
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sathish Muthu | + 1562 word(s) | 1562 | 2021-11-22 10:17:40 | | | |
| 2 | Beatrix Zheng | Meta information modification | 1562 | 2021-11-23 10:29:19 | | |
Video Upload Options
Cellular therapy offers regeneration which curbs osteoarthritis of the knee. Among cellular therapies, mesenchymal stromal cells (MSCs) are readily isolated from various sources as culture expanded and unexpanded cellular population which are used as therapeutic products. Though MSCs possess a unique immunological and regulatory profile through cross-talk between MSCs and immunoregulatory cells (T cells, NK cells, dendritic cells, B cells, neutrophils, monocytes, and macrophages), they provide an immunotolerant environment when transplanted to the site of action.
1. Introduction
Osteoarthritis of the knee, also called degenerative arthritis or osteoarthrosis, is a multi-factorial, slowly progressing disease of the synovial joint [1]. It is primarily a non-inflammatory degenerative disorder of the knee which is characterized by the progressive degenerative process leading to irreversible articular cartilage destruction [1][2]. Among all musculoskeletal diseases, osteoarthritis of the knee poses significant health, economic and social burden to the aging population, hence it is prudent to exactly define and locate the etiological agent to contemplate an effective management strategy [3]. A total of 15% of the world population is affected with osteoarthritis of the knee which leads to the major cause of activity limitation [4]. Understanding the molecular and cellular components of the pathophysiology of osteoarthritis of the knee is of paramount importance to establish the cell-based therapy to formulate the disease-modifying strategy [2][5].
Osteoarthritis of the knee results due to degradation of the articular cartilage and the components of the bone matrix [4]. The earliest pathomechanism in the progression of cartilage loss is due to the loss of type 2 collagen and aggrecans in the cartilage tissue [6]. In the early stage of knee osteoarthritis, natural killer (NK) cells and macrophages play a significant role in pathogenesis and intervening these cross-talks pose a potential breakthrough in the management of osteoarthritis of the knee [7]. Osteoarthritis of the knee is characterized by increased levels of pro-inflammatory cytokines such as IL-1 and TNF-α, raised expression of TGF-β, and the activation of matrix metalloproteinases (MMPs), ultimately leading to chondrocyte senescence [8][9].
Cytotherapy or cell-based therapies have been developed in the past decade for management of early knee osteoarthritis by utilizing the stem cells harvested from various donor tissues such as bone marrow, adipose tissue, placenta, synovium, dental tissues, peripheral blood, and rarely from embryonal tissues [10][11]. In particular, mesenchymal stromal cell (MSC)-based cellular therapy demonstrate a promising tool for regenerating the degenerated cartilage in the osteoarthritic knee [12][13][14][15]. A large number of published literature states that the regenerative capacity of MSCs is due to paracrine factors released by MSCs rather than the pluripotent nature of MSCs to differentiate into cells of the desired tissue [16][17]. The ease of availability from multiple sources makes MSCs the versatile cell-based therapy of choice in regenerating tissues [18]. Despite the promising results seen in vitro and in vivo studies, the application of MSCs are limited due to the risk of teratoma formation, limited viability of differentiated cells and rejection of the cells post transplantation [19][20][21][22].
There is no available evidence to prove that engrafted MSCs in osteoarthritis of the knee are differentiated into cartilage cells. These engrafted MSCs work in a paracrine fashion and stimulate the tissue-specific residual and resilient cells for tissue regeneration and repair [23][24]. MSCs act by empowerment rather than replacement [25][26]. The engrafted MSCs possess a stromal environment that contains both cellular and molecular components. 26 The combined action of cytokines and chemokines drive MSC to induce tissue regeneration by proliferation and differentiation of resident stem cells [27].
2. Immunomodulation by Living MSCs
3. Immunomodulation by Apoptotic MSCs
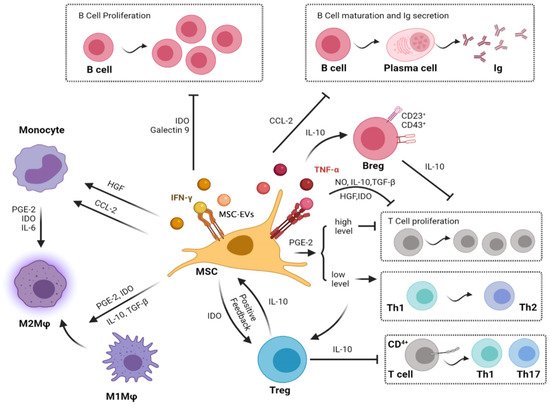
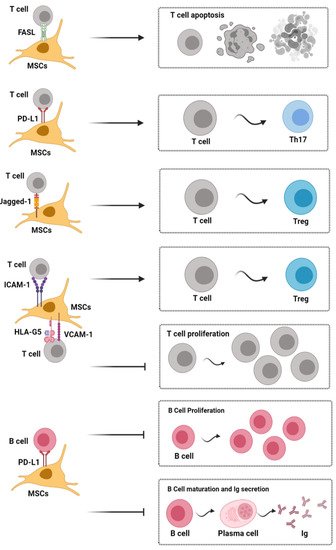
4. Cross-Talk between MSCs and Immunological Cells in Cartilage Regeneration
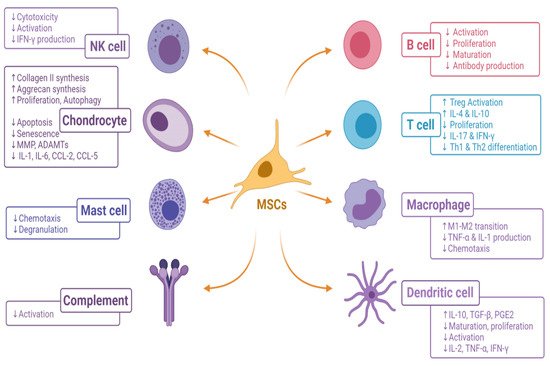
References
- Mobasheri, A.; Batt, M. An update on the pathophysiology of osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 333–339.
- Grässel, S.; Muschter, D. Recent advances in the treatment of osteoarthritis. F1000Research 2020, 9, 325.
- Bitton, R. The economic burden of osteoarthritis. Am. J. Manag. Care 2009, 15, S230–S235.
- Man, G.; Mologhianu, G. Osteoarthritis pathogenesis—A complex process that involves the entire joint. J. Med. Life 2014, 7, 37–41.
- Grässel, S.; Aszodi, A. Osteoarthritis and Cartilage Regeneration: Focus on Pathophysiology and Molecular Mechanisms. Int. J. Mol. Sci. 2019, 20, 6156.
- Cooke, M.E.; Lawless, B.M.; Jones, S.W.; Grover, L.M. Matrix degradation in osteoarthritis primes the superficial region of cartilage for mechanical damage. Acta Biomater. 2018, 78, 320–328.
- Mora, J.C.; Przkora, R.; Cruz-Almeida, Y. Knee osteoarthritis: Pathophysiology and current treatment modalities. J. Pain Res. 2018, 11, 2189–2196.
- Wojdasiewicz, P.; Poniatowski, Ł.A.; Szukiewicz, D. The Role of Inflammatory and Anti-Inflammatory Cytokines in the Pathogenesis of Osteoarthritis. Mediat. Inflamm. 2014, 2014, e561459.
- Sokolove, J.; Lepus, C.M. Role of inflammation in the pathogenesis of osteoarthritis: Latest findings and interpretations. Ther. Adv. Musculoskelet. Dis. 2013, 5, 77–94.
- Roseti, L.; Desando, G.; Cavallo, C.; Petretta, M.; Grigolo, B. Articular Cartilage Regeneration in Osteoarthritis. Cells 2019, 8, 1305.
- Jeyaraman, M.; Muthu, S.; Ganie, P.A. Does the Source of Mesenchymal Stem Cell Have an Effect in the Management of Osteoarthritis of the Knee? Meta-Analysis of Randomized Controlled Trials. Cartilage 2020, 1947603520951623.
- Muthu, S.; Jeyaraman, M.; Jain, R.; Gulati, A.; Jeyaraman, N.; Prajwal, G.S.; Mishra, P.C. Accentuating the sources of mesenchymal stem cells as cellular therapy for osteoarthritis knees—A panoramic review. Stem Cell Investig. 2021, 8.
- Zhao, L.; Kaye, A.D.; Abd-Elsayed, A. Stem Cells for the Treatment of Knee Osteoarthritis: A Comprehensive Review. Pain Physician 2018, 21, 229–242.
- Stem cell application for osteoarthritis in the knee joint: A minireview. World J. Stem Cells 2014, 6, 629–636.
- Jeyaraman, M.; Muthu, S.; Jeyaraman, N.; Ranjan, R.; Jha, S.K.; Mishra, P. Synovium Derived Mesenchymal Stromal Cells (Sy-MSCs): A Promising Therapeutic Paradigm in the Management of Knee Osteoarthritis. JOIO 2021.
- Fitzsimmons, R.E.B.; Mazurek, M.S.; Soos, A.; Simmons, C.A. Mesenchymal Stromal/Stem Cells in Regenerative Medicine and Tissue Engineering. Stem Cells Int. 2018, 2018, e8031718.
- Murphy, M.B.; Moncivais, K.; Caplan, A.I. Mesenchymal stem cells: Environmentally responsive therapeutics for regenerative medicine. Exp. Mol. Med. 2013, 45, e54.
- Marquez-Curtis, L.A.; Janowska-Wieczorek, A.; McGann, L.E.; Elliott, J.A.W. Mesenchymal stromal cells derived from various tissues: Biological, clinical and cryopreservation aspects. Cryobiology 2015, 71, 181–197.
- Herberts, C.A.; Kwa, M.S.; Hermsen, H.P. Risk factors in the development of stem cell therapy. J. Transl. Med. 2011, 9, 29.
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 68.
- Zhou, T.; Yuan, Z.; Weng, J.; Pei, D.; Du, X.; He, C.; Lai, P. Challenges and advances in clinical applications of mesenchymal stromal cells. J. Hematol. Oncol. 2021, 14, 24.
- Lukomska, B.; Stanaszek, L.; Zuba-Surma, E.; Legosz, P.; Sarzynska, S.; Drela, K. Challenges and Controversies in Human Mesenchymal Stem Cell Therapy. Stem Cells Int. 2019, 2019, e9628536.
- Liang, X.; Ding, Y.; Zhang, Y.; Tse, H.-F.; Lian, Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: Current status and perspectives. Cell Transplant. 2014, 23, 1045–1059.
- Maacha, S.; Sidahmed, H.; Jacob, S.; Gentilcore, G.; Calzone, R.; Grivel, J.-C.; Cugno, C. Paracrine Mechanisms of Mesenchymal Stromal Cells in Angiogenesis. Stem Cells Int. 2020, 2020, e4356359.
- Qi, K.; Li, N.; Zhang, Z.; Melino, G. Tissue regeneration: The crosstalk between mesenchymal stem cells and immune response. Cell Immunol. 2018, 326, 86–93.
- Costa-Almeida, R.; Calejo, I.; Gomes, M.E. Mesenchymal Stem Cells Empowering Tendon Regenerative Therapies. Int. J. Mol. Sci. 2019, 20, 3002.
- Shi, Y.; Hu, G.; Su, J.; Li, W.; Chen, Q.; Shou, P.; Xu, C.; Chen, X.; Huang, Y.; Zhu, Z.; et al. Mesenchymal stem cells: A new strategy for immunosuppression and tissue repair. Cell Res. 2010, 20, 510–518.
- Wang, M.; Yuan, Q.; Xie, L. Mesenchymal Stem Cell-Based Immunomodulation: Properties and Clinical Application. Stem Cells Int. 2018, 2018, 3057624.
- Lim, J.-Y.; Im, K.-I.; Lee, E.-S.; Kim, N.; Nam, Y.-S.; Jeon, Y.-W.; Cho, S.-G. Enhanced immunoregulation of mesenchymal stem cells by IL-10-producing type 1 regulatory T cells in collagen-induced arthritis. Sci. Rep. 2016, 6, 26851.
- Weiss, A.R.R.; Dahlke, M.H. Immunomodulation by Mesenchymal Stem Cells (MSCs): Mechanisms of Action of Living, Apoptotic, and Dead MSCs. Front. Immunol. 2019, 10, 1191.
- Ham, O.; Lee, C.Y.; Kim, R.; Lee, J.; Oh, S.; Lee, M.Y.; Kim, J.; Hwang, K.-C.; Maeng, L.-S.; Chang, W. Therapeutic Potential of Differentiated Mesenchymal Stem Cells for Treatment of Osteoarthritis. Int. J. Mol. Sci. 2015, 16, 14961–14978.
- Zhao, X.; Zhao, Y.; Sun, X.; Xing, Y.; Wang, X.; Yang, Q. Immunomodulation of MSCs and MSC-Derived Extracellular Vesicles in Osteoarthritis. Front. Bioeng. Biotechnol. 2020, 8.
- Kangari, P.; Talaei-Khozani, T.; Razeghian-Jahromi, I.; Razmkhah, M. Mesenchymal stem cells: Amazing remedies for bone and cartilage defects. Stem Cell Res. Ther. 2020, 11, 492.
- Jiang, W.; Xu, J. Immune modulation by mesenchymal stem cells. Cell Prolif. 2019, 53.
- Ankrum, J.A.; Ong, J.F.; Karp, J.M. Mesenchymal stem cells: Immune evasive, not immune privileged. Nat. Biotechnol. 2014, 32, 252–260.
- Wang, Y.; Huang, J.; Gong, L.; Yu, D.; An, C.; Bunpetch, V.; Dai, J.; Huang, H.; Zou, X.; Ouyang, H.; et al. The Plasticity of Mesenchymal Stem Cells in Regulating Surface HLA-I. iScience 2019, 15, 66–78.
- Ghannam, S.; Bouffi, C.; Djouad, F.; Jorgensen, C.; Noël, D. Immunosuppression by mesenchymal stem cells: Mechanisms and clinical applications. Stem Cell Res. Ther. 2010, 1, 2.
- Jo, C.H.; Gil Lee, Y.; Shin, W.H.; Kim, H.; Chai, J.W.; Jeong, E.C.; Kim, J.E.; Shim, H.; Shin, J.S.; Shin, I.S.; et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: A proof-of-concept clinical trial. Stem Cells. 2014, 32, 1254–1266.
- Najar, M.; Martel-Pelletier, J.; Pelletier, J.-P.; Fahmi, H. Mesenchymal Stromal Cell Immunology for Efficient and Safe Treatment of Osteoarthritis. Front. Cell Dev. Biol. 2020, 8, 567813.
- Ma, S.; Xie, N.; Li, W.; Yuan, B.; Shi, Y.; Wang, Y. Immunobiology of mesenchymal stem cells. Cell Death Differ. 2014, 21, 216–225.
- Osińska, I.; Popko, K.; Demkow, U. Perforin: An important player in immune response. Cent. Eur. J. Immunol. 2014, 39, 109–115.
- Mancuso, P.; Murphy, M.J.; Barry, F. Immunomodulatory effect of mesenchymal stem cells following intra-articular injection in a model of osteoarthritis: A potential role for apoptosis. Osteoarthr. Cartil. 2017, 25, S386–S387.
- Ye, J.; Xie, C.; Wang, C.; Huang, J.; Yin, Z.; Heng, B.C.; Chen, X.; Shen, W. Promoting musculoskeletal system soft tissue regeneration by biomaterial-mediated modulation of macrophage polarization. Bioact. Mater. 2021, 6, 4096–4109.
- Gavin, C.; Meinke, S.; Heldring, N.; Heck, K.A.; Achour, A.; Iacobaeus, E.; Höglund, P.; Le Blanc, K.; Kadri, N. The Complement System Is Essential for the Phagocytosis of Mesenchymal Stromal Cells by Monocytes. Front. Immunol. 2019, 10, 2249.
- Hu, C.; Zhao, L.; Wu, D.; Li, L. Modulating autophagy in mesenchymal stem cells effectively protects against hypoxia- or ischemia-induced injury. Stem Cell Res. Ther. 2019, 10, 120.
- Liu, J.; Ding, Y.; Liu, Z.; Liang, X. Senescence in Mesenchymal Stem Cells: Functional Alterations, Molecular Mechanisms, and Rejuvenation Strategies. Front. Cell Dev. Biol. 2020, 8, 258.




