Cellular therapy offers regeneration which curbs osteoarthritis of the knee. Among cellular therapies, mesenchymal stromal cells (MSCs) are readily isolated from various sources as culture expanded and unexpanded cellular population which are used as therapeutic products. Though MSCs possess a unique immunological and regulatory profile through cross-talk between MSCs and immunoregulatory cells (T cells, NK cells, dendritic cells, B cells, neutrophils, monocytes, and macrophages), they provide an immunotolerant environment when transplanted to the site of action.
1. Introduction
Osteoarthritis of the knee, also called degenerative arthritis or osteoarthrosis, is a multi-factorial, slowly progressing disease of the synovial joint [1]. It is primarily a non-inflammatory degenerative disorder of the knee which is characterized by the progressive degenerative process leading to irreversible articular cartilage destruction [1][2][1,2]. Among all musculoskeletal diseases, osteoarthritis of the knee poses significant health, economic and social burden to the aging population, hence it is prudent to exactly define and locate the etiological agent to contemplate an effective management strategy [3]. A total of 15% of the world population is affected with osteoarthritis of the knee which leads to the major cause of activity limitation [4]. Understanding the molecular and cellular components of the pathophysiology of osteoarthritis of the knee is of paramount importance to establish the cell-based therapy to formulate the disease-modifying strategy [2][5][2,5].
Osteoarthritis of the knee results due to degradation of the articular cartilage and the components of the bone matrix [4]. The earliest pathomechanism in the progression of cartilage loss is due to the loss of type 2 collagen and aggrecans in the cartilage tissue [6]. In the early stage of knee osteoarthritis, natural killer (NK) cells and macrophages play a significant role in pathogenesis and intervening these cross-talks pose a potential breakthrough in the management of osteoarthritis of the knee [7]. Osteoarthritis of the knee is characterized by increased levels of pro-inflammatory cytokines such as IL-1 and TNF-α, raised expression of TGF-β, and the activation of matrix metalloproteinases (MMPs), ultimately leading to chondrocyte senescence [8][9][8,9].
Cytotherapy or cell-based therapies have been developed in the past decade for management of early knee osteoarthritis by utilizing the stem cells harvested from various donor tissues such as bone marrow, adipose tissue, placenta, synovium, dental tissues, peripheral blood, and rarely from embryonal tissues [10][11][10,11]. In particular, mesenchymal stromal cell (MSC)-based cellular therapy demonstrate a promising tool for regenerating the degenerated cartilage in the osteoarthritic knee [12][13][14][15][12,13,14,15]. A large number of published literature states that the regenerative capacity of MSCs is due to paracrine factors released by MSCs rather than the pluripotent nature of MSCs to differentiate into cells of the desired tissue [16][17][16,17]. The ease of availability from multiple sources makes MSCs the versatile cell-based therapy of choice in regenerating tissues [18]. Despite the promising results seen in vitro and in vivo studies, the application of MSCs are limited due to the risk of teratoma formation, limited viability of differentiated cells and rejection of the cells post transplantation [19][20][21][22][19,20,21,22].
There is no available evidence to prove that engrafted MSCs in osteoarthritis of the knee are differentiated into cartilage cells. These engrafted MSCs work in a paracrine fashion and stimulate the tissue-specific residual and resilient cells for tissue regeneration and repair [23][24][23,24]. MSCs act by empowerment rather than replacement [25][26][25,26]. The engrafted MSCs possess a stromal environment that contains both cellular and molecular components. 26 The combined action of cytokines and chemokines drive MSC to induce tissue regeneration by proliferation and differentiation of resident stem cells [27].
2. Immunomodulation by Living MSCs
Immunomodulation by MSCs is through direct cell-to-cell contact (membrane-bound proteins and receptors) and by paracrine signaling of macromolecules (cytokines, chemokines, and trophic factors) in the environment
[28]. Interleukin-10 (IL-10) is a pleiotropic immunomodulatory cytokine that regulates innate and adaptive immune systems. The interplay between regulatory cytokines and IL-10 establishes a tolerogenic environment suitable for the inhibition of T-cells. IL-10 plays a significant positive feedback loop by enhancing proliferative and molecular changes when MSCs are infused
[29][30][29,30].
MSCs exhibit a varied spectrum of immunoregulatory capabilities in both innate and adaptive immune systems. Due to the prevailing inflammatory environment, specific toll-like receptor (TLR) ligands affect the MSC-based cellular therapy in the management of osteoarthritis of the knee
[31][32][33][31,32,33]. In the early stages of knee osteoarthritis, TLR2/4 on MSC surface may result in polarization into MSC1, which promotes further immunogenic response to protect joint tissues in osteoarthritis pathogenesis
[32]. In later stages of knee osteoarthritis of the knee, TLR3 accumulation leads to polarization into MSC2 which further prevents articular surface damage in the osteoarthritis of the knee
[32]. Failure of inflammatory attenuation leads to the continuation of the vicious cycle in osteoarthritis pathogenesis.
In such a pro-inflammatory milieu, the quiescent resident MSCs are attenuated and may not be induced in the lines of regeneration of the articular cartilage and hence infusion of MSCs into the knee joint may not only repair and regenerate the joint cartilage but also help in stimulating the resident MSCs to attain joint homeostasis in favor of regeneration. MSCs suppress host immune responses by inhibiting immune cells proliferation and maturation in a non-MHC restricted manner
[34]. MSCs are considered to be hypoimmunogenic, which express low levels of HLA 1 and are deprived of HLA class II and co-stimulatory molecule expression
[35][36][35,36]. Numerous studies have also demonstrated MSCs to suppress the activity of a broad range of immune cells, including T cells, natural killer (NK) cells, dendritic cells DCs), B cells, neutrophils, monocytes, macrophages
[37].
3. Immunomodulation by Apoptotic MSCs
There is no consensus on the viability of MSCs to exert immunomodulatory effects in the local or systemic environment. Once administered, MSCs undergo radical biological and morphological changes and disappear from the site of injection within a few days of intra-articular injection
[38]. Despite the disappearance of administered MSCs from the site, they elaborate the cytokines and chemokines which work by paracrine signaling and induce the quiescent and resident MSCs to enhance the significant therapeutic effects
[23]. Hence the dead MSCs exert a non-specific immunomodulatory environment in the osteoarthritis of the knee through apoptosis, complement-mediated cell death, autophagy, and senescence
[39]. The paracrine and the contact immunomodulatory effects of MSCs on the immune cells are given in
Figure 1 and
Figure 2 respectively.
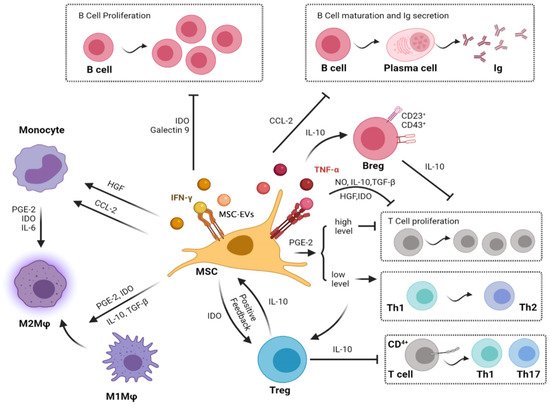
Figure 1. Paracrine immunomodulatory effects of the mesenchymal stromal cell on immune cells. [Note: MSCs have their immunomodulatory effects on Macrophages, T cells, and B cells related to osteoarthritis via the secretion of various cytokines through MSC-derived exosomal vesicles. MSCs inhibit the migration of macrophages and regulate their polarization by inducing transformation from M1 to M2. MSCs inhibit the proliferation of T cells and activation of inflammatory TH1 and TH17 cells. In turn, MSCs induce immunosuppressive Tregs. Moreover, MSCs decrease B-cell activation and proliferation and attenuate immunoglobulin production].
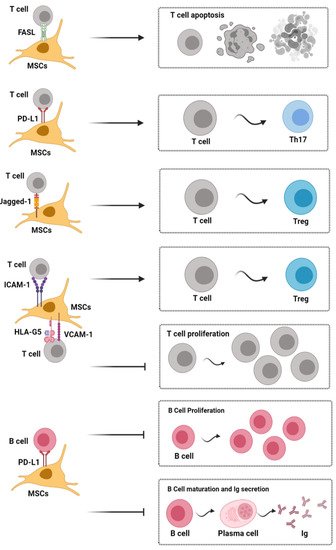
Figure 2. Contact dependent immunoregulatory effects of mesenchymal stromal cells on immune cells. [Note: Fas/FasL signal activation induces apoptosis of inflammatory T cells; Jagged-1/Notch-1 signal activation can induce CD4+ T cells to differentiate into Tregs, and PD-1/PD-L1 signaling effectively represses TH17 differentiation and inhibits the proliferation and maturation of B cells. Moreover, HLA-G5, ICAM-1, and VCAM-1 are also needed for MSCs to exert their immunosuppressive effects upon T cells]. HLA-G5, human leukocyte antigen-G5; ICAM-1, intercellular adhesion molecule-1; Ig, immunoglobulin; MSC, mesenchymal stem cells; PD-L1, programmed death-1 ligand; Th, helper T cell; Treg, regulatory T cell; VCAM-1, vascular cell adhesion molecule-1.
Apoptotic MSCs interact with immune cells directly by apoptosis and indirectly by phagocytosis. In DIRECT mechanism, apoptotic or dead MSCs create an immunosuppressive or immune-privileged environment by secreting IL-10 and TGF-β and inhibit lipopolysaccharide (LPS) stimulated macrophages by the secretion of IL-1β and TNF-α
[40]. In INDIRECT mechanism, phagocytes eliminate MSCs resulting in hyporesponsiveness to LPS and switching off the immunological cellular environment to an anti-inflammatory environment
[30]. Upon cell-to-cell contact, T cells release perforin and granzyme B and induce apoptosis of infused MSCs
[41]. IDO, a soluble factor, secreted by phagocytes is responsible for immunomodulation
[42]. Mancuso et al. emphasized that apoptotic MSCs retrieved from osteoarthritis of the knee exhibited higher immunosuppressive effects than viable MSCs
[42].
Complement cascade induces phagocytosis and recruits progenitor cells to the site of injury. Complement systems exhibit an interplay between MSCs to influence the biological features of engrafted cells. When a complement molecule binds to MSC, it induces phagocytosis by macrophages and in turn, M2 polarization occurs to establish a tolerogenic environment
[43]. With the rapid clearance of MSCs from the systemic circulation, a therapeutic response being observed was due to the interplay between complement and monocyte-phagocyte system
[44]. During normal development, autophagy modulate cellular homeostasis and remodeling potential. MSCs attenuate autophagy and promote cellular survival, proliferation, differentiation, and restoration of functional tissues in the degenerative environment
[45]. Senescent (aged) MSCs exhibit minimal proliferation, differentiation, immunoregulatory and secretory ability in osteoarthritis of the knee
[46].
Breg, regulatory B cell; CCL-2, C-C motif chemokine ligand 2; HGF, hepatocyte growth factor; IDO, indoleamine-2,3-dioxygenase; IL, interleukin; Ig, immunoglobulin; IFN-γ, interferon-gamma; MSC, mesenchymal stromal cell; MSC-EVs, Mesenchymal stromal cell-derived exosomal vesicles; Mφ, macrophages; NO, nitric oxide; PGE2, prostaglandin E2; Th, helper T cell; Treg, regulatory T cell; TGF-β, transforming growth factor-beta.
4. Cross-Talk between MSCs and Immunological Cells in Cartilage Regeneration
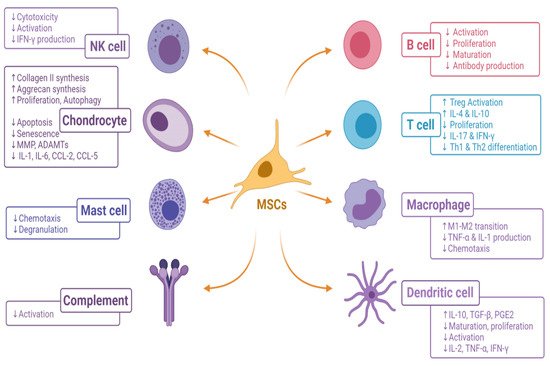
The immunomodulatory effect of MSCs on the immune cells in the osteoarthritis of the knee is given in Figure 3.
Figure 3. Immunomodulatory effects of MSCs on immune cells in osteoarthritis of the knee. [ADAMTs, A Disintegrin and Metalloproteinase with Thrombospondin motifs; CCL, C-C motif chemokine ligand; IL, interleukin; IFN-γ, interferon-gamma; M, macrophage; MMP, matrix metalloproteinase; MSC, mesenchymal stromal cell; PGE2, prostaglandin E2; Th, helper T cell; Treg, regulatory T cell; TGF-β, transforming growth factor-beta].