Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maria João Matos | + 1871 word(s) | 1871 | 2021-11-19 02:44:55 | | | |
| 2 | Yvaine Wei | Meta information modification | 1871 | 2021-11-19 10:59:26 | | | | |
| 3 | Yvaine Wei | Meta information modification | 1871 | 2021-11-25 04:01:08 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Matos, M.J. 3-Phenylcoumarins as a Privileged Scaffold in Medicinal Chemistry. Encyclopedia. Available online: https://encyclopedia.pub/entry/16190 (accessed on 27 February 2026).
Matos MJ. 3-Phenylcoumarins as a Privileged Scaffold in Medicinal Chemistry. Encyclopedia. Available at: https://encyclopedia.pub/entry/16190. Accessed February 27, 2026.
Matos, Maria João. "3-Phenylcoumarins as a Privileged Scaffold in Medicinal Chemistry" Encyclopedia, https://encyclopedia.pub/entry/16190 (accessed February 27, 2026).
Matos, M.J. (2021, November 19). 3-Phenylcoumarins as a Privileged Scaffold in Medicinal Chemistry. In Encyclopedia. https://encyclopedia.pub/entry/16190
Matos, Maria João. "3-Phenylcoumarins as a Privileged Scaffold in Medicinal Chemistry." Encyclopedia. Web. 19 November, 2021.
Copy Citation
3-Phenylcoumarins are a family of heterocyclic molecules that are widely used in both organic and medicinal chemistry. 3-Phenylcoumarins have been used by several research groups in the search for new chemical entities with potential in the discovery of new therapeutic solutions for several diseases. The versatility and chemical properties of this scaffold have been attracting the attention of researchers all over the world.
3-phenylcoumarins
1. Introduction
The versatility and chemical properties of 3-Phenylcoumarins have been attracting the attention of researchers all over the world. Different molecular spots that may be modified, with different reactivities, allow for a huge number of different derivatives with different properties. This scaffold can be considered an isostere of the isoflavone in which the carbonyl group is translated from position 4 to position 2 on the pyran ring (Figure 1). Isoflavones are produced almost exclusively by the members of the bean family Fabaceae (Leguminosae). It can also be considered a coumarin-resveratrol hybrid. Resveratrol (3,5,4-trihydroxy-trans-stilbene) is a stilbenoid, a type of natural phenol, and a phytoalexin produced by several plants (Figure 1). The stilbenoids share most of their biosynthetic pathway with chalcones.
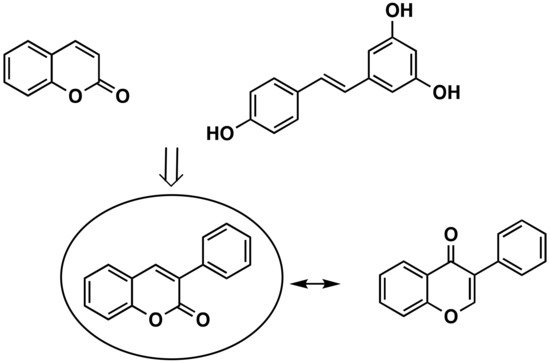
Figure 1. Coumarin, trans-resveratrol, 3-phenylcoumarin and isoflavone chemical structures.
Coumarins, the basic structure of 3-phenylcoumarins, are a group of substances of natural or synthetic origin that are highly studied and have a great variety of pharmacological interests. In addition, a connection of 3-phenylcoumarins can also be established (although it is more from a structural or steric point of view) with steroid hormones, especially with estrogens, due to the aromatization of the A ring. For these reasons, 3-phenylcoumarins (Figure 1) are considered a privileged scaffold in medicinal chemistry.
2. Presence of 3-Phenylcoumarins in Nature
The naturally-occurring 3-phenylcoumarins that have been published in the past decade are listed in Table 1. Mucodianin A was isolated from the vine stems of Mucuna birdwoodiana [1]. The 3-(4-ethynylphenyl)-4-formylcoumarin has been isolated from a methanol extract of the red ants of ChangBai Mountain, Tetramorium sp. [2]. Pterosonin F was isolated from the heartwood of Pterocarpus soyauxii [3]. Sphenostylisin A was isolated from the root bark of Sphenostylis marginata using a bioactivity-guided isolation approach. It is worth highlighting that this compound is a potent NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) inhibitor, displaying different physiological functions. This compound is also overexpressed in some cancer cells [4]. 2′,4′-Dinitro-3-phenylcoumarin was isolated from Rhizophora mucronata [5]. Selaginolide A was found in Selaginella rolandi-principis [6]. Glycycoumarin and licorylcoumarin (Figure 2) are two 3-phenylcoumarins previously isolated from Glycyrrhiza uralensis or glabra (Licorice), on which there are several studies in the past decade [7][8].
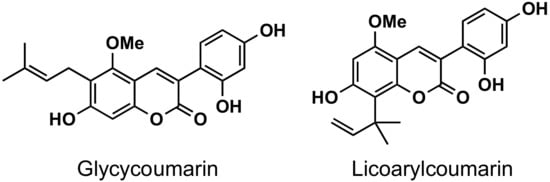
Figure 2. Chemical structures of glycycoumarin and licoarylcoumarin.
Table 1. Naturally-occurring 3-phenylcoumarins identified in the past decade.
| Compound | Origin | Reference |
|---|---|---|
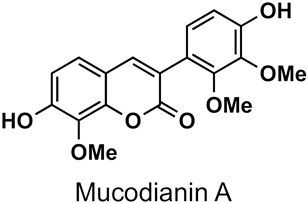 |
Mucuna birdwoodiana | [1] |
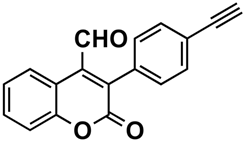 |
Tetramorium sp. | [2] |
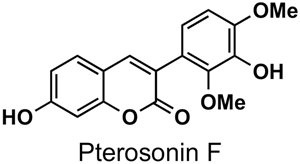 |
Pterocarpus soyauxii | [3] |
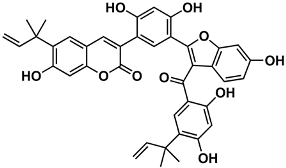 |
Sphenostylis marginata | [4] |
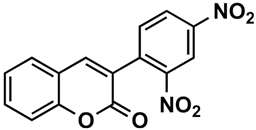 |
Rhizophora mucronata | [5] |
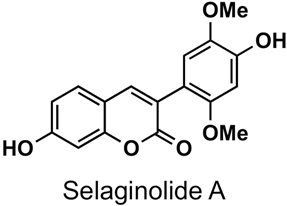 |
Selaginella rolandi-principis | [6] |
3. Pharmacological Interest of 3-Arylcoumarins
3.1. Alzheimer’s Disease and AChE
Several series of simple 3-phenylcoumarins have been studied to prevent and treat Alzheimer’s disease and its complications, showing high activity toward AChE and MAO, together with antioxidant activity (Figure 3). Among a studied series of polyhydroxy 3-phenylcoumarins, 3-(3′,4′-dihydroxyphenyl)-7,8-dihydroxycoumarin (1) stands out with IC50 inhibition values 3 μM and 27 μM against AChE and MAO-B, respectively [9]. This research group also studied a series of benzamide derivatives at the position 4′, resulting in the compound 2, the best of the series, with an AChE inhibition IC50 of 0.09 μM.
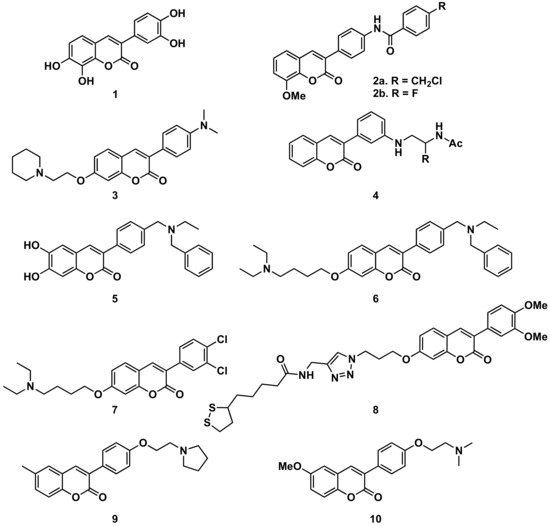
Also, from 3-(3′-aminophenyl)coumarin (Ki = 146 μM), a non-peptidic drug-like β-secretase 1 (BACE-1) inhibitor, the hit fragment containing the N-acylated ethane-1,2-diamine motif has been identified as a directing probe to pick inhibitory fragments for the S1 pocket of the aspartic protease BACE-1, leading to the most interesting compound (compound 4, with a biphenylmethyl residue) with a Ki of 3.7 μM [10]. Separating the amine from the benzene ring at position 3 of the 3-phenylcoumarins by a methylene group proved to be an interesting strategy to obtain more active compounds. When this amine is a bulky amine as a N,N-dibenzyl(N-ethyl)amine fragment, several compounds of a studied series proved to act on three relevant targets in Alzheimer’s disease: σ-1 receptor (σ1R), BACE1 and AChE. These also show potent neurogenic properties, good antioxidant capacity and favorable central nervous system (CNS) permeability. Compound 5 must be highlighted within the series [11]. Compounds with the same substitution at position 4′, and a 7-aminoalkoxy chain, formed a series of products presenting multitarget interest for the treatment of the middle stage of Alzheimer’s disease. A non-neurotoxic dual AChE/BuChE inhibitor, compound 6, which is also a nanomolar human AChE inhibitor, turned out to be a significant inhibitor of Aβ42 self-aggregation activity, being also a promising neuroprotective agent [12]. A series of compounds with 7-aminoalkoxy-3-phenylcoumarins, presenting simple substitutions on the phenyl at position 3, were studied, identifying compound 7 as the most potent compound against AChE (IC50 = 0.27 μM). Kinetic and molecular modelling studies proved that compound 7 works in a mixed-type approach, and interacts concomitantly with the catalytic active site (CAS) and the peripheral anionic site (PAS) of AChE. In addition, compound 7 blocks β-amyloid (Aβ) self-aggregation with a ratio of 44% at 100 μM, and significantly protects rat pheochromocytoma (PC12) cells from hydrogen peroxide (H2O2)-damage in a dose-dependent way [13]. The 7-substitution of 3-phenylcoumarins has also been used as a building block for a novel series of coumarin-lipoic acid conjugates, resulting in compound 8, the most potent AChE inhibitor, showing a good inhibitory effect on Aβ-aggregation and intracellular ROS formation, as well as the ability of selective bio-metal chelation and neuroprotection against H2O2- and Aβ1-42-induced cytotoxicity [14][15]. Interesting to note is the comparison between two compounds prepared in a study on Alzheimer’s disease, being 6-substituted 3-arylcoumarins. Compounds 9 and 10, with the substituents in the same positions, and small structural differences between them, do not offer great differences in their activities and selectivity against cholinesterases or towards the inhibition of self-induced Aβ42-aggregation; however, they do offer important differences in selectivity against human MAO-A and MAO-B which are worthy of further study [16].

Figure 3. 3-Phenylcoumarins in Alzheimer’s disease: AChE inhibitors.
3.2. Inflammation
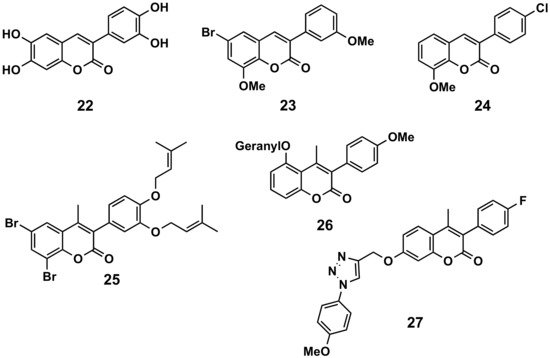
In a comparative study, 6,7-disubstituted compounds proved to down-modulate the Fc-gamma (Fcγ) receptor-mediated neutrophil oxidative metabolization more efficiently than compounds disubstituted at positions 5 and 7, and hydroxylated compounds down-modulated this function more strongly than their acetylated counterparts. The most interesting compound turned out to be compound 22 and, even better, their 3′,4′-methylenedioxyphenyl derivative, which may be a prototype for the development of novel immunomodulating drugs to treat immune complex-mediated inflammatory diseases (Figure 4) [17]. A series of 34 3-phenylcoumarins has been evaluated in lipopolysaccharide-activated mouse macrophage RAW264.7 cells. 6-Bromo-8-methoxy-3-(3′-methoxyphenyl)coumarin (23), a dimethoxybromine derivative, exhibited nitric oxide production inhibitory activity, with an IC50 of 6.9 μM [18]. Some simple derivatives such as the 3-(4-chlorophenyl)-8-methoxycoumarin (24) showed interesting soybean lipoxygenase inhibitory activities due to the importance they may have in anti-inflammatory processes [19]. Prenyloxycoumarin 25 displayed the best combined inhibition of lipid peroxidation (100%) and soybean lipoxygenase (IC50 = 37 μM) amongst the studied series of coumarins and thiocoumarins [20]. The geranyloxy-derived compound 26 also exhibited good soybean lipoxygenase inhibition (IC50 = 10 μM) [21]. Finally, searching for anti-inflammatory activities, but also antimicrobial and antioxidant properties, a series of 3-phenylcoumarins with a 1,2,3-triazole 1,4-disubstituted residue attached by an ether to the position 7 of the scaffold, has been prepared. Compound 27 proved to be the most interesting pharmacological profile, with an IC50 of 15.78 μM (by the egg-albumin), slightly higher than diclofenac (IC50 = 17.52 μM) [22].

Figure 4. 3-Phenylcoumarins in inflammation.
3.3. Oxidation
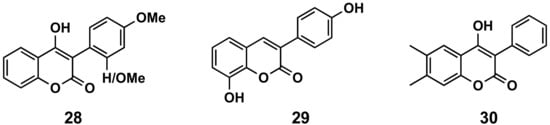
2′- or 4′-Methoxy derivatives of 4-hydroxy-3-phenylcoumarins (compound 28, Figure 5) have shown higher antioxidant capacity than 4-hydroxycoumarin, measuring their capacity to scavenge two different radicals: 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azinobis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS). These molecules also display protecting effects towards the β-carotene-linoleic acid co-oxidation enzymically induced by lipoxygenase [23]. From a series of hydroxylated 3-phenylcoumarin derivatives [24][25], 8-hydroxy-3-(4′-hydroxyphenyl)coumarin (29, Figure 14) has been the most interesting molecule, showing an oxygen radical absorbance capacity fluorescein (ORAC-FL) of 13.5, capacity of scavenging hydroxyl radicals of 100%, capacity of scavenging DPPH radicals of 65.9% and capacity of scavenging superoxide radicals of 71.5%, as well as being a potential candidate for preventing or minimizing the free radicals’ overproduction in oxidative-stress related diseases [24]. Simple 3-phenylcoumarins (in many cases associated with other cycles) have been identified as inhibitors of the function of nicotinamide adenine dinucleotide phosphate (NADPH) and quinone reductase (NQO1), with multiple cellular functions as detoxifying enzymes, as well as chaperone proteins. These compounds proved to be even more potent and less toxic than dicoumarol, and more efficient for inhibiting the toxicity of chemotherapeutic drug EO9 [26][27]. 4-Hydroxy-6,7-dimethyl-3-phenylcoumarin (30, Figure 14) stands out with an IC50 of 660 nM in the presence of bovine serum albumin (BSA) [27]. Finally, QSAR predictive models for antioxidant activity of new coumarin derivatives have also been described as an interesting tool in drug discovery [28].

Figure 5. 3-Phenylcoumarins in oxidation.
3.4. Cardiovascular Diseases
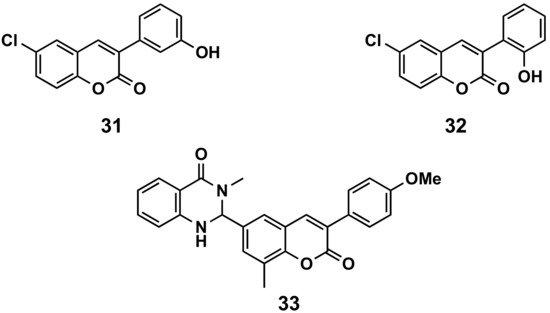
A series of 6-halo-3-hydroxyphenylcoumarins has been evaluated for their vasorelaxation activity in intact rat aorta rings pre-contracted with phenylephrine, as well as for their inhibitory effects on platelet aggregation induced by thrombin in washed human platelets (Figure 6). These compounds proved to relax the vascular smooth muscle in a concentration-dependent manner. Compound 31 presents an IC50 of 36.6 μM against a concretion induced by phenylephrine. Some of the compounds showed a platelet antiaggregatory activity that was up to 30 times higher than that shown by trans-resveratrol, used as control, i.e., compound 32 (IC50 = 6.41 μM) [29]. The niacin receptor 1 (GPR109a) is a receptor that inhibits lipolytic and atherogenic activity and induces vasodilatation. A series of coumarin-dihydroquinazolinone conjugates has been evaluated for its agonist potential, displaying, in compound 33, robust agonist action to GPR109a with an EC50 < 11 nM. Further, the efficacy of the active compound has been corroborated by in vivo assays, showing the animals reduced body weight in a diet-induced obese mice model. Compound 33 proved to reduce leptin in blood plasma and total serum cholesterol [30].

Figure 6. 3-Phenylcoumarins in cardiovascular diseases.
4. Other Interests: Fluorescent Probes
The 3-phenylcoumarins are a privileged scaffold not only for their interesting and varied specific pharmacological activities, but also for other physicochemical properties and, in particular, for their potential as fluorescence probes. Due to the interesting and large number of studies that exist on this in recent years, they deserve an independent review. In the current overview, very succinct examples have been selected which are considered the most significant. Fluorescent biosensors have been developed to enable imaging and monitoring of a variety of metabolites and cellular events. This can be done by direct visualization and analysis; however, 3-phenylcoumarins also offer enormous possibilities in biorthogonal fluorogenic reactions, which allow not only the visualization of a fixed situation but also, in many cases, to follow the transformations throughout complex metabolic processes [31][32]. These compounds can be useful as fluorescent biosensors for the detection of hydroxyl radicals, and therefore can be potentially applied in the diagnosis of oxidative stress in the human body [33], metal cations such as Fe3+ [34] or anions such as carbonate [35]. 3-Phenylcoumarins can be used to analyze and study different biochemical compounds such as flavin [36][37] or anatomical/physiological states, such as the state of neuronal myelination [38], histamine released by mast cells [39], access to mitochondria [40] and others, which can be identified very directly with some metabolic problem, which in turn can be linked to a disease or disorder. On occasion, this may allow or facilitate the study of new pharmacological agents in oxidation/reduction processes [41], but also in more specific processes such as estrogen receptors and breast cancer [42], bioinorganic anticancer compounds [43], MAOs [44], etc. For these functions, they can also be associated with other groups or molecules, as there are examples with fluorenes or xanthenes [45], tetrazines [46], rhodamine [47] or with different polymers [48].
References
- Gong, T.; Wang, D.X.; Yang, Y.; Liu, P.; Chen, Y.; Yu, D.Q. A novel 3-arylcoumarin and three new 2-arylbenzofurans from Mucuna birdwoodiana. Chem. Pharm. Bull. 2010, 58, 254–256.
- Song, Z.W.; Liu, P.; Yin, W.P.; Jiang, Y.L.; Ren, Y.L. Isolation and identification of antibacterial neo-compounds from the red ants of ChangBai Mountain, Tetramorium sp. Bioorg. Med. Chem. Lett. 2012, 22, 2175–2181.
- Su, Z.; Wang, P.; Yuan, W.; Li, S. Flavonoids and 3-arylcoumarin from Pterocarpus soyauxii. Planta Med. 2013, 79, 487–491.
- Li, J.; Pan, L.; Deng, Y.; Muñoz-Acuña, U.; Yuan, C.; Lai, H.; Chai, H.; Chagwedera, T.E.; Farnsworth, N.R.; de Blanco, E.J.C.; et al. Bioactive Modified Isoflavonoid Constituents of the Root Bark of Sphenostylis marginata ssp. erecta. J. Org. Chem. 2013, 78, 10166–10177.
- Asbin, M.X.; Syed, A.M.; Anuradha, V.; Moorthi, M. Identification of bioactive compounds from Rhizophora mucronata methanolic leaf extract by GC-MS analysis. Int. J. Pharm. Sci. Res. 2020, 11, 5268–5272.
- Nguyen, D.T.; To, D.C.; Tran, T.T.; Tran, M.H.; Nguyen, P.H. PTP1B and α-glucosidase inhibitors from Selaginella rolandi-principis and their glucose uptake stimulation. J. Nat. Med. 2021, 75, 186–193.
- El-Saber, B.G.; Beshbishy, A.M.; El-Mleeh, A.; Abdel-Daim, M.M.; Devkota, H.P. Traditional uses, bioactive chemical constituents, and pharmacological and toxicological activities of Glycyrrhiza glabra L. (fabaceae). Biomolecules 2020, 10, 352.
- Zhang, E.; Yin, S.; Zhao, S.; Zhao, C.; Yan, M.; Fan, L.; Hu, H. Protective effects of glycycoumarin on liver diseases. Phytother. Res. 2020, 34, 1191–1197.
- Jie, Y.; Pingping, Z.; Yuheng, H.; Teng, L.; Jie, S.; Xiaojing, W. Synthesis and biological evaluation of 3-arylcoumarins as potential anti-Alzheimer’s disease agents. J. Enzym. Inhib. Med. Chem. 2019, 34, 651–656.
- Fernández-Bachiller, M.I.; Horatscheck, A.; Lisurek, M.; Rademann, J. Alzheimer’s Disease: Identification and Development of β-Secretase (BACE-1) Binding Fragments and Inhibitors by Dynamic Ligation Screening (DLS). ChemMedChem 2013, 8, 1041–1056.
- Tarozzi, A.; Bartolini, M.; Piazzi, L.; Valgimigli, L.; Amorati, R.; Bolondi, C.; Djemil, A.; Mancini, F.; Andrisano, V.; Rampa, A. From the dual function lead AP2238 to AP2469, a multi-target-directed ligand for the treatment of Alzheimer’s disease. Pharmacol. Res. Perspect. 2014, 2, e00023.
- Montanari, S.; Bartolini, M.; Neviani, P.; Belluti, F.; Gobbi, S.; Pruccoli, L.; Tarozzi, A.; Falchi, F.; Andrisano, V.; Miszta, P.; et al. Multitarget Strategy to Address Alzheimer’s Disease: Design, Synthesis, Biological Evaluation, and Computational Studies of Coumarin-Based Derivatives. ChemMedChem 2016, 11, 1296–1308.
- Abdshahzadeh, H.; Golshani, M.; Nadri, H.; Saberi Kia, I.; Abdolahi, Z.; Forootanfar, H.; Ameri, A.; Tueylue, K.T.; Ayazgok, B.; Jalili-Baleh, L.; et al. 3-aryl coumarin derivatives bearing aminoalkoxy moiety as multi-target-directed ligands against Alzheimer’s disease. Chem. Biodivers. 2019, 16, e1800436.
- Jalili-Baleh, L.; Forootanfar, H.; Tüylü Küçükkılınç, T.; Nadri, H.; Abdolahi, Z.; Ameri, A.; Jafari, M.; Ayazgok, B.; Baeeri, M.; Mahban Rahimifard, M.; et al. Design, synthesis and evaluation of novel multi-target-directed ligands for treatment of Alzheimer’s disease based on coumarin and lipoic acid scaffolds. Eur. J. Med. Chem. 2018, 152, 600–614.
- Theodosis-Nobelos, P.; Papagiouvannis, G.; Tziona, P.; Rekka, E.A. Lipoic acid. Kinetics and pluripotent biological properties and derivatives. Mol. Biol. Rep. 2021, 48, 6539–6550.
- Wang, Z.M.; Li, X.M.; Xue, G.M.; Xu, W.; Wang, X.B.; Kong, L.Y. Synthesis and evaluation of 6-substituted 3-arylcoumarin derivatives as multifunctional acetylcholinesterase/monoamine oxidase B dual inhibitors for the treatment of Alzheimer’s disease. RSC Adv. 2015, 5, 104122–104137.
- Andrade, M.F.; Kabeya, L.M.; Bortot, L.O.; dos Santos, G.B.; Santos, E.O.L.; Albiero, L.R.; Figueiredo-Rinhel, A.S.G.; Carvalho, C.A.; Azzolini, A.E.C.S.; Caliri, A.; et al. The 3-phenylcoumarin derivative 6,7-dihydroxy-3--coumarin down-modulates the FcγR- and CR-mediated oxidative metabolism and elastase release in human neutrophils: Possible mechanisms underlying inhibition of the formation and release of neutrophil extracellular traps. Free Radic. Biol. Med. 2018, 115, 421–435.
- Pu, W.; Lin, Y.; Zhang, J.; Wang, F.; Wang, C.; Zhang, G. 3-Arylcoumarins: Synthesis and potent anti-inflammatory activity. Bioorg. Med. Chem. Lett. 2014, 24, 5432–5434.
- Rahmani-Nezhad, S.; Khosravani, L.; Saeedi, M.; Divsalar, K.; Firoozpour, L.; Pourshojaei, Y.; Sarrafi, Y.; Nadri, H.; Moradi, A.; Mahdavi, M.; et al. Synthesis and Evaluation of Coumarin-Resveratrol Hybrids as 15-Lipoxygenase Inhibitors. Synth. Commun. 2015, 45, 741–749.
- Roussaki, M.; Zelianaios, K.; Kavetsou, E.; Hamilakis, S.; Hadjipavlou-Litina, D.; Kontogiorgis, C.; Liargkova, T.; Detsi, A. Structural modifications of coumarin derivatives: Determination of antioxidant and lipoxygenase (LOX) inhibitory activity. Bioorg. Med. Chem. 2014, 22, 6586–6594.
- Kavetsou, E.; Katopodi, A.; Argyri, L.; Chainoglou, E.; Pontiki, E.; Hadjipavlou-Litina, D.; Chroni, A.; Detsi, A. Novel 3-aryl-5-substituted-coumarin analogues: Synthesis and bioactivity profile. Drug Dev. Res. 2020, 81, 456–469.
- Dharavath, R.; Nagaraju, N.; Reddy, M.R.; Ashok, D.; Sarasija, M.; Vijjulatha, M.; Vani, T.; Jyothi, K.; Prashanthi, G. Microwave-assisted synthesis, biological evaluation and molecular docking studies of new coumarin-based 1,2,3-triazoles. RSC Adv. 2020, 10, 11615–11623.
- Rodríguez, S.A.; Nazareno, M.A.; Baumgartner, M.T. Effect of different C3-aryl substituents on the Antioxidant activity of 4-hydroxycoumarin derivatives. Bioorg. Med. Chem. 2011, 19, 6233–6238.
- Matos, M.J.; Pérez-Cruz, F.; Vazquez-Rodriguez, S.; Uriarte, E.; Santana, L.; Borges, F.; Olea-Azar, C. Remarkable antioxidant properties of a series of hydroxy-3-arylcoumarins. Bioorg. Med. Chem. 2013, 2, 3900–3906.
- Matos, M.J.; Mura, F.; Vazquez-Rodriguez, S.; Borges, F.; Santana, L.; Uriarte, E.; Olea-Azar, C. Study of coumarin-resveratrol hybrids as potent antioxidant compounds. Molecules 2015, 20, 3290–3308.
- Nolan, K.A.; Scott, K.A.; Barnes, J.; Doncaster, J.; Whitehead, R.C.; Stratford, I.J. Pharmacological inhibitors of NAD(P)H quinone oxidoreductase, NQO1: Structure/activity relationships and functional activity in tumour cells. Nolan. Biochem. Pharmacol. 2010, 80, 977–981.
- Scott, K.A.; Barnes, J.; Whitehead, R.C.; Stratford, I.J.; Nolan, K.A. Inhibitors of NQO1: Identification of compounds more potent than dicoumarol without associated off-target effects. Biochem. Pharmacol. 2011, 81, 355–363.
- Erzincan, P.; Saçan, M.T.; Yüce-Dursun, B.; Daniş, Ö.; Demir, S.; Erdem, S.S.; Ogan, A. QSAR models for antioxidant activity of new coumarin derivatives. SAR QSAR Environ. Res. 2015, 26, 721–737.
- Quezada, E.; Delogu, G.; Picciau, C.; Santana, L.; Podda, G.; Borges, F.; García-Morales, V.; Viña, D.; Orallo, F. Synthesis and Vasorelaxant and Platelet Antiaggregatory Activities of a New Series of 6-Halo-3-phenylcoumarins. Molecules 2010, 15, 270–279.
- Singh, L.R.; Kumar, A.; Upadhyay, A.; Gupta, S.; Palanati, G.R.; Sikka, K.; Siddiqi, M.I.; Yadav, P.N.; Sashidhara, K.V. Discovery of coumarin-dihydroquinazolinone analogs as niacin receptor 1 agonist with in-vivo anti-obesity efficacy. Eur. J. Med. Chem. 2018, 152, 208–222.
- Cambray, S.; Bandyopadhyay, A.; Gao, J. Fluorogenic diazaborine formation of semicarbazide with designed coumarin derivatives. Chem. Commun. 2017, 53, 12532–12535.
- Meimetis, L.G.; Carlson, J.C.T.; Giedt, R.J.; Kohler, R.H.; Weissleder, R. Ultrafluorogenic Coumarin-Tetrazine Probes for Real-Time Biological Imaging. Angew. Chem. Int. Ed. 2014, 53, 7531–7534.
- Zhang, B.; Xu, L.; Zhou, Y.; Zhang, W.; Wang, Y.; Zhu, Y. Synthesis and activity of a coumarin-based fluorescent probe for hydroxyl radical detection. Luminescence 2020, 35, 305–311.
- Kaya, E.N.; Yuksel, F.; Ozpinar, G.A.; Bulut, M.; Durmus, M. 7-Oxy-3-(3,4,5-trimethoxyphenyl)coumarin substituted phthalonitrile derivatives as fluorescent sensors for detection of Fe3+ ions: Experimental and theoretical study. Sens. Actuators B Chem. 2014, 194, 377–388.
- Elmas, S.N.K.; Sukriye, N.; Ozen, F.; Koran, K.; Gorgulu, A.O.; Sadi, G.; Yilmaz, I.; Erdemir, S. Selective and sensitive fluorescent and colorimetric chemosensor for detection of CO3−2 anions in aqueous solution and living cells. Talanta 2018, 188, 614–622.
- Lee, D.N.; Bae, S.; Han, K.; Shin, I.S.; Kim, S.K.; Hong, J.I. Electrostatic Modification for Promotion of Flavin-Mediated Oxidation of a Probe for Flavin Detection. Chem. Eur. J. 2017, 23, 16078–16084.
- Lee, D.N.; Kim, E.; Lee, J.H.; Kim, J.S.; Kang, C.; Hong, J.I. Flavin-mediated photo-oxidation for the detection of mitochondrial flavins. Chem. Commun. 2016, 52, 13487–13490.
- Wang, C.; Wu, C.; Zhu, J.; Miller, R.H.; Wang, Y. Design, Synthesis, and Evaluation of Coumarin-Based Molecular Probes for Imaging of Myelination. J. Med. Chem. 2011, 54, 2331–2340.
- Oshikawa, Y.; Furuta, K.; Tanaka, S.; Ojida, A. Cell Surface-Anchored Fluorescent Probe Capable of Real-Time Imaging of Single Mast Cell Degranulation Based on Histamine-Induced Coordination Displacement. Anal. Chem. 2016, 88, 1526–1529.
- Yang, Z.; He, Y.; Lee, J.H.; Park, N.; Suh, M.; Chae, W.S.; Cao, J.; Peng, X.; Jung, H.; Kang, C.; et al. A Self-Calibrating Bipartite Viscosity Sensor for Mitochondria. J. Am. Chem. Soc. 2013, 135, 9181–9185.
- Hanthorn, J.J.; Haidasz, E.; Gebhardt, P.; Pratt, D.A. A versatile fluorescence approach to kinetic studies of hydrocarbon autoxidations and their inhibition by radical-trapping Antioxidants. Chem. Commun. 2012, 48, 10141–10143.
- Yang, L.; Hu, Z.; Luo, J.; Tang, C.; Zhang, S.; Ning, W.; Dong, C.; Huang, J.; Liu, X.; Zhou, H.B. Dual functional small molecule fluorescent probes for image-guided estrogen receptor-specific targeting coupled potent antiproliferative potency for breast cancer therapy. Bioorg. Med. Chem. 2017, 25, 3531–3539.
- Ali, M.; Dondaine, L.; Adolle, A.; Sampaio, C.; Chotard, F.; Richard, P.; Denat, F.; Bettaieb, A.; Le Gendre, P.; Laurens, V.; et al. Anticancer Agents: Does a Phosphonium Behave Like a Gold(I) Phosphine Complex? Let a “Smart” Probe Answer. J. Med. Chem. 2015, 58, 4521–4528.
- Zhang, Y.; Xu, Y.; Tan, S.; Xu, L.; Qian, X. Rapid and sensitive fluorescent probes for monoamine oxidases B to A at low concentrations. Tetrahedron Lett. 2012, 53, 6881–6884.
- Zhang, H.; Liu, X.; Gong, Y.; Yu, T.; Zhao, Y. Synthesis and characterization of SFX-based coumarin derivatives for OLEDs. Dye. Pigm. 2021, 185, 108969.
- Galeta, J.; Dzijak, O.J.; Dracinsky, M.; Vrabel, M. A Systematic Study of Coumarin–Tetrazine Light-Up Probes for Bioorthogonal Fluorescence Imaging. Chem. Eur. J. 2020, 26, 9945–9953.
- Ong, J.X.; Pang, V.Y.T.; Tng, L.M.; Ang, W.H. Pre-Assembled Coumarin-Rhodamine Scaffold for Ratiometric Sensing of Nitric Oxide and Hypochlorite. Chem. Eur. J. 2018, 24, 1870–1876.
- Ye, D.; Wang, L.; Li, H.; Zhou, J.; Cao, D. Synthesis of coumarin-containing conjugated polymer for naked-eye detection of DNA and cellular imaging. Sens. Actuators B Chem. 2013, 181, 234–243.
More
Information
Subjects:
Others; Chemistry, Medicinal
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Revisions:
3 times
(View History)
Update Date:
25 Nov 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





