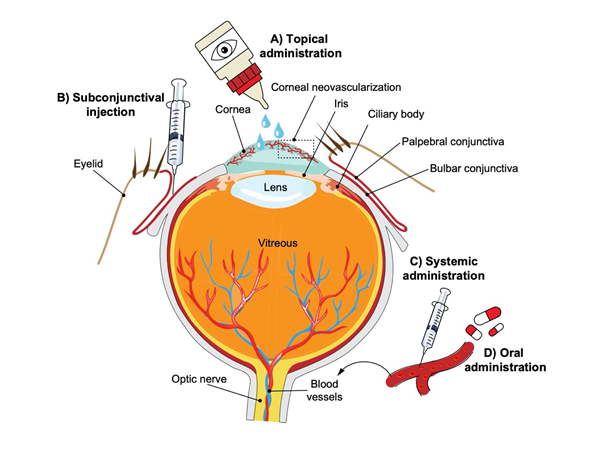
Figure 1. Schematic representation of the human eye and routes of administration for preclinical corneal drug delivery. (A) Noninvasive topical administration (eye drops), (B) subconjunctival injection given underneath the conjunctiva lining the eyelid, (C) systemic administration as intravenous injection, intraperitoneal injection or implanted osmotic pump, and (D) oral administration (gavage).
CoNV arises due to a variety of insults including hypoxic injury and ocular surface inflammation due to trauma, infection, chemical burns, and immunological disease.[2] The increased vascular permeability of these new vessels leads to chronic corneal edema, lipid exudation, inflammation, and scar formation, thus compromising corneal transparency and potentially resulting in permanent vision loss.[2] The exact incidence and prevalence of CoNV are unknown, but CoNV is present in many cases of corneal disease, which is the 4th leading cause of blindness globally after cataract, glaucoma and age-related macular degeneration according to the World Health Organization.[3] CoNV is also a common complication of corneal infections such as chlamydial infection, which is estimated to blind 4.9 million people due to scarring and vascularization.[4]
Several medical and surgical options for treating CoNV exist. The mainstay treatment for CoNV is to suppress the inflammatory response with administration of topical steroids such as dexamethasone. Steroids suppress actively proliferating corneal vessels through their anti-inflammatory properties, which include inhibition of cell chemotaxis, proinflammatory cytokines, and prostaglandin synthesis.[5] However, steroids only provide incomplete suppression of CoNV[6] and are associated with major side effects such as corneal thinning, ocular hypertension, cataracts, and increased risk of infection.[7] Additional CoNV therapies consist of off-label use of anti-VEGF antibodies, such as bevacizumab, which has shown efficacy in treatment of other vascular ocular diseases such as age-related macular degeneration.[8] Bevacizumab has shown promising results in treating CoNV; however, partial efficacy, resistance, and side effects consisting of corneal thinning and reduced epithelial healing[9][10] have limited its use. Thus, there is need for safer and more effective therapies for CoNV.
The search for novel drugs that block pathologic neovascularization has led to the study of numerous natural products and small-molecule inhibitors with varying biological mechanisms. Compared to large-molecule biologics, small molecules have the advantage of having various administration routes such as oral and topical (Figure 1). Additionally, they can potentially target multiple pathways and have favorable absorption, distribution, metabolism, excretion, and toxicity (ADMET) characteristics.[11] The efficacy of large-molecule biologics and other synthetic anti-VEGF therapies in the treatment of CoNV has been reviewed elsewhere.[12]
2. Models of CoNV
A majority of human CoNV cases are associated with ocular surface inflammation. Therefore, to study corneal hemangiogenesis, lymphangiogenesis, and tissue response to different therapies, multiple in vitro and in vivo models of CoNV have been developed. In vitro models of angiogenesis use cultured endothelial cells to test cell proliferation, migration, and tube formation in response to different compounds.[13][14] Human umbilical vein endothelial cells (HUVECs) are the most frequently used cell type for in vitro studies, although they are not a perfect surrogate of corneal endothelium. One model used to study angiogenesis in vivo is the corneal micropocket angiogenesis assay. This requires two adjacent micropocket incisions to be made in the mid cornea near the limbus for the implantation of a pellet of VEGF or b-FGF to stimulate angiogenesis while a pellet of the target antiangiogenic agent is inserted in the other micropocket.[15] This model is used to study the influence of specific molecules/proteins on angiogenesis[16] and is commonly used as a surrogate to study neovascularization in the context of other pathological processes such as cancer.
Chemical cauterization and suture placement models are the two most commonly used models for studying CoNV. Both of these model types have an inflammatory component, which mimics closely the complex nature of CoNV in human disease.[16] Chemical cauterization models induce CoNV by application of alkali (1N NaOH) or silver/potassium nitrate to the center of mouse, rat, or rabbit cornea for a short time followed by flushing with saline.[17] CoNV can be evaluated at 7–14 days after the procedure. In the suture-induced model, 7–0 silk or 10–0 nylon sutures are placed intrastromally in rabbit or rat/mouse cornea respectively. This results in CoNV response 7 days after surgery. Finally, some studies use the corneal de-epithelialization model. In this model, scraping of the corneal epithelium from limbus to limbus is used to induce CoNV.[18]
3. Future Directions
CoNV is a common sequela of numerous corneal insults from hypoxia and inflammatory conditions. The mechanisms involved in the induction of CoNV are complex and are regulated by a multitude of pro and antiangiogenic factors in the cornea. A majority of CoNV cases involve inflammatory conditions and VEGF is arguably one of the main factors involved in the development of NV. However, despite the usefulness of corticosteroids and anti-VEGF agents in suppressing CoNV their partial efficacy and side effect profile reveal the need for novel therapies. The next step for many experimental compounds would be to compare their effects on CoNV to current therapies such as dexamethasone and bevacizumab. More of this work would help to identify the small molecules that are comparable to or better than current CoNV therapies, and narrow the long list of potential CoNV treatments.
Overall, small molecules have some significant advantages over large molecules in the treatment of CoNV. Biologic pharmaceuticals are drastically more costly than small molecules, therefore resulting in high consumer cost.[19] One reason for this is that analytical characterization of biologics is extremely challenging, requiring combination of numerous methods to ensure their stability and purity[20] whereas small molecules can be structurally verified through high resolution analytical techniques such as NMR spectroscopy.[21] In terms of drug delivery, small molecules can offer greater partitioning across ocular barriers. Oral administration is one of the most common methods of delivery that is noninvasive and patient compliance friendly.[22] However, biologics have poor uptake from the gut and poor absorption across ocular barriers, and therefore oral and other systemic delivery of such large size molecules are typically avoided when oral administration of small molecules can offer more favorable partitioning across ocular barriers.[23] Small molecules can also be formulated as topical eye drops allowing for targeted drug administration to the clinically accessible cornea. Topical administration has the advantage of being noninvasive, with low systemic absorption. Eye drops are easy to administer, and have fairly high compliance rates.[24] Additionally, many of the side effects associated with systemic administration of medications can be avoided with topical eye drop administration. The disadvantage to topical application is that there is decreased bioavailability of the drug by this route. Nanoparticles or other advanced formulation of small molecules may allow for better inhibition of CoNV.
Many natural products such as the polyphenols and phytochemicals show suppression of CoNV; however, their exact antiangiogenic mechanisms and target proteins in CoNV inhibition are not yet known. This is largely due to the non-selectivity of natural products that allows for multitarget disease treatment. While further studies are needed to better understand the mechanism of natural products in CoNV, their non-selectivity provides a potential advantage of regulating multiple pathways that may be involved in the complex pathogenesis of CoNV.
Natural products have been studied for their antiangiogenic effects in multiple other disease states. For instance, many antiangiogenic natural products have been tested for their effect on cancer and posterior segment ocular angiogenesis.[25][26] More of those molecules that show potential in treating other angiogenic diseases could be tested in CoNV as well. Moreover, many CoNV studies focus on inhibiting the common pathways associated with vessel proliferation such as VEGF. However, other pathways have been discovered to be involved in angiogenesis. Therefore, these could be potential unexplored targets for blocking CoNV.
Polypharmacology can also have therapeutic benefits. Multitarget and combination therapies have the advantage of impacting different pathways involved in the pathogenesis of CoNV. Future development of effective CoNV therapies could benefit from combining existing methods with drug discovery of small molecules, repurposed drugs and natural products. Our current knowledge of the application of natural products and numerous small-molecule inhibitors in treating CoNV is limited to in vitro and preclinical in vivo studies but offers exciting potential in future translational studies to investigate their clinical efficacy and safety.
References
- Dimitri T. Azar; CORNEAL ANGIOGENIC PRIVILEGE: ANGIOGENIC AND ANTIANGIOGENIC FACTORS IN CORNEAL AVASCULARITY, VASCULOGENESIS, AND WOUND HEALING (AN AMERICAN OPHTHALMOLOGICAL SOCIETY THESIS). Transactions of the American Ophthalmological Society 2005, 104, 264-302.
- Jin-Hong Chang; Eric E. Gabison; Takuji Kato; Dimitri T. Azar; Corneal neovascularization. Current Opinion in Ophthalmology 2001, 12, 242-249, 10.1097/00055735-200108000-00002.
- Blindness and Vision Impairment Prevention . World Health Organization. Retrieved 2020-8-14
- Pineda, R.. World corneal blindness. In Foundations of Corneal Disease; Springer: New York, NY, USA, 2020; pp. 299-305.
- Deepak Gupta; Chris Illingworth; Treatments for Corneal Neovascularization: A Review. Cornea 2011, 30, 927-938, 10.1097/ico.0b013e318201405a.
- Claus Cursiefen; H Wenkel; Peter Martus; A Langenbucher; N X Nguyen; Berthold Seitz; Michael Küchle; Gottfried O. Naumann; Impact of short-term versus long-term topical steroids on corneal neovascularization after non-high-risk keratoplasty.. Graefe's Archive for Clinical and Experimental Ophthalmology 2001, 239, 514-521, 10.1007/s004170100313.
- Abdullah Al-Torbak; Abdullrahman Al-Amri; Michael D. Wagoner; Deep Corneal Neovascularization After Implantation With Intrastromal Corneal Ring Segments. American Journal of Ophthalmology 2005, 140, 926-927, 10.1016/j.ajo.2005.05.020.
- I Krebs; Shilla Lie; Ulrike Stolba; Florian Zeiler; Stefan Felke; Susanne Binder; Efficacy of intravitreal bevacizumab (Avastin®) therapy for early and advanced neovascular age-related macular degeneration. Acta Ophthalmologica 2009, 87, 611-617, 10.1111/j.1755-3768.2008.01312.x.
- Sang Woo Kim; Byung Jin Ha; E K Kim; Hungwon Tchah; Tae-Im Kim; The Effect of Topical Bevacizumab on Corneal Neovascularization. Ophthalmology 2008, 115, e33-e38, 10.1016/j.ophtha.2008.02.013.
- Ozdemir Ozdemir; Özgül Altintaş; Levent Altıntaş; Berna Özkan; Cigdem Akdag; Nurşen Yüksel; Comparison of the effects of subconjunctival and topical anti-VEGF therapy (bevacizumab) on experimental corneal neovascularization. Arquivos Brasileiros de Oftalmologia 2013, 77, 209-213, 10.5935/0004-2749.20140054.
- Timothy W. Corson; Craig M Crews; Molecular Understanding and Modern Application of Traditional Medicines: Triumphs and Trials. Cell 2007, 130, 769-774, 10.1016/j.cell.2007.08.021.
- Jin-Hong Chang; Nitin K. Garg; Elisa Lunde; Kyu-Yeon Han; Sandeep Jain; Dimitri T. Azar; Corneal neovascularization: an anti-VEGF therapy review.. Survey of Ophthalmology 2012, 57, 415-29, 10.1016/j.survophthal.2012.01.007.
- Chun-Chi Liang; Ann Y Park; Jun-Lin Guan; In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nature Protocols 2007, 2, 329-333, 10.1038/nprot.2007.30.
- J. A. Madri; B M Pratt; A M Tucker; Phenotypic modulation of endothelial cells by transforming growth factor-beta depends upon the composition and organization of the extracellular matrix.. The Journal of Cell Biology 1988, 106, 1375-1384, 10.1083/jcb.106.4.1375.
- Michael S. Rogers; Amy E Birsner; Robert J D'amato; The mouse cornea micropocket angiogenesis assay. Nature Protocols 2007, 2, 2545-2550, 10.1038/nprot.2007.368.
- Sandra R. Montezuma; Demetrios G. Vavvas; Joan W Miller; Review of the Ocular Angiogenesis Animal Models. Seminars in Ophthalmology 2008, 24, 52-61, 10.1080/08820530902800017.
- Janette M. Mahoney; L. David Waterbury; Drug effects on the neovascularization response to silver nitrate cauterization of the rat cornea. Current Eye Research 1984, 4, 531-535, 10.3109/02713688508999984.
- Sonali Pal-Ghosh; Gauri Tadvalkar; Rosalyn A Jurjus; James D. Zieske; Mary Ann Stepp; BALB/c and C57BL6 mouse strains vary in their ability to heal corneal epithelial debridement wounds. Experimental Eye Research 2008, 87, 478-486, 10.1016/j.exer.2008.08.013.
- Huy X. Ngo; Sylvie Garneau-Tsodikova; What are the drugs of the future?. MedChemComm 2017, 9, 757-758, 10.1039/c8md90019a.
- Steven A. Berkowitz; John R. Engen; Jeffrey R. Mazzeo; Graham B. Jones; Analytical tools for characterizing biopharmaceuticals and the implications for biosimilars. Nature Reviews Drug Discovery 2012, 11, 527-540, 10.1038/nrd3746.
- Toshihiko Sugiki; Kyoko Furuita; Toshimichi Fujiwara; Chojiro Kojima; Current NMR Techniques for Structure-Based Drug Discovery. Molecules 2018, 23, 148, 10.3390/molecules23010148.
- Jitendra; P. K. Sharma; Sumedha Bansal; Arunabha Banik; Noninvasive Routes of Proteins and Peptides Drug Delivery. Indian Journal of Pharmaceutical Sciences 2011, 73, 367-375, 10.4103/0250-474X.95608.
- Ripal Gaudana; Hari Krishna Ananthula; Ashwin Parenky; Abhirup Mandal; Ocular Drug Delivery. The AAPS Journal 2010, 12, 348-360, 10.1208/s12248-010-9183-3.
- Kishore Cholkar; Sulabh P. Patel; Aswani Dutt Vadlapudi; Abhirup Mandal; Novel Strategies for Anterior Segment Ocular Drug Delivery. Journal of Ocular Pharmacology and Therapeutics 2013, 29, 106-123, 10.1089/jop.2012.0200.
- El Bairi Khalid; El-Meghawry El-Kenawy Ayman; Heshu Rahman; Guaadaoui Abdelkarim; Agnieszka Najda; Natural products against cancer angiogenesis. Tumor Biology 2016, 37, 14513-14536, 10.1007/s13277-016-5364-8.
- Rania S. Sulaiman; Halesha D. Basavarajappa; Timothy W Corson; Natural product inhibitors of ocular angiogenesis.. Experimental Eye Research 2014, 129, 161-71, 10.1016/j.exer.2014.10.002.