
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Francisco J. Barrantes | + 3089 word(s) | 3089 | 2021-11-26 09:13:59 | | | |
| 2 | Lindsay Dong | + 484 word(s) | 3573 | 2021-11-29 08:47:45 | | |
Video Upload Options
Compartmentalization of the membrane is essential for cells to perform highly specific tasks and spatially constrained biochemical functions in topographically defined areas. These membrane lateral heterogeneities range from nanoscopic dimensions, often involving only a few molecular constituents, to micron-sized mesoscopic domains resulting from the coalescence of nanodomains. Short-lived domains lasting for a few milliseconds coexist with more stable platforms lasting from minutes to days. This panoply of lateral domains subserves the great variety of demands of cell physiology, particularly high for those implicated in signaling. The dendritic spine, a subcellular structure of neurons at the receiving (postsynaptic) end of central nervous system excitatory synapses, exploits this compartmentalization principle. In its most frequent adult morphology, the mushroom-shaped spine harbors neurotransmitter receptors, enzymes, and scaffolding proteins tightly packed in a volume of a few femtoliters. In addition to constituting a mesoscopic lateral heterogeneity of the dendritic arborization, the dendritic spine postsynaptic membrane is further compartmentalized into spatially delimited nanodomains that execute separate functions in the synapse.
1. Introduction
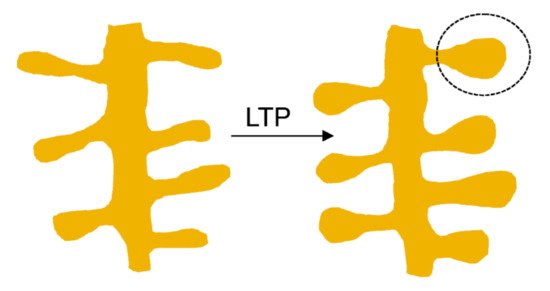
2. Dendritic Spines, Discrete Subcellular Compartment of the Neuronal Membrane
3. Cannabinoids and Cannabinoid Receptors
4. Cholinergic Signaling Contribution to Glutamatergic Receptor Compartmentalization
5. Cannabinoids and Nicotinic Receptors
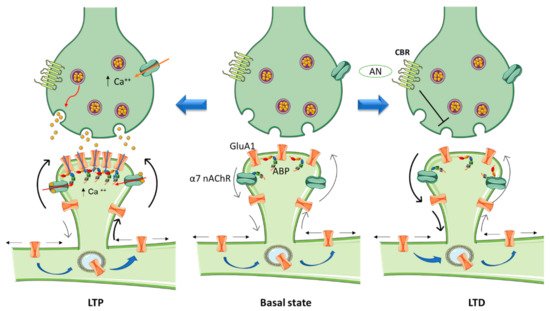
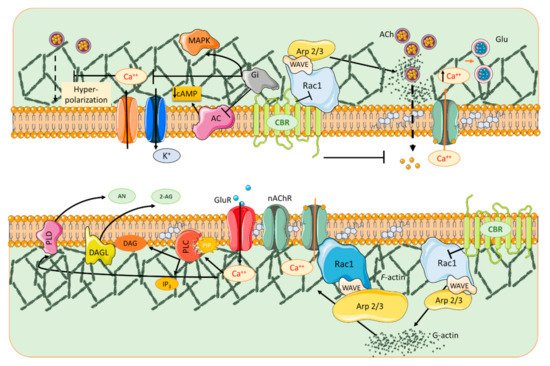
In the activated spine, Ca2+ entry through postsynaptic α7 nAChRs [47] or NMDA receptors and/or voltage-gated Ca2+ channels [48] can promote the activation of multiple signaling pathways with specific spatio-temporal patterns that orchestrate and regulate different aspects of cytoskeletal dynamics in the stimulated spines. Within the spine, Ca2+ binds to calmodulin (CaM), a Ca2+-binding protein which subsequently activates Ca2+/CaM-dependent kinases and phosphatases such as CaMKII and calcineurin [49][50]. CaMKII activates small GTPases and these in turn further modulate several downstream kinases [51] that have the ability to activate many ABPs, including Cofilin and Arp2/3, two proteins that play essential roles in actin remodeling [52][53]. Thus cannabinoids, through rac1/WAVE 1 modulation and α7 nAChR activation, contribute to actin structural remodeling of dendritic spines. Whereas activation of Rac1/WAVE1 induces a depletion of mushroom type spines, α7 nAChR activation contributes to the formation and maturation of dendritic spines (Figure 1).
6. Importance of Lipids in Dendritic Spine Compartmentalization
Impact of the Lipid Microenvironment on nAChRs and CBRs
7. Contribution of Actin Dynamics to the Compartmentalization of the Dendritic Spine
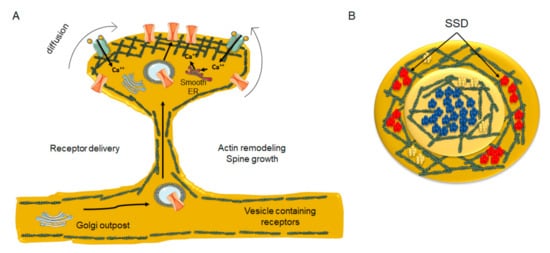
8. Nanodomain Cluster Organization Emerges as a Common Organizing Principle at CNS Synapses
9. Concluding Remarks
References
- Brusés, J.L.; Chauvet, N.; Rutishauser, U. Membrane lipid rafts are necessary for the maintenance of the (alpha)7 nicotinic acetylcholine receptor in somatic spines of ciliary neurons. J. Neurosci. 2001, 21, 504–512.
- Hayashi, T.; Su, T.-P. Sigma-1 receptors at galactosylceramide-enriched lipid microdomains regulate oligodendrocyte differentiation. Proc. Natl. Acad. Sci. USA 2004, 101, 14949–14954.
- Allen, J.A.; Halverson-Tamboli, R.A.; Rasenick, M.M. Lipid raft microdomains and neurotransmitter signalling. Nat. Rev. Neurosci. 2007, 8, 128–140.
- Egawa, J.; Pearn, M.L.; Lemkuil, B.P.; Patel, P.M.; Head, B.P. Membrane lipid rafts and neurobiology: Age-related changes in membrane lipids and loss of neuronal function. J. Physiol. 2016, 594, 4565–4579.
- Nagappan, G.; Lu, B. Activity-dependent modulation of the BDNF receptor TrkB: Mechanisms and implications. Trends Neurosci. 2005, 28, 464–471.
- Fernandes, C.C.; Berg, D.K.; Gómez-Varela, D. Lateral mobility of nicotinic acetylcholine receptors on neurons is determined by receptor composition, local domain, and cell type. J. Neurosci. 2010, 30, 8841–8851.
- Dunaevsky, A.; Tashiro, A.; Majewska, A.; Mason, C.; Yuste, R. Developmental regulation of spine motility in the mammalian central nervous system. Proc. Natl. Acad. Sci. USA 1999, 96, 13438–13443.
- Heine, M.; Holcman, D. Asymmetry between Pre- and Postsynaptic Transient Nanodomains Shapes Neuronal Communication. Trends Neurosci. 2020, 43, 182–196.
- Hering, H.; Sheng, M. Dentritic spines: Structure, dynamics and regulation. Nat. Rev. Neurosci. 2001, 2, 880–888.
- Trommald, M.; Hulleberg, G. Dimensions and density of dendritic spines from rat dentate granule cells based on reconstructions from serial electron micrographs. J. Comp. Neurol. 1997, 377, 15–28.
- Arellano, J.I.; Benavides-Piccione, R.; Defelipe, J.; Yuste, R. Ultrastructure of dendritic spines: Correlation between synaptic and spine morphologies. Front. Neurosci. 2007, 1, 131–143.
- Harris, K.M.; Stevens, J.K. Dendritic spines of CA 1 pyramidal cells in the rat hippocampus: Serial electron microscopy with reference to their biophysical characteristics. J. Neurosci. 1989, 9, 2982–2997.
- Noguchi, J.; Nagaoka, A.; Watanabe, S.; Ellis-Davies, G.C.R.; Kitamura, K.; Kano, M.; Matsuzaki, M.; Kasai, H. In vivo two-photon uncaging of glutamate revealing the structure-function relationships of dendritic spines in the neocortex of adult mice. J. Physiol. 2011, 589, 2447–2457.
- Matsuzaki, M.; Ellis-Davies, G.C.R.; Nemoto, T.; Miyashita, Y.; Iino, M.; Kasai, H. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat. Neurosci. 2001, 4, 1086–1092.
- Citri, A.; Malenka, R.C. Synaptic Plasticity: Multiple Forms, Functions, and Mechanisms. Neuropsychopharmacology 2008, 33, 18–41.
- Bliss, T.V.P.; Cooke, S.F. Long-term potentiation and long-term depression: A clinical perspective. Clinics 2011, 66 (Suppl. 1), 3–17.
- Matsuzaki, M.; Honkura, N.; Ellis-Davies, G.C.R.; Kasai, H. Structural basis of long-term potentiation in single dendritic spines. Nature 2004, 429, 761–766.
- Lang, C.; Barco, A.; Zablow, L.; Kandel, E.R.; Siegelbaum, S.A.; Zakharenko, S.S. Transient expansion of synaptically connected dendritic spines upon induction of hippocampal long-term potentiation. Proc. Natl. Acad. Sci. USA 2004, 101, 16665–16670.
- Zhou, Q.; Homma, K.J.; Poo, M. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron 2004, 44, 749–757.
- Nägerl, U.V.; Eberhorn, N.; Cambridge, S.B.; Bonhoeffer, T. Bidirectional activity-dependent morphological plasticity in hippocampal neurons. Neuron 2004, 44, 759–767.
- Tønnesen, J.; Katona, G.; Rózsa, B.; Nägerl, U.V. Spine neck plasticity regulates compartmentalization of synapses. Nat. Neurosci. 2014, 17, 678–685.
- Vanderklish, P.W.; Edelman, G.M. Dendritic spines elongate after stimulation of group 1 metabotropic glutamate receptors in cultured hippocampal neurons. Proc. Natl. Acad. Sci. USA 2002, 99, 1639–1644.
- Tønnesen, J.; Nägerl, U.V. Dendritic Spines as Tunable Regulators of Synaptic Signals. Front. Psychiatry 2016, 7, 101.
- Müller, W.; Connor, J.A. Dendritic spines as individual neuronal compartments for synaptic Ca2+ responses. Nature 1991, 354, 73–76.
- Guthrie, P.B.; Segal, M.; Kater, S.B. Independent regulation of calcium revealed by imaging dendritic spines. Nature 1991, 354, 76–80.
- Honigmann, A.; Pralle, A. Compartmentalization of the Cell Membrane. J. Mol. Biol. 2016, 428, 4739–4748.
- Hohmann, T.; Feese, K.; Ghadban, C.; Dehghani, F.; Grabiec, U. On the influence of cannabinoids on cell morphology and motility of glioblastoma cells. PLoS ONE 2019, 14, e0212037.
- Njoo, C.; Agarwal, N.; Lutz, B.; Kuner, R. The Cannabinoid Receptor CB1 Interacts with the WAVE1 Complex and Plays a Role in Actin Dynamics and Structural Plasticity in Neurons. PLOS Biol. 2015, 13, e1002286.
- Ladarre, D.; Roland, A.B.; Biedzinski, S.; Ricobaraza, A.; Lenkei, Z. Polarized cellular patterns of endocannabinoid production and detection shape cannabinoid signaling in neurons. Front. Cell. Neurosci. 2014, 8, 426.
- Leterrier, C.; Lainé, J.; Darmon, M.; Boudin, H.; Rossier, J.; Lenkei, Z. Constitutive activation drives compartment-selective endocytosis and axonal targeting of type 1 cannabinoid receptors. J. Neurosci. 2006, 26, 3141–3153.
- Nimchinsky, E.A.; Sabatini, B.L.; Svoboda, K. Structure and function of dendritic spines. Annu. Rev. Physiol. 2002, 64, 313–353.
- Carvalho, A.F.; Reyes, B.A.S.; Ramalhosa, F.; Sousa, N.; Van Bockstaele, E.J. Repeated administration of a synthetic cannabinoid receptor agonist differentially affects cortical and accumbal neuronal morphology in adolescent and adult rats. Brain Struct. Funct. 2016, 221, 407–419.
- Spiga, S.; Lintas, A.; Diana, M. Altered Mesolimbic Dopamine System in THC Dependence. Curr. Neuropharmacol. 2011, 9, 200–204.
- Spiga, S.; Lintas, A.; Migliore, M.; Diana, M. Altered architecture and functional consequences of the mesolimbic dopamine system in cannabis dependence. Addict. Biol. 2010, 15, 266–276.
- Monday, H.R.; Bourdenx, M.; Jordan, B.A.; Castillo, P.E. Cb1-receptor-mediated inhibitory ltd triggers presynaptic remodeling via protein synthesis and ubiquitination. Elife 2020, 9, 1–25.
- Scott, L.; Zelenin, S.; Malmersjö, S.; Kowalewski, J.M.; Markus, E.Z.; Nairn, A.C.; Greengard, P.; Brismar, H.; Aperia, A. Allosteric changes of the NMDA receptor trap diffusible dopamine 1 receptors in spines. Proc. Natl. Acad. Sci. USA 2006, 103, 762–767.
- Cepeda, C.; Levine, M.S. Where do you think you are going? The NMDA-D1 receptor trap. Sci. STKE 2006, 2006, pe20.
- Calabresi, P.; Gubellini, P.; Centonze, D.; Picconi, B.; Bernardi, G.; Chergui, K.; Svenningsson, P.; Fienberg, A.A.; Greengard, P. Dopamine and cAMP-regulated phosphoprotein 32 kDa controls both striatal long-term depression and long-term potentiation, opposing forms of synaptic plasticity. J. Neurosci. 2000, 20, 8443–8451.
- Kerr, J.N.; Wickens, J.R. Dopamine D-1/D-5 receptor activation is required for long-term potentiation in the rat neostriatum in vitro. J. Neurophysiol. 2001, 85, 117–124.
- Sawaguchi, T.; Goldman-Rakic, P.S. The role of D1-dopamine receptor in working memory: Local injections of dopamine antagonists into the prefrontal cortex of rhesus monkeys performing an oculomotor delayed-response task. J. Neurophysiol. 1994, 71, 515–528.
- Fiorentini, C.; Gardoni, F.; Spano, P.; Di Luca, M.; Missale, C. Regulation of dopamine D1 receptor trafficking and desensitization by oligomerization with glutamate N-methyl-D-aspartate receptors. J. Biol. Chem. 2003, 278, 20196–20202.
- Maurer, S.V.; Williams, C.L. The Cholinergic System Modulates Memory and Hippocampal Plasticity via Its Interactions with Non-Neuronal Cells. Front. Immunol. 2017, 8, 1489.
- Prado, V.F.; Janickova, H.; Al-Onaizi, M.A.; Prado, M.A.M. Cholinergic circuits in cognitive flexibility. Neuroscience 2017, 345, 130–141.
- Wang, L.; Almeida, L.E.F.; Spornick, N.A.; Kenyon, N.; Kamimura, S.; Khaibullina, A.; Nouraie, M.; Quezado, Z.M.N. Modulation of social deficits and repetitive behaviors in a mouse model of autism: The role of the nicotinic cholinergic system. Psychopharmacology 2015, 232, 4303–4316.
- Pidoplichko, V.I.; DeBiasi, M.; Williams, J.T.; Dani, J.A. Nicotine activates and desensitizes midbrain dopamine neurons. Nature 1997, 390, 401–404.
- Corrigall, W.A.; Franklin, K.B.; Coen, K.M.; Clarke, P.B. The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology 1992, 107, 285–289.
- Dani, J.A.; Bertrand, D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 699–729.
- Sabatini, B.L.; Oertner, T.G.; Svoboda, K. The life cycle of Ca(2+) ions in dendritic spines. Neuron 2002, 33, 439–452.
- Fujii, H.; Inoue, M.; Okuno, H.; Sano, Y.; Takemoto-Kimura, S.; Kitamura, K.; Kano, M.; Bito, H. Nonlinear decoding and asymmetric representation of neuronal input information by CaMKIIα and calcineurin. Cell Rep. 2013, 3, 978–987.
- Chang, J.-Y.; Parra-Bueno, P.; Laviv, T.; Szatmari, E.M.; Lee, S.-J.R.; Yasuda, R. CaMKII Autophosphorylation Is Necessary for Optimal Integration of Ca(2+) Signals during LTP Induction, but Not Maintenance. Neuron 2017, 94, 800–808.
- Murakoshi, H.; Wang, H.; Yasuda, R. Local, persistent activation of Rho GTPases during plasticity of single dendritic spines. Nature 2011, 472, 100.
- Borovac, J.; Bosch, M.; Okamoto, K. Regulation of actin dynamics during structural plasticity of dendritic spines: Signaling messengers and actin-binding proteins. Mol. Cell. Neurosci. 2018, 91, 122–130.
- Costa, J.F.; Dines, M.; Lamprecht, R. The Role of Rac GTPase in Dendritic Spine Morphogenesis and Memory. Front. Synaptic Neurosci. 2020, 12, 12.
- Baier, C.J.; Fantini, J.; Barrantes, F.J. Disclosure of cholesterol recognition motifs in transmembrane domains of the human nicotinic acetylcholine receptor. Sci. Rep. 2011, 1, 69.
- Fantini, J.; Di Scala, C.; Evans, L.S.; Williamson, P.T.F.; Barrantes, F.J. A mirror code for protein-cholesterol interactions in the two leaflets of biological membranes. Sci. Rep. 2016, 6, 21907.
- Oddi, S.; Dainese, E.; Fezza, F.; Lanuti, M.; Barcaroli, D.; De Laurenzi, V.; Centonze, D.; Maccarrone, M. Functional characterization of putative cholesterol binding sequence (CRAC) in human type-1 cannabinoid receptor. J. Neurochem. 2011, 116, 858–865.
- Hua, T.; Vemuri, K.; Pu, M.; Qu, L.; Han, G.W.; Wu, Y.; Zhao, S.; Shui, W.; Li, S.; Korde, A.; et al. Crystal Structure of the Human Cannabinoid Receptor CB(1). Cell 2016, 167, 750–762.
- Krishna Kumar, K.; Shalev-Benami, M.; Robertson, M.J.; Hu, H.; Banister, S.D.; Hollingsworth, S.A.; Latorraca, N.R.; Kato, H.E.; Hilger, D.; Maeda, S.; et al. Structure of a Signaling Cannabinoid Receptor 1-G Protein Complex. Cell 2019, 176, 448–458.
- Allende, M.L.; Zhu, H.; Kono, M.; Hoachlander-Hobby, L.E.; Huso, V.L.; Proia, R.L. Genetic defects in the sphingolipid degradation pathway and their effects on microglia in neurodegenerative disease. Cell. Signal. 2021, 78, 109879.
- Hering, H.; Lin, C.C.; Sheng, M. Lipid rafts in the maintenance of synapses, dendritic spines, and surface AMPA receptor stability. J. Neurosci. 2003, 23, 3262–3271.
- Carrasco, P.; Sahún, I.; McDonald, J.; Ramírez, S.; Jacas, J.; Gratacós, E.; Sierra, A.Y.; Serra, D.; Herrero, L.; Acker-Palmer, A.; et al. Ceramide levels regulated by carnitine palmitoyltransferase 1C control dendritic spine maturation and cognition. J. Biol. Chem. 2012, 287, 21224–21232.
- Hammond, G.R.V.; Schiavo, G. Polyphosphoinositol lipids: Under-PPInning synaptic function in health and disease. Dev. Neurobiol. 2007, 67, 1232–1247.
- Richards, D.A.; Mateos, J.M.; Hugel, S.; De Paola, V.; Caroni, P.; Gähwiler, B.H.; McKinney, R.A. Glutamate induces the rapid formation of spine head protrusions in hippocampal slice cultures. Proc. Natl. Acad. Sci. USA 2005, 102, 6166–6171.
- Ueda, Y.; Hayashi, Y. PIP3 regulates Spinule formation in dendritic spines during structural long-term potentiation. J. Neurosci. 2013, 33, 11040–11047.
- Yin, H.L.; Janmey, P.A. Phosphoinositide Regulation of the Actin Cytoskeleton. Annu. Rev. Physiol. 2003, 65, 761–789.
- Kumar, V.; Zhang, M.X.; Swank, M.W.; Kunz, J.; Wu, G.Y. Regulation of dendritic morphogenesis by Ras-PI3K-Akt-mTOR and Ras-MAPK signaling pathways. J. Neurosci. 2005, 25, 11288–11299.
- Kelleher, R.J., 3rd; Govindarajan, A.; Tonegawa, S. Translational regulatory mechanisms in persistent forms of synaptic plasticity. Neuron 2004, 44, 59–73.
- McLaughlin, S.; Murray, D. Plasma membrane phosphoinositide organization by protein electrostatics. Nature 2005, 438, 605–611.
- Calabrese, B.; Halpain, S. Essential role for the PKC target MARCKS in maintaining dendritic spine morphology. Neuron 2005, 48, 77–90.
- Horne, E.A.; Dell’Acqua, M.L. Phospholipase C is required for changes in postsynaptic structure and function associated with NMDA receptor-dependent long-term depression. J. Neurosci. 2007, 27, 3523–3534.
- Arroyo, A.I.; Camoletto, P.G.; Morando, L.; Sassoe-Pognetto, M.; Giustetto, M.; Van Veldhoven, P.P.; Schuchman, E.H.; Ledesma, M.D. Pharmacological reversion of sphingomyelin-induced dendritic spine anomalies in a Niemann Pick disease type A mouse model. EMBO Mol. Med. 2014, 6, 398–413.
- Stoffel, W. Functional analysis of acid and neutral sphingomyelinases in vitro and in vivo. Chem. Phys. Lipids 1999, 102, 107–121.
- Franco-Villanueva, A.; Fernández-López, E.; Gabandé-Rodríguez, E.; Bañón-Rodríguez, I.; Esteban, J.A.; Antón, I.M.; Ledesma, M.D. WIP modulates dendritic spine actin cytoskeleton by transcriptional control of lipid metabolic enzymes. Hum. Mol. Genet. 2014, 23, 4383–4395.
- Barrantes, F.J. Structural basis for lipid modulation of nicotinic acetylcholine receptor function. Brain Res. Brain Res. Rev. 2004, 47, 71–95.
- Barrantes, F.J. Cholesterol effects on nicotinic acetylcholine receptor. J. Neurochem. 2007, 103, 72–80.
- Baier, C.J.; Gallegos, C.E.; Levi, V.; Barrantes, F.J. Cholesterol modulation of nicotinic acetylcholine receptor surface mobility. Eur. Biophys. J. 2010, 39, 213–227.
- Mosqueira, A.; Camino, P.A.; Barrantes, F.J. Antibody-induced crosslinking and cholesterol-sensitive, anomalous diffusion of nicotinic acetylcholine receptors. J. Neurochem. 2020, 152, 663–674.
- Mosqueira, A.; Camino, P.A.; Barrantes, F.J. Cholesterol modulates acetylcholine receptor diffusion by tuning confinement sojourns and nanocluster stability. Sci. Rep. 2018, 8, 11974.
- Pediconi, M.F.; Gallegos, C.E.; De Los Santos, E.B.; Barrantes, F.J. Metabolic cholesterol depletion hinders cell-surface trafficking of the nicotinic acetylcholine receptor. Neuroscience 2004, 128, 239–249.
- Borroni, V.; Kamerbeek, C.; Pediconi, M.F.; Barrantes, F.J. Lovastatin Differentially Regulates α7 and α4 Neuronal Nicotinic Acetylcholine Receptor Levels in Rat Hippocampal Neurons. Molecules 2020, 25, 4838.
- Baier, C.J.; Barrantes, F.J. Sphingolipids are necessary for nicotinic acetylcholine receptor export in the early secretory pathway. J. Neurochem. 2007, 101, 1072–1084.
- Kapus, A.; Janmey, P. Plasma membrane--cortical cytoskeleton interactions: A cell biology approach with biophysical considerations. Compr. Physiol. 2013, 3, 1231–1281.
- Kusumi, A.; Nakada, C.; Ritchie, K.; Murase, K.; Suzuki, K.; Murakoshi, H.; Kasai, R.S.; Kondo, J.; Fujiwara, T. Paradigm shift of the plasma membrane concept from the two-dimensional continuum fluid to the partitioned fluid: High-speed single-molecule tracking of membrane molecules. Annu. Rev. Biophys. Biomol. Struct. 2005, 34, 351–378.
- Andrade, D.M.; Clausen, M.P.; Keller, J.; Mueller, V.; Wu, C.; Bear, J.E.; Hell, S.W.; Lagerholm, B.C.; Eggeling, C. Cortical actin networks induce spatio-temporal confinement of phospholipids in the plasma membrane--a minimally invasive investigation by STED-FCS. Sci. Rep. 2015, 5, 11454.
- Fujiwara, T.; Ritchie, K.; Murakoshi, H.; Jacobson, K.; Kusumi, A. Phospholipids undergo hop diffusion in compartmentalized cell membrane. J. Cell Biol. 2002, 157, 1071–1081.
- Auth, T.; Gov, N.S. Diffusion in a fluid membrane with a flexible cortical cytoskeleton. Biophys. J. 2009, 96, 818–830.
- Hotulainen, P.; Hoogenraad, C.C. Actin in dendritic spines: Connecting dynamics to function. J. Cell Biol. 2010, 189, 619–629.
- Kwik, J.; Boyle, S.; Fooksman, D.; Margolis, L.; Sheetz, M.P.; Edidin, M. Membrane cholesterol, lateral mobility, and the phosphatidylinositol 4,5-bisphosphate-dependent organization of cell actin. Proc. Natl. Acad. Sci. USA 2003, 100, 13964–13969.
- Cingolani, L.A.; Goda, Y. Actin in action: The interplay between the actin cytoskeleton and synaptic efficacy. Nat. Rev. Neurosci. 2008, 9, 344–356.
- Nelson, J.C.; Stavoe, A.K.H.; Colón-Ramos, D.A. The actin cytoskeleton in presynaptic assembly. Cell Adh. Migr. 2013, 7, 379–387.
- Michel, K.; Müller, J.A.; Oprişoreanu, A.-M.; Schoch, S. The presynaptic active zone: A dynamic scaffold that regulates synaptic efficacy. Exp. Cell Res. 2015, 335, 157–164.
- Rust, M.B.; Maritzen, T. Relevance of presynaptic actin dynamics for synapse function and mouse behavior. Exp. Cell Res. 2015, 335, 165–171.
- Bartol, T.M.; Bromer, C.; Kinney, J.; Chirillo, M.A.; Bourne, J.N.; Harris, K.M.; Sejnowski, T.J. Nanoconnectomic upper bound on the variability of synaptic plasticity. Elife 2015, 4, e10778.
- Bourne, J.N.; Chirillo, M.A.; Harris, K.M. Presynaptic ultrastructural plasticity along CA3→CA1 axons during long-term potentiation in mature hippocampus. J. Comp. Neurol. 2013, 521, 3898–3912.
- Meyer, D.; Bonhoeffer, T.; Scheuss, V. Balance and stability of synaptic structures during synaptic plasticity. Neuron 2014, 82, 430–443.
- Gundelfinger, E.D.; Fejtova, A. Molecular organization and plasticity of the cytomatrix at the active zone. Curr. Opin. Neurobiol. 2012, 22, 423–430.
- Matz, J.; Gilyan, A.; Kolar, A.; McCarvill, T.; Krueger, S.R. Rapid structural alterations of the active zone lead to sustained changes in neurotransmitter release. Proc. Natl. Acad. Sci. USA 2010, 107, 8836–8841.
- Monday, H.R.; Castillo, P.E. Closing the gap: Long-term presynaptic plasticity in brain function and disease. Curr. Opin. Neurobiol. 2017, 45, 106–112.
- Runge, K.; Cardoso, C.; de Chevigny, A. Dendritic Spine Plasticity: Function and Mechanisms. Front. Synaptic Neurosci. 2020, 12, 36.
- Nakahata, Y.; Yasuda, R. Plasticity of Spine Structure: Local Signaling, Translation and Cytoskeletal Reorganization. Front. Synaptic Neurosci. 2018, 10, 29.
- Holcman, D.; Triller, A. Modeling synaptic dynamics driven by receptor lateral diffusion. Biophys. J. 2006, 91, 2405–2415.
- Yang, X.; Specht, C.G. Subsynaptic Domains in Super-Resolution Microscopy: The Treachery of Images. Front. Mol. Neurosci. 2019, 12, 161.
- Hruska, M.; Henderson, N.; Le Marchand, S.J.; Jafri, H.; Dalva, M.B. Synaptic nanomodules underlie the organization and plasticity of spine synapses. Nat. Neurosci. 2018, 21, 671–682.
- Wegner, W.; Mott, A.C.; Grant, S.G.N.; Steffens, H.; Willig, K.I. In vivo STED microscopy visualizes PSD95 sub-structures and morphological changes over several hours in the mouse visual cortex. Sci. Rep. 2018, 8, 219.
- Moretto, E.; Longatti, A.; Murru, L.; Chamma, I.; Sessa, A.; Zapata, J.; Hosy, E.; Sainlos, M.; Saint-Pol, J.; Rubinstein, E.; et al. TSPAN5 Enriched Microdomains Provide a Platform for Dendritic Spine Maturation through Neuroligin-1 Clustering. Cell Rep. 2019, 29, 1130–1146.
- Choquet, D.; Triller, A. The role of receptor diffusion in the organization of the postsynaptic membrane. Nat. Rev. Neurosci. 2003, 4, 251–265.
- Triller, A.; Choquet, D. New concepts in synaptic biology derived from single-molecule imaging. Neuron 2008, 59, 359–374.
- Blanpied, T.A.; Scott, D.B.; Ehlers, M.D. Dynamics and regulation of clathrin coats at specialized endocytic zones of dendrites and spines. Neuron 2002, 36, 435–449.
- Rácz, B.; Blanpied, T.A.; Ehlers, M.D.; Weinberg, R.J. Lateral organization of endocytic machinery in dendritic spines. Nat. Neurosci. 2004, 7, 917–918.
- Lu, J.; Helton, T.D.; Blanpied, T.A.; Rácz, B.; Newpher, T.M.; Weinberg, R.J.; Ehlers, M.D. Postsynaptic positioning of endocytic zones and AMPA receptor cycling by physical coupling of dynamin-3 to Homer. Neuron 2007, 55, 874–889.
- Groc, L.; Choquet, D. Measurement and characteristics of neurotransmitter receptor surface trafficking (Review). Mol. Membr. Biol. 2008, 25, 344–352.
- Kusters, R.; van der Heijden, T.; Kaoui, B.; Harting, J.; Storm, C. Forced transport of deformable containers through narrow constrictions. Phys. Rev. E. Stat. Nonlin. Soft Matter Phys. 2014, 90, 33006.
- Wang, J.; Fourriere, L.; Gleeson, P.A. Local Secretory Trafficking Pathways in Neurons and the Role of Dendritic Golgi Outposts in Different Cell Models. Front. Mol. Neurosci. 2020, 13, 597391.
- Frotscher, M.; Studer, D.; Graber, W.; Chai, X.; Nestel, S.; Zhao, S. Fine structure of synapses on dendritic spines. Front. Neuroanat. 2014, 8, 94.




