Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | ALZAIDI MOHAMMED AWAD MOHAMMED AWAD | + 3433 word(s) | 3433 | 2021-11-12 09:29:47 | | | |
| 2 | Jessie Wu | + 49 word(s) | 3482 | 2021-11-19 10:12:29 | | | | |
| 3 | Jessie Wu | + 49 word(s) | 3482 | 2021-11-19 10:17:29 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Awad, A.M.A. Plant Extracts as Natural Antioxidants in Meat. Encyclopedia. Available online: https://encyclopedia.pub/entry/16170 (accessed on 07 February 2026).
Awad AMA. Plant Extracts as Natural Antioxidants in Meat. Encyclopedia. Available at: https://encyclopedia.pub/entry/16170. Accessed February 07, 2026.
Awad, Alzaidi Mohammed Awad. "Plant Extracts as Natural Antioxidants in Meat" Encyclopedia, https://encyclopedia.pub/entry/16170 (accessed February 07, 2026).
Awad, A.M.A. (2021, November 18). Plant Extracts as Natural Antioxidants in Meat. In Encyclopedia. https://encyclopedia.pub/entry/16170
Awad, Alzaidi Mohammed Awad. "Plant Extracts as Natural Antioxidants in Meat." Encyclopedia. Web. 18 November, 2021.
Copy Citation
Plant extracts are rich in various bioactive compounds exerting antioxidants effects, such as phenolics, catechins, flavonoids, quercetin, anthocyanin, tocopherol, rutin, chlorogenic acid, lycopene, caffeic acid, ferulic acid, p-coumaric acid, vitamin C, protocatechuic acid, vitamin E, carotenoids, β-carotene, myricetin, kaempferol, carnosine, zeaxanthin, sesamol, rosmarinic acid, carnosic acid, and carnosol.
Natural Antioxidants
Plant Extracts
1. Introduction
The meat industry’s focus is shifting towards antioxidants that are novel, efficient, economical, green, or natural alternatives to potentially harmful synthetic preservatives. There has been an ever-increasing effort by the meat industry to explore novel, effective and edible antioxidants and antimicrobials compounds obtained from natural sources. Recently, there have been renewed interests in applying natural compounds (plant extracts, herbs, spices) in food preservation. Since ancient times, these additives have been added to foods as flavorings/seasonings, folk medicine, and food preservatives owing to antioxidant, bacteriostatic or bactericidal properties of polyphenolic compounds, and essential oils. The consumption of these compounds is known to exert beneficial effects on consumer’s health in addition to their preservative role. These herbs, spices, essential oils, or plant extracts exhibit various protective actions against the occurrence of diseases, and thus are widely used as potential green alternatives to food additives, preservatives, and dietary supplements.
Natural antioxidants are safe to consume, available in large quantities at a relatively lower price than their synthetic counterparts, and can be extracted and applied in the meat industry [1]. These compounds are abundantly present in the vegetable kingdom, such as in spices, herbs, and essential oils, with their concentration varying in throughout a plant, for example bark in the cinnamon and arjuna trees, the roots of liquorice, rosemary, cloves, and grape seeds, and the leaves of oregano and tea. Natural antioxidants are recognized as nutraceutical ingredients or supplements that can be used for raw meat and/or meat products [2]. The commonly used spices and herbs (such as oregano, rosemary, cinnamon, aniseed, fennel, basil, garlic, and ginger) are rich in phenolic compounds like phenolic acids, phenolic diterpenes, flavonoids, volatile oils, carnosic, caffeic, and chlorogenic acid. In their B rings, these polyphenols have 30–40 dihydroxy groups, and galloyl ester in the C rings of flavonoids can bind iron, making these compounds very potent antioxidants [3]. Phenolic compounds are lipid-soluble, owing to the hydroxyl group present in their chemical structure; they react with microbes’ cellular membranes, leading to loss of cell membrane integrity and, thus, antimicrobial effect. Fruits such as apple, plum, grape, pomegranate, and several types of berries, such as blueberries, cranberries, and bearberries, which are all good sources of antioxidants, owing to their high concentrations of flavonoids.
2. Plant Extracts as Natural Antioxidants
At present, various plant extracts are increasingly being explored in meat products as potential natural antioxidants for improving oxidative stability due to the potent antioxidant potential of these compounds in the meat matrix. The antioxidant attributes of these plant extracts are due to flavonoids (flavanol, flavones, anthocyanins), phenolic acid (hydroxybenzoic, hydroxycinnamic acids), diterpenes tannins (hydrolyzable and condensed tannins), stilbenes, coumarins, lignans, quinones, curcuminoids, and others including phenolic alkaloids, phenolic glycosides, phenolic terpenoids, and essential oil) [4][5]. Tea phenolics, such as epigallocatechin gallate (EGCG), have antioxidant potential in terms of their having electron reduction (550 mV) potential comparable to alpha-tocopherols or vitamin E (480 mV) [6].
The antioxidant potential of these plant extracts can be measured by assessing their free radical-scavenging ability or by measuring the compounds associated with lipid and protein peroxidation. The free radical-scavenging ability of the plant extract is measured spectrophotometrically by using stable radicals, such as 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical-scavenging activity or percent inhibition or 2-2-azinobis-3ethylbenthiazoline-6-sulphonic acid (ABTS) radical cation activity or percent inhibition, by measuring the ability of antioxidants to quench stable DPPH+ or ABTS+ radical cations, respectively, in ferric reducing antioxidant power (FRAP) by assessing the reduction of ferric-TPTZ (2,4,6-tri(2-pyridyl)-1,3,5-triazine (TPTZ) into ferrous from ferric thiocyanate assay (FTC), nitric oxide scavenging (NOS), aldehyde/carboxylic acid assay (ACA), superoxide scavenging, oxygen radical absorbance capacity (ORAC) and total antioxidant capacity (TAC). The antioxidant potential of the plant extract can also be measured by various compounds associated with lipid peroxidation (malonaldehyde formation, thiobarbituric acid reacting substances [TBARS], peroxide value [PV], free fatty acids [FFA]), protein oxidation (carbonyl formation, free thiols, conjugated diene) and beta carotene bleaching assay. Figure 1 depicts the antioxidant activity of the bioactive compounds extracted from plant biomass.
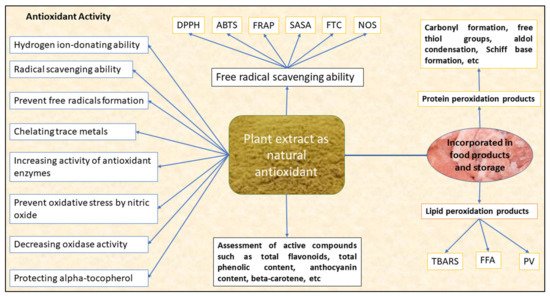
Figure 1. Overview of the antioxidant activity of the bioactive compounds extracted from a plant biomass source [7][8]. DPPH-1,1-diphenyl-2-picrylhydrazyl radical-scavenging activity, ABTS-2-2-azinobis-3ethylbenthiazoline-6-sulphonic acid radical cation activity, FRAP—ferric reducing antioxidant power, SASA-superoxide anion-scavenging ability, FTC—ferric thiocyanate assay, NOS—nitric oxide scavenging, TBARS-thiobarbituric acid-reacting substances, FFA—free fatty acids, PV—peroxide value.
3. Plant Extracts as Natural Antioxidants in Meat
The most common method of applying these extracts in meat processing is mixing them with water (mostly for water-soluble extracts; for organic solvent-assisted extracts, it is advised to mix in vegetable oil or fat) during preparation. It ensures the homogenous distribution of extracts in the product. The overall oxidative stability of products depends upon the concentration of the extracts; the higher the extract concentration, the greater the antioxidant effect. Phenolics, catechins, flavonoids, quercetin, anthocyanin, tocopherol, rutin, chlorogenic acid, lycopene, caffeic acid, ferulic acid, p-coumaric acid, vitamin C, protocatechuic acid, vitamin E, carotenoids, β-carotene, myricetin, kaempferol, chrysin, carnosine, zeaxanthin, sesamol, rosmarinic acid, chlorophyll, carnosic acid, carnosol, and gallic acid are compounds, present in plants, possessing antioxidant potential [9].
The important bioactive compounds present in various plant biomass and their meat application as natural antioxidants are described next.
Moringa oleifera a common vegetable in South Asian and African countries, is widely explored for its use as natural preservatives, owing to its various bioactive compounds viz. rhamnetin, isoquercitrin, kaempferol, kaempferitrin, saponins, triterpenoids, tannins, anthraquinones, alkaloids, and terpenoids [10], with concentration varying with the maturity of the plant and climatic and geographical conditions. M. oleifera is a rich source of protein, provitamins, vitamin C, A and E, zinc, calcium, iron, and potassium along with anti-cancerous agents such as glycerol-1-9-octadecanoate, glucosinolates, isothiocyanates, and glycoside compounds.
Rosemary contains a high amount of rosmarinic acid, carnosol, and carnosic acid in extract, and eucalyptol, α-pinene-bornyl acetate and camphor in rosemary essential oil [11][12]. Rosemary leaves were reported as a rich source of vitamin C, as much as as 18.51 g/100 g raw materials and extracts (0.26 mg/100 mL aqueous, 0.34 mg/100 mL alcoholic, and 0.36 mg/100 mL acetonic) have been reportedly obtained [13]. Monoterpenes hydrocarbons, esters, oxygenated sesquiterpenes, phenol, sesquiterpene hydrocarbons, oxygenated monoterpenes, ketones, and alcohol are the primary flavoring compounds of rosemary [12]. Generally, camphor, caryophyllene, borneol, bornyl acetate, and verbenone are chief compounds present in rosemary extracts. Peng et al. [14] advocated the application of supercritical fluids for the extraction of rosemary extracts, as it is associated with saving time, maximizing yield, and inhibiting the conversion of carnosic acid into carnosol. For rosemary stems, flowers, and leaves extracts, a suitable extraction methodology must be adopted, as lipophillic solvents and water (in the case of fresh samples were reported to result in a significant loss of phenols due to the action of phenoloxidase enzyme [15][16].
Arjuna or Arjun tree (Terminalia arjuna) bark, stem, leaves, roots, and fruits are a rich source of various beneficial bioactive compounds (polyphenols, flavonoids, triterpenoids, tannins, glycosides, sitosterol) and minerals. The Arjuna tree bark was reported to have the highest concentration of flavonoids such as arjunolone, quercetin, flavones, kaempferol, baicalein, and pelargonidin. Ethanolic and aqueous arjuna fruit extracts have been reported to contain a good amount of total phenolics (11.04–16.53 mg gallic acid equivalents/g), exerting significant scavenging activity (50.02–58.62%) [17].
Cinnamon bark is considered a promising antioxidant source, even exhibiting antioxidant potential comparable to synthetic antioxidants [18][19]. Most of the bark available in the market is Ceylon bark; dried bark (cork and cortex) of shoots of tree Cinnamomum zeylanicum F. lauraceae, containing approximately 0.5–1.0 % of volatile oil made up of 50.5% cinnamaldehyde, 8.7% cinnamic acid, methoxycinnamaldehyde and cinnamyl acetate, and 4.7% eugenol [18]. Chan et al. [20] reported ahigher antioxidant efficacy of deodorized cinnamon in meatballs, and noted significantly reduced lipid oxidation without causing any detrimental effects to its sensory attributes. Spices in the Lamiaceae family contain high rosmarinic acid, the major phenol (ranging from 1086–2563 mg/100 g of dry-weight) [21]. Rosmarinic acid possesses strong antioxidant activity due to two ortho-dihydroxy groups in its structure. This compound can act as an antioxidant and control the oxidation of low-density lipoproteins (LDLs). Rosmarinic acid also possesses anti-inflammatory activity by stimulating interleukin-10 (IL-10) secretion [22].
Several studies have confirmed the anti-inflammatory activity of oregano extracts, which are aqueous extracts observed to inhibit cyclooxygenase-2 (COX-2) secretion in epithelial carcinoma cells [23], and to exhibit anti-inflammatory properties by controlling stress-induced gastritis and hypersensitivity [24]. Oxidative stress leads to incorrect protein folding in the endoplasmic reticulum. Kaempferol, an aglycone flavonoid widely present in aloe vera, ivy gourd, saffron coccus and Peking spurge, is known to prevent hepatocellular carcinoma by controlling oxidative stress caused by reactive oxygen species [25]. Five bioflavonoids obtained from moss fern (Selaginella doederleinii) were observed to inhibit non-small cell lung cancer cells by suppressing XIAP and survivin expression, increasing the upregulation of caspase-3/cleaved-caspase-3, inducing cell apoptosis in A549 cells with low toxicity to non-cancer cells MRC-5 cells [26]. The Kalmia angustifolia extract exerted antioxidant, anti-inflammatory and anti-aging effects, at concentrations up to 200 μg/mL, by enhancing the expression of elastin and collagen-1 [27]. Flavonoids, such as apigenin, myricetin, and luteolin, were observed to exert anti-cancer effects against a range of human epithelial cancers by selectively reducing the viability of cancer cells, the alteration of ROS signaling, and the arrest of cell multiplication [28]. The extract of Polyalthia spp. is known to exert antioxidant, anti-ulcer, anti-plasmodial, anti-cancer, anti-microbial and anti-inflammatory effects due to the inhibition of COX-2 activity, inhibiting downstream prostaglandin E2 (PGE2) production, and inhibiting focal adhesion kinase, phosphoinositide 3-kinase, 3-hydroxy-3-methylglutaryl co-enzyme A reductase, and dipeptidyl peptidase 4 [29].
Clove exerts the most potent antioxidant activity among all spices and condiments commonly used in the food industry. Carnosic acid and carnosol, tocopherols, carotenoids, and sterols are important active ingredients present in herbs. Clove extracts mainly contain eugenol, caryophyllene, and eugenyl acetate. These two compounds have strong antioxidant potential and are considered the main bioactive compounds responsible for the increased antioxidant activity of clove, equivalent to vitamin E [30]. While processing, n-hexane solvent was recorded to produce a better yield, with high antioxidant activity (total flavonoid content- 15.54 mg GAE/g, total polyphenol content-54.05 mg GAE/g, FRAP = 0.69 mg/mL, DPPH = 0.29 mg/mL), and antimicrobial activity as compared with extracts obtained with other solvents (alcohol, water, and petroleum ether) [31]. Clove extract, at 0.25%, reported exerting antimicrobial activity with an 8.8 mm-to-9.27 mm minimum inhibitory concentration, as seen in S. typhimurium and E. coli, respectively [31].
Citrus byproducts/coproducts viz. peel and pulp are a rich source of various active ingredients, such as dietary fiber, minerals, vitamins, organic acids, flavonoids (ferulic, sinapic acids, and chlorogenic), phenolics (hesperetin, hesperidium, diosmin, and narirutin), carotenoids (carotene, zeaxanthin, lutein) [32][33][34][35]. These compounds are applied in the meat industry due to their associated health effects, such as antimicrobial, antioxidant, anticancer, anti-allergic, and antihypertensive effects [36]. The antioxidant activity of different citrus extracts varies with the methodology of their extraction, their fruit type, their environmental conditions, such as soil type and climate, their fruit-ripening stage, and their harvesting time [37][38]. Nayak et al. [39] reported the high antioxidant potential of Citrus sinensis peel extracts obtained by using microwave and ultrasound-assisted accelerated extraction technology at 337.16–433.09 mL/L DPPH (50% inhibition). Aqueous and methanolic extracts of lemon pomace had high antioxidant potential, wherein the methanolic extract exhibited higher antioxidant potential as compared with the aqueous extract, as measured in terms of DPPH assay (0.17, 0.13 mg Trolox equivalents/g, respectively) and ABTS (0.403, 0.458 mg Trolox equivalents/g, respectively) [40].
Catechin, present in green tea, is a group of flavonoids (flavan-3-ols), especially epicatechin, epicatechin-3-gallate, epigallocatechin (EGC), and epigallocatechin-3-gallate (EGCG), with EGC and EGCG regarded as the main active principle in green tea, and which is studied for its various antioxidant effects in food processing. The incorporation of tea catechins at 300 ppm in beef, duck, ostrich, pork, and chicken markedly reduced their TBARS values during refrigerated storage and was noted to exert 2–4 times higher antioxidant potential as compared with vitamin E [41]. Catechins reduced the production of putrescine and tyramine in dry, fermented pork sausage. Green tea catechins (GTC) and green coffee antioxidants (GCA) in linseed oil and fish oil, added in pork sausage at 200 mg/kg, decreased the lipid peroxidation of the sausages during seven days refridgerated storage. A significantly decreased lipid oxidation rate and higher organoleptic attributes were recorded in the fish- oil-substituted sausages [42].
Grape seed extract has a larege amount of polyphenols (such as epicatechin, gallic acid, resveratrol, and procyanidin dimers) and was reported to exert high antioxidant efficacy [43]. The antioxidant activity of grape seed is reported to be 20–50 times greater vitamins E and C [44], due to its possessing proanthocyanidins and oligomers of flavan-3-ol units. Grape seed extract (GSE) is the richest known source of polyphenolic compounds (catechins, flavanols, phenolic acids, anthocyanins, and proanthocyanidins) and has 20–50 times more antioxidant potential as compared with vitamins E and C, respectively [45][46]. Libera et al. [47] prepared grape seed extract by heating a grape seed slurry in water at 50–60 °C under high pressure (100–175 hPa or 10000–17500 bar). The authors evaluated the antioxidant potential of grape seed extract by incorporating it into pork neck containing Lactobacillus rhamnosus LOCK900 and reported that the lipid oxidation (PV-1% oleic acid and TBARS-0.46 mg MDA/kg) in the extract-incorporated samples was comparable to samples containing sodium ascorbate (PV-0.9% oleic acid and TBARS-0.53 mg MDA/kg).
The aqueous extract of Cantharellus cibarius hads high antioxidant potential due to its high concentrations of polyphenols, beta-carotene, and ascorbic acid, but has lower ABTS radical scavenging potency than vitamin C in cases where the extract is prepared by the water-decoction method i.e., first smashing and grinding, followed by boiling in water to obtain the extract [48][49]. The incorporation of Cantharellus cibarius water decoctions during the preparation of frankfurters, at 0.75- and 1.5% levels, resulted in significantly reducing the total plate counts with potent inhibitory action against Candida albicans, and improved the sensory attributes of the frankfurters during 60 days of storage under refrigerated conditions [49].
The ethanolic extract of mesquite leaf of Proposis was reported to exert high antioxidant potential due to its total phenolic content (278.5 mg GAE/g) and total flavonoid content (226.8 mg RE/g). The incorporation of the extract (0.05–0.10%) during the preparation of pork patties resulted in significantly improved oxidative stability of the treated patties as measured by theie marked reduction in TBARS value (90%) and conjugated dienes (40%) as compared with the positive control, i.e., patties with BHT [50]. The Carob (Ceratonia siliqua L.) tree is an underutilized tree of the Mediterranean region. Carob has high dietary fiber content and has 1.2–7.0% polyphenolic compounds, especially catechins, myricetin, rutin, and gallic acid [51].
The various plant extracts utilized in meat as natural antioxidants are presented in Table 1.
Table 1. Plant extracts as a natural antioxidant in meat.
| Plant Source | Extraction Protocol | Experimental Design (Level, Meat Product, Storage Temp, Days) | Significant Outcome (Extract Quality and Its Antioxidant Effect on Incorporation in Meat) |
Reference |
|---|---|---|---|---|
| Cinnamon barks | Ethanol (90%), 60 °C, 9 h | 0.25%, chevon rolls, 4 ± 1 °C, 35 days | Overall acceptability of treated rolls was higher than control, significantly (p < 0.05) lower TBARS, FFA, PV, SPC, and psychrophilic count | [52][53][54] |
| Papaya leaves | Ethanol (60%), 65 °C, 15 min | 0.5%, chevon emulsion, 4 ± 1 °C, 9 days | TBARS, FFA and PV (p < 0.05) higher in control than treatments | [55][56] |
| Terminalia arjuna bark | Ethanol (60%), 10 min at 75 °C | 1.0%, pork emulsion, 4 ± 1 °C, 9 days |
2.5-fold reduction in TBARS value than control (0.79 from 1.75 mg malonaldehyde/kg), better colour stability (L *, a *, b * values) |
[57][58] |
| Terminalia arjuna fruit | Ethanol-water (60:40), 27 °C ± 1 °C, overnight, vortex shaking at 400 rpm for 8 h | 1.0%, ground pork, 4 ± 1 °C, 9 days | Higher total phenolics (16.53 mg GAE/g), DPPH IC50—10.37 μg/mL, FRAP-1.33, Metmyoglobin content comparable to BHT added sample and significantly lower than control | [17] |
| Oregano vulgare leaves | Ethanol (60%), 80 °C, 10 min | 1.0%, chevon emulsion, 4 ± 1 °C, 9 days | Total phenolic content-328.71 mg GAE/100 g, SASA-44.49%, DPPH activity-30.72%, improving oxidative and microbial quality of chevon meat | [55] |
| Clove buds | Ethanol (95%), 12 h at 100 rpm, residue again re-extracted | 0.25%, 0.5%, 1.0%, 2.0%, Chinese-style sausage, 4 °C, 21 days | Concentration dependence effectiveness in controlling lipid and protein oxidation, better retention of textural and sensory attributes during storage | [59] |
| Watermelon rind | Ethanol (95%) 25 °C, 24 h at 200 rpm | 0.10%, pork patties, 4 ± 1 °C, 28 days | DPPH (% inhibition)-77.46, ABTS (% inhibition)-75.57, FRAP (mM of Fe++ equivalent/mL)-77.5 and SASA (% inhibition)-47.5; zone of inhibition for S. aureus-5.68 mm | [60] |
| Sea buckthorn seeds | Methanol (60%), 55 °C, 20 min | 0.30%, ground pork, 4 ± 1 °C, 9 days | TPC-128.23 mgGAE/g, DPPH-66.11% inhibition, ABTS-87.13% inhibition, significantly lower TBARS, FFA and PV in treated samples | [61] |
| Moringa oleifera leaves | Water for 18–20 h at 40–50 °C | 0.10%, goat meat patties, 4 ± 1 °C, 15 days | TPC-48.36 mgGAE/g, TFC-31.42 mg/g, Lower TBARS value on 15 th day of storage in treated sample-0.53 mg malonaldehyde/kg | [62] |
| Boiled distilled water, 5 min | 450–600 ppm, raw and cooked patties | TPC-60.78–70.27 mg/g, non-significant reduction in metmyoglobin formation in control and treated samples during storage | [62][63] | |
| Ginger rhizomes, potato peel, seeds of fenugreek | Ethanol (90%), room temperature, 1 h at freeze dried −60 °C | 500–1000 ppm, ground beef patties, 5, 25 & 37 °C, 12 days | Ginger rhizome extract has the highest antioxidant (% inhibition)-(77.4) followed by fenugreek seeds (71.4) and potato peel (59.5) | [64] |
| Garlic ginger and onion | Water, 40 °C,30 min, Ultrasonic extractor (200 W, 40 kHz) | 5–10% ginger-garlic-onion, stewed pork, 4 °C, 12 days | Synergistic effect of combinations of extracts, storage life extended to 5–6 days | [65] |
| Leaves of hyssop and rosemary | Dimethyl sulfoxide for 5 h at ambient temperature | Solution with 5.8 pH, cooked pork meat, 4 °C, 8 days | Hyssop and rosemary extract inhibit lipid oxidation and metmyoglobin formation | [66] |
| Leaves of myrtle, lemon balm, rosemary and nettle | De-ionized water ambient temperature, 15 min | 10% each extract, ground beef, 20 ± 2 °C, 120 days | Inhibited lipid oxidation (lemon and nettle-23–24% lower peroxide value; myrtle and rosemary-33–41%) and protected colour | [67] |
| Green tea and grape seed | Boiling water, 10 min | 500, 3000, 6000 ppm, Baladi goat meat, 5 °C, 9 days | Lower antioxidant capacity of green tea extract (7.5 h) than grape seed extract (9.4 h), plant extract increased the induction time | [68] |
| Red grape pomace | Methanol ambient temp, 10 min, sudden pressure changes to 5 × 103 Pa (N/m2), rotatory evaporator at 200 rpm at 50 °C | 0.06 g/100 g, pork burger, 4 °C, 6 days | TPC-546.0, total anthocyanins-1783.5 mg/L, antioxidant capacity-141.8 mmol/L Trolox, the application of instantaneous high-low pressure increased the extract yield | [69] |
| Wine residues | Aqueous acetone (50%), ambient temperature | 7–15 g/100 g, dried minced pork slice, room temperature, 21 days | Decreased hexanal, TBARS (up to 108%), carbonyls, sulfhydryl loss | [19] |
| Mustard leave kimchi | Ethanol (70%), room temperature, overnight | 0.05%, 0.1% & 0.2%, ground pork, 4 °C, 14 days | Extract at 0.1% and 0.2% having antioxidant effect equal to 0.02% ascorbic acid. MDA concentration below 0.5 mg/kg at the end of storage | [70] |
| Lotus rhizome knot (LRK) and leaf (LL) | Aqueous, room temperature, overnight | 3%, bovine and porcine meat, 4 °C, 10 days | TPC-(LRK-17.0 gGAE/100 g, LL-34.9 g GAE/100 g), TTC-(LRK-13.02 gGAE/100 g, LL-6.02 gGAE/100 g), TFC-(LRK-7.96 g rutin euivalent/100 g, LL-33.0 g rutin equivalent/100 g) | [71] |
| Curry berry | Boiled water for 2 h followed by centrifuge at 5000 rpm for 10 min | 2.5–5.0%, raw chicken meat homogenate, 4 ± 1 °C, 48 days | TPC-9.5 mg TAE/gdw, TFC-11.9 mgCE/gdw; the extract incorporation inhibited oxidative changes in meat | [72] |
| Lychee fruit pericarp | Boiled distilled water, 1 h | 0.50, 1.0 and 1.5%, sheep meat nuggets, 4 ± 1 °C, 12 days | TPC-18.36 mgGAE/g, high anthocyanins content, the extract has good antioxidant potential. | [73] |
| Byproducts of olive, pomegranate, tomato and grape | Water, 60 °C, 2.5 h | 0.1%, lamb patties, 4 ± 1 °C, 7 days | Water extracts exhibited antimicrobial and antioxidant potential, red grape and olive extract (1000 mg/kg) in patties reduced microbial counts | [74] |
| Bamboo shoot | Boiled water with 1% NaCl, 10 min | 6% kordoi juice and 4% aqueous extract, pork nuggets, 4 ± 1 °C, 35 days | TPC-246 mg GAC/100 g, Ascorbic acid-4.1 mg AAE/100 g, The incorporation of extract and kordoi juice extended storage life from 21 days to 35 days |
[75] |
| Colombian berry | Ethanol-water (50:50 v/v), solvent-solute ratio (5:1), 4 °C, lyophilized (0.18 bar, −50 °C) | 250, 500 and 750 ppm, pork patties, 2 ± 1 °C, 9 days, 15–20 lux value | TPC-83976 mg/kg, total anthocyanin content-29077.5 mg/kg, making upto 35%. Extract improved colour stability and oxidative stability in dose dependent manner. | [76] |
| Petals blue pea flower | Spray-dried, vacuum packaged | 0.02–0.16% w/w, pork patties, 4 ± 1 °C, 12 days | TPC-28.8 mgGAE/g, TEAC value of cooked patties-0.10–0.167 mg TE/g; Addition of 0.16% extract protect lipid and protein oxidation during storage | [77] |
| Bee pollen | Ethanol, 40 °C, 1 h, 150 rpm, lyophilized | 0.02%, pork sausage, 4 ± 1 °C, 30 days | TPC-19.69 mgGAE/g, 10 mg/mL can neutralise 91.93% of beta carotene. | [78] |
| Monkfruit | Water, 200 W ultrasound power, 80 °C, 2 h | 7–15 g/100 g, dried minced pork | 98.51% DPPH inhibition at 200 g/L, 34.93% mongroside in extract. Extract delayed hexanal formation, TBARS, carbonyls and sulphydryl loss | [19] |
| Jabuticaba | Water, 60 °C, 6 h, microencapsulated | 2–4%, fresh pork sausage | TPC-15.63 mg GAE/mg, FRAP-20.51μmol equivalent Trolox/g, Extract added fresh sausage as natural colorant had an antimicrobial and antioxidant effect |
[79] |
| Peanut skin |
Ethanol (80%), 60 °C, 50 min; followed by 15 min sonication at ambient temperature | 3.0%, chicken patties 1 ± 1 °C, 15 days | TPC-32.6 mg GAE/g, FRAP-of 26.5 μmol Trolox equivalent/g. Decreased a * values (p < 0.05) and reduced lipid oxidation, with 0.97 malondialdehyde (MDA)/kg as compared with 19 mg MDA/kg |
[80] |
TBARS-thiobarbituric acid reactive substances, FFA-free fatty acid, PV-peroxide vale, DPPH—1,1-diphenyl-2-picrylhydrazyl, ABTS-2-2-azinobis-3ethylbenthiazoline-6-sulphonic acid, GAE–gallic acid equivalent, SPC–standard plate count, MDA–malondialdehyde, FRAP—ferric reducing antioxidant power, SASA–superoxide anion radical scavenging assay, TPC–total flavonoid content, TFC–total flavonoid content, TTC–total tannin content, TAE—tannin acid equivalent, AAE—ascorbic acid equivalent, TEAC value–Trolox equivalence antioxidant capacity, * p < 0.05.
References
- Kumar, P.; Chatli, M.K.; Mehta, N.; Malav, O.P.; Verma, A.K.; Kumar, D.; Rathour, M. Antioxidant and antimicrobial efficacy of sapota powder in pork patties stored under different packaging conditions. Korean J. Food Sci. Anim. Resour. 2018, 38, 593.
- Jiang, J.; Xiong, Y.L. Natural antioxidants as food and feed additives to promote health benefits and quality of meat products: A review. Meat Sci. 2016, 120, 107–117.
- Chu, S.-C.; Chen, C. Effects of origins and fermentation time on the antioxidant activities of kombucha. Food Chem. 2006, 98, 502–507.
- Kumar, P.; Verma, A.K.; Umaraw, P.; Mehta, N.; Rajeev, R. Natural extracts are very promising: They are a novel green alternative to synthetic preservatives for the meat industry. Fleischwirtsch. Int. J. Meat Prod. Meat Process. 2020, 3, 48–57.
- Efenberger-Szmechtyk, M.; Nowak, A.; Czyzowska, A. Plant extracts rich in polyphenols: Antibacterial agents and natural preservatives for meat and meat products. Crit. Rev. Food Sci. Nutr. 2021, 61, 149–178.
- Dai, F.; Chen, W.-F.; Zhou, B. Antioxidant synergism of green tea polyphenols with alpha-tocopherol and L-ascorbic acid in SDS micelles. Biochimie 2008, 90, 1499–1505.
- Moon, J.-K.; Shibamoto, T. Antioxidant assays for plant and food components. J. Agric. Food Chem. 2009, 57, 1655–1666.
- Kumar, P.; Verma, A.K.; Umaraw, P.; Mehta, N.; Malav, O.P. Plant phenolics as natural preservatives in food system. In Plant Phenolics in Sustainable Agriculture; Lone, R., Shuab, R., Kamili, A.N., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 367–406. ISBN 978-981-15-4890-1.
- Lee, S.Y.; Lee, D.Y.; Kim, O.Y.; Kang, H.J.; Kim, H.S.; Hur, S.J. Overview of studies on the use of natural antioxidative materials in meat products. Food Sci. Anim. Resour. 2020, 40, 863–880.
- Singh, T.P.; Singh, P.; Kumar, P. Drumstick (Moringa oleifera) as a food additive in livestock products. Nutr. Food Sci. 2015, 45.
- Arranz, E.; Mes, J.; Wichers, H.J.; Jaime, L.; Mendiola, J.A.; Reglero, G.; Santoyo, S. Anti-inflammatory activity of the basolateral fraction of Caco-2 cells exposed to a rosemary supercritical extract. J. Funct. Foods 2015, 13, 384–390.
- Teixeira, B.; Marques, A.; Ramos, C.; Neng, N.R.; Nogueira, J.M.F.; Saraiva, J.A.; Nunes, M.L. Chemical composition and antibacterial and antioxidant properties of commercial essential oils. Ind. Crops Prod. 2013, 43, 587–595.
- Dumbrava, D.G.; Moldovan, C.; Raba, D.-N.; Popa, M.-V. Vitamin C, chlorophylls, carotenoids and xanthophylls content in some basil (Ocimum basilicum L.) and rosemary (Rosmarinus officinalis L.) leaves extracts. J. Agroaliment. Process. Technol. 2012, 18, 253–258.
- Peng, C.-H.; Su, J.-D.; Chyau, C.-C.; Sung, T.-Y.; Ho, S.-S.; Peng, C.-C.; Peng, R.Y. Supercritical fluid extracts of rosemary leaves exhibit potent anti-inflammation and anti-tumor effects. Biosci. Biotechnol. Biochem. 2007, 71, 2223–2232.
- Bellumori, M.; Michelozzi, M.; Innocenti, M.; Congiu, F.; Cencetti, G.; Mulinacci, N. An innovative approach to the recovery of phenolic compounds and volatile terpenes from the same fresh foliar sample of Rosmarinus officinalis L. Talanta 2015, 131, 81–87.
- Mulinacci, N.; Innocenti, M.; Bellumori, M.; Giaccherini, C.; Martini, V.; Michelozzi, M. Storage method, drying processes and extraction procedures strongly affect the phenolic fraction of rosemary leaves: An HPLC/DAD/MS study. Talanta 2011, 85, 167–176.
- Chauhan, P.; Pradhan, S.R.; Das, A.; Nanda, P.K.; Bandyopadhyay, S.; Das, A.K. Inhibition of lipid and protein oxidation in raw ground pork by Terminalia arjuna fruit extract during refrigerated storage. Asian Australas. J. Anim. Sci. 2019, 32, 265–273.
- Mathew, S.; Abraham, T.E. Studies on the antioxidant activities of cinnamon (Cinnamomum verum) bark extracts, through various in vitro models. Food Chem. 2006, 94, 520–528.
- Cheng, J.R.; Liu, X.M.; Zhang, Y.S.; Zhang, Y.H.; Chen, Z.Y.; Tang, D.B.; Wang, J.Y. Protective effects of Momordica grosvenori extract against lipid and protein oxidation-induced damage in dried minced pork slices. Meat Sci. 2017, 133, 26–35.
- Chan, K.W.; Khong, N.M.H.; Iqbal, S.; Ch’ng, S.E.; Younas, U.; Babji, A.S. Cinnamon bark deodorised aqueous extract as potential natural antioxidant in meat emulsion system: A comparative study with synthetic and natural food antioxidants. J. Food Sci. Technol. 2014, 51, 3269–3276.
- Shan, B.; Cai, Y.Z.; Sun, M.; Corke, H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J. Agric. Food Chem. 2005, 53, 7749–7759.
- Mueller, M.; Hobiger, S.; Jungbauer, A. Anti-inflammatory activity of extracts from fruits, herbs and spices. Food Chem. 2010, 122, 987–996.
- Lemay, M. Anti-inflammatory phytochemicals: In vitro and ex vivo evaluation. Phytochemicals 2006, 1, 41–60.
- Yoshino, K.; Higashi, N.; Koga, K. Antioxidant and antiinflammatory activities of oregano extract. J. Health Sci. 2006, 52, 169–173.
- Sharma, N.; Biswas, S.; Al-Dayan, N.; Alhegaili, A.S.; Sarwat, M. Antioxidant role of kaempferol in prevention of hepatocellular carcinoma. Antioxidants 2021, 10, 1419.
- Kang, F.; Zhang, S.; Chen, D.; Tan, J.; Kuang, M.; Zhang, J.; Zeng, G.; Xu, K.; Zou, Z.; Tan, G. Biflavonoids from Selaginella doederleinii as potential antitumor agents for intervention of non-small cell lung cancer. Molecules 2021, 26, 5401.
- Grenier, A.; Legault, J.; Pichette, A.; Jean, L.; Bélanger, A.; Pouliot, R. Antioxidant, anti-inflammatory, and anti-aging potential of a Kalmia angustifolia extract and identification of some major compounds. Antioxidants 2021, 10, 1373.
- Tavsan, Z.; Kayali, H.A. Flavonoids showed anticancer effects on the ovarian cancer cells: Involvement of reactive oxygen species, apoptosis, cell cycle and invasion. Biomed. Pharmacother. 2019, 116, 109004.
- Chen, Y.-C.; Chia, Y.-C.; Huang, B.-M. Phytochemicals from Polyalthia species: Potential and implication on anti-oxidant, anti-inflammatory, anti-cancer, and chemoprevention activities. Molecules 2021, 26, 5369.
- El-Maati, M.F.A.; Mahgoub, S.A.; Labib, S.M.; Al-Gaby, A.M.A.; Ramadan, M.F. Phenolic extracts of clove (Syzygium aromaticum) with novel antioxidant and antibacterial activities. Eur. J. Integr. Med. 2016, 8, 494–504.
- Ishaq, A.; Syed, Q.A.; Khan, M.I.; Zia, M.A. Characterising and optimising antioxidant and antimicrobial properties of clove extracts against food-borne pathogenic bacteria. Int. Food Res. J. 2019, 26, 1165–1172.
- Multari, S.; Licciardello, C.; Caruso, M.; Martens, S. Monitoring the changes in phenolic compounds and carotenoids occurring during fruit development in the tissues of four citrus fruits. Food Res. Int. 2020, 134, 109228.
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition, antioxidant potential and health benefits of citrus peel. Food Res. Int. 2020, 132, 109114.
- Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Citrus peels waste as a source of value-added compounds: Extraction and quantification of bioactive polyphenols. Food Chem. 2019, 295, 289–299.
- Czech, A.; Zarycka, E.; Yanovych, D.; Zasadna, Z.; Grzegorczyk, I.; Kłys, S. Mineral content of the pulp and peel of various citrus fruit cultivars. Biol. Trace Elem. Res. 2020, 193, 555–563.
- Nieto, G.; Fernández-López, J.; Pérez-Álvarez, J.A.; Peñalver, R.; Ros-Berruezo, G.; Viuda-Martos, M. Valorization of citrus co-products: Recovery of bioactive compounds and application in meat and meat products. Plants 2021, 10, 1069.
- Radović, M.; Milatović, D.; Tešić, Ž.; Tosti, T.; Gašić, U.; Dojčinović, B.; Dabić Zagorac, D. Influence of rootstocks on the chemical composition of the fruits of plum cultivars. J. Food Compos. Anal. 2020, 92, 103480.
- Abeysinghe, D.C.; Li, X.; Sun, C.; Zhang, W.; Zhou, C.; Chen, K. Bioactive compounds and antioxidant capacities in different edible tissues of citrus fruit of four species. Food Chem. 2007, 104, 1338–1344.
- Nayak, B.; Dahmoune, F.; Moussi, K.; Remini, H.; Dairi, S.; Aoun, O.; Khodir, M. Comparison of microwave, ultrasound and accelerated-assisted solvent extraction for recovery of polyphenols from Citrus sinensis peels. Food Chem. 2015, 187, 507–516.
- Papoutsis, K.; Pristijono, P.; Golding, J.B.; Stathopoulos, C.E.; Bowyer, M.C.; Scarlett, C.J.; Vuong, Q.V. Optimisation of aqueous extraction conditions for the recovery of phenolic compounds and antioxidants from lemon pomace. Int. J. Food Sci. Technol. 2016, 51, 2009–2018.
- Tan, C.Y.; Zhong, R.Z.; Tan, Z.L.; Han, X.F.; Tang, S.X.; Xiao, W.J.; Sun, Z.H.; Wang, M. Dietary inclusion of tea catechins changes fatty acid composition of muscle in goats. Lipids 2011, 46, 239–247.
- Valencia, I.; O’Grady, M.N.; Ansorena, D.; Astiasarán, I.; Kerry, J.P. Enhancement of the nutritional status and quality of fresh pork sausages following the addition of linseed oil, fish oil and natural antioxidants. Meat Sci. 2008, 80, 1046–1054.
- Shi, C.; Cui, J.; Yin, X.; Luo, Y.; Zhou, Z. Grape seed and clove bud extracts as natural antioxidants in silver carp (Hypophthalmichthys molitrix) fillets during chilled storage: Effect on lipid and protein oxidation. Food Control 2014, 40, 134–139.
- Shi, J.; Yu, J.; Pohorly, J.E.; Kakuda, Y. Polyphenolics in grape seeds—biochemistry and functionality. J. Med. Food 2003, 6, 291–299.
- Hygreeva, D.; Pandey, M.C.; Chauhan, O.P. Effect of high-pressure processing on quality characteristics of precooked chicken patties containing wheat germ oil wheat bran and grape seed extract. J. Food Process. Preserv. 2017, 41, e12980.
- Silván, J.M.; Mingo, E.; Hidalgo, M.; de Pascual-Teresa, S.; Carrascosa, A.V.; Martinez-Rodriguez, A.J. Antibacterial activity of a grape seed extract and its fractions against Campylobacter spp. Food Control 2013, 29, 25–31.
- Libera, J.; Latoch, A.; Wójciak, K.M. Utilization of grape seed extract as a natural antioxidant in the technology of meat products inoculated with a probiotic strain of LAB. Foods 2020, 9, 103.
- Dimitrijevic, M.; Jovanovic, V.S.; Cvetkovic, J.; Mihajilov-Krstev, T.; Stojanovic, G.; Mitic, V. Screening of antioxidant, antimicrobial and antiradical activities of twelve selected Serbian wild mushrooms. Anal. Methods 2015, 7, 4181–4191.
- Novakovic, S.; Djekic, I.; Klaus, A.; Vunduk, J.; Djordjevic, V.; Tomović, V.; Šojić, B.; Kocić-Tanackov, S.; Lorenzo, J.M.; Barba, F.J.; et al. The Effect of Cantharellus cibarius addition on quality characteristics of frankfurter during refrigerated storage. Foods 2019, 8, 635.
- Ramírez-Rojo, M.I.; Vargas-Sánchez, R.D.; Torres-Martínez, B.; Torres-Martínez, B.D.M.; Torrescano-Urrutia, G.R.; Lorenzo, J.M.; Sánchez-Escalante, A. Inclusion of ethanol extract of mesquite leaves to enhance the oxidative stability of pork patties. Foods 2019, 8, 631.
- Goulas, V.; Georgiou, E. Utilization of carob fruit as sources of phenolic compounds with antioxidant potential: Extraction optimization and application in food models. Foods 2020, 9, 20.
- Rathour, M.; Malav, O.P.; Kumar, P.; Chatli, M.K.; Mehta, N. Storage stability of chevon rolls incorporated with ethanolic extracts of aloe vera and cinnamon bark at refrigeration temperature (4 ± 1 °C). J. Anim. Res. 2017, 7, 183–190.
- Rathour, M.; Malav, O.P.; Kumar, P.; Chatli, M.K.; Mehta, N. Standardization of protocols for extraction of aloe vera and cinnamon bark extracts. J. Anim. Res. 2017, 7, 175–182.
- Rathour, M.; Malav, O.P.; Kumar, P.; Chatli, M.K.; Mehta, N. Functional chevon rolls fortified with cinnamon bark and Aloe-vera powder extracts. Haryana Vet. 2019, 58, 1–5.
- Jagtap, N.S.; Wagh, R.V.; Chatli, M.K.; Malav, O.P.; Kumar, P.; Mehta, N. Chevon meat storage stability infused with response surface methodology optimized Origanum vulgare leaf extracts. Agric. Res. 2020, 9, 464.
- Jagtap, N.S.; Wagh, R.V.; Chatli, M.K.; Kumar, P.; Malav, O.P.; Mehta, N. Optimisation of extraction protocol for Carica papaya L. to obtain phenolic rich phyto-extract with prospective application in chevon emulsion system. J. Food Sci. Technol. 2019, 56, 3456.
- Birla, R.; Malav, O.; Wagh, R.; Mehta, N.; Kumar, P.; Chatli, M. Storage stability of pork emulsion incorporated with Arjuna (Terminalia arjuna) tree bark extract. Int. J. Livest. Res. 2019, 9, 1.
- Bishnoi, S.; Ahlawat, S.S. Development of buffalo meat rolls incorporated with aloe vera gel and arjun tree bark extract. Haryana Vet. 2015, 54, 174–177.
- Zhang, H.; Peng, X.; Li, X.; Wu, J.; Guo, X. The application of clove extract protects chinesestyle sausages against oxidation and quality deterioration. Korean J. Food Sci. Anim. Resour. 2017, 37, 114–122.
- Kumar, P.; Mehta, N.; Malav, O.P.; Kumar Chatli, M.; Rathour, M.; Kumar Verma, A. Antioxidant and antimicrobial efficacy of watermelon rind extract (WMRE) in aerobically packaged pork patties stored under refrigeration temperature (4 ± 1 °C). J. Food Process. Preserv. 2018, 42, e13757.
- Wagh, R.V.; Chatli, M.K. Response surface optimization of extraction protocols to obtain phenolic rich antioxidant from sea buckthorn and their potential application into model meat system. J. Food Sci. Technol. 2017, 54, 1565–1576.
- Das, A.K.; Rajkumar, V.; Verma, A.K.; Swarup, D. Moringa oleiferia leaves extract: A natural antioxidant for retarding lipid peroxidation in cooked goat meat patties. Int. J. food Sci. Technol. 2012, 47, 585–591.
- Muthukumar, M.; Naveena, B.M.; Vaithiyanathan, S.; Sen, A.R.; Sureshkumar, K. Effect of incorporation of Moringa oleifera leaves extract on quality of ground pork patties. J. Food Sci. Technol. 2014, 51, 3172–3180.
- Mansour, E.H.; Khalil, A.H. Evaluation of antioxidant activity of some plant extracts and their application to ground beef patties. Food Chem. 2000, 69, 135–141.
- Cao, Y.; Gu, W.; Zhang, J.; Chu, Y.; Ye, X.; Hu, Y.; Chen, J. Effects of chitosan, aqueous extract of ginger, onion and garlic on quality and shelf life of stewed-pork during refrigerated storage. Food Chem. 2013, 141, 1655–1660.
- Fernández-López, J.; Sevilla, L.; Sayas-Barberá, E.; Navarro, C.; Marín, F.; Pérez-Alvarez, J.A. Evaluation of the antioxidant potential of hyssop (Hyssopus officinalis L.) and Rosemary (Rosmarinus officinalis L.) extracts in cooked pork meat. J. Food Sci. 2003, 68, 660–664.
- Akarpat, A.; Turhan, S.; Ustun, N.S. Effects of hot-water extracts from myrtle, rosemary, nettle and lemon balm leaves on lipid oxidation and colour of beef patties during frozen storage. J. Food Process. Preserv. 2008, 32, 117–132.
- Rababah, T.M.; Ereifej, K.I.; Alhamad, M.N.; Al-Qudah, K.M.; Rousan, L.M.; Al-Mahasneh, M.A.; Al-u’datt, M.H.; Yang, W. Effects of green tea and grape seed and TBHQ on physicochemical properties of baladi goat meats. Int. J. Food Prop. 2011, 14, 1208–1216.
- Garrido, M.D.; Auqui, M.; Martí, N.; Linares, M.B. Effect of two different red grape pomace extracts obtained under different extraction systems on meat quality of pork burgers. LWT Food Sci. Technol. 2011, 44, 2238–2243.
- Lee, M.-A.; Choi, J.-H.; Choi, Y.-S.; Han, D.-J.; Kim, H.-Y.; Shim, S.-Y.; Chung, H.-K.; Kim, C.-J. The antioxidative properties of mustard leaf (Brassica juncea) kimchi extracts on refrigerated raw ground pork meat against lipid oxidation. Meat Sci. 2010, 84, 498–504.
- Huang, B.; He, J.; Ban, X.; Zeng, H.; Yao, X.; Wang, Y. Antioxidant activity of bovine and porcine meat treated with extracts from edible lotus (Nelumbo nucifera) rhizome knot and leaf. Meat Sci. 2011, 87, 46–53.
- Yogesh, K.; Jha, S.N.; Yadav, D.N. Antioxidant Activities of Murraya koenigii (L.) spreng berry extract: Application in refrigerated (4 ± 1 °C) stored meat homogenates. Agric. Res. 2012, 1, 183–189.
- Das, A.K.; Rajkumar, V.; Nanda, P.K.; Chauhan, P.; Pradhan, S.R.; Biswas, S. Antioxidant efficacy of litchi (Litchi chinensis Sonn.) pericarp extract in sheep meat nuggets. Antioxidants 2016, 5, 16.
- Andrés, A.I.; Petrón, M.J.; Adámez, J.D.; López, M.; Timón, M.L. Food by-products as potential antioxidant and antimicrobial additives in chill stored raw lamb patties. Meat Sci. 2017, 129, 62–70.
- Thomas, R.; Jebin, N.; Saha, R.; Sarma, D.K. Antioxidant and antimicrobial effects of kordoi (Averrhoa carambola) fruit juice and bamboo (Bambusa polymorpha) shoot extract in pork nuggets. Food Chem. 2016, 190, 41–49.
- Vargas-Ramella, M.; Lorenzo, J.M.; Zamuz, S.; Valdés, M.E.; Moreno, D.; Balcázar, M.C.G.; Fernández-Arias, J.M.; Reyes, J.F.; Franco, D. The Antioxidant effect of Colombian berry (Vaccinium meridionale Sw.) extracts to prevent lipid oxidation during pork patties shelf-life. Antioxidants 2021, 10, 1290.
- Pasukamonset, P.; Kwon, O.; Adisakwattana, S. Oxidative stability of cooked pork patties incorporated with clitoria ternatea extract (blue pea flower petal) during refrigerated storage. J. Food Process. Preserv. 2017, 41, e12751.
- De Florio Almeida, J.; dos Reis, A.S.; Heldt, L.F.S.; Pereira, D.; Bianchin, M.; de Moura, C.; Plata-Oviedo, M.V.; Haminiuk, C.W.I.; Ribeiro, I.S.; da Luz, C.F.P.; et al. Lyophilized bee pollen extract: A natural antioxidant source to prevent lipid oxidation in refrigerated sausages. LWT Food Sci. Technol. 2017, 76, 299–305.
- Baldin, J.C.; Michelin, E.C.; Polizer, Y.J.; Rodrigues, I.; de Godoy, S.H.S.; Fregonesi, R.P.; Pires, M.A.; Carvalho, L.T.; Fávaro-Trindade, C.S.; de Lima, C.G.; et al. Microencapsulated jabuticaba (Myrciaria cauliflora) extract added to fresh sausage as natural dye with antioxidant and antimicrobial activity. Meat Sci. 2016, 118, 15–21.
- Munekata, P.E.S.; Calomeni, A.V.; Rodrigues, C.E.C.; Fávaro-Trindade, C.S.; Alencar, S.M.; Trindade, M.A. Peanut skin extract reduces lipid oxidation in cooked chicken patties. Poult. Sci. 2015, 94, 442–446.
More
Information
Subjects:
Food Science & Technology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.9K
Entry Collection:
Extraction Techniques in Sample Preparation
Revisions:
3 times
(View History)
Update Date:
19 Nov 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




