
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Panagiotis Dorovinis | + 1613 word(s) | 1613 | 2021-10-25 04:18:42 | | | |
| 2 | Lindsay Dong | + 124 word(s) | 1737 | 2021-11-22 04:25:33 | | |
Video Upload Options
Resection of the caudate lobe of the liver is considered a highly challenging type of liver resection due to the region’s intimacy with critical vascular structures and deep anatomic location inside the abdominal cavity. Laparoscopic resection of the caudate lobe is considered one of the most challenging laparoscopic liver procedures.
1. Introduction
2. Safety and Efficacy of Laparoscopic Caudate Lobectomy
2.1. Long-Term Outcomes
| Author | Follow-Up (Months) |
Adjuvant Chemotherapy (%) | Recurrence, N (%) | Overall Survival (Months) | Disease-Free Survival (Months) |
|---|---|---|---|---|---|
| Cheung [17] |
12 | 1 (100%) |
No | 12 | 12 |
| Wan et al. [18] |
6 | 1 (100%) |
No | 6 | 6 |
| Dulucq et al. [19] |
7 | 1 (100%) |
No | 7 | 7 |
| Ho et al. [20] |
1 | n/a | No | 1 | 1 |
| Oh et al. [21] |
54.6 (12.9–86.7) a |
1 (25%) |
2 (50%) |
54.6 (12.9–86.7) a |
32 (9–55) a |
| Li et al. [22] |
8 a | n/a | n/a | 8 a | 8 a |
| Chen et al. [23] |
13 (3–56) a |
n/a | n/a | 13 (3–56) a |
n/a |
| Kyriakides et al. [24] | n/a | No | No | n/a | n/a |
| Parikh et al. [25] | 43(4–149) a | n/a | n/a | n/a | n/a |
| Sun et al. [26] | n/a | n/a | 13.3% | n/a | n/a |
| Ruzzenente et al. [27] | n/a | n/a | n/a | n/a | n/a |
| Capelle et al. [15] |
14(10–23) a | n/a | 129(54.5%) | 85% c | 10 |
2.2. Comparative Studies
3. Summary
LCL is a safe and efficient alternative to open resection for selected patients when performed by experienced hepatobiliary surgeons. This minimally invasive approach combines a low overall morbidity rate of 16.3%, as well as low mortality. Comparative studies also substantiate these outcomes, reporting lower morbidity between laparoscopic and open caudate lobectomy. Moreover, aside from being a safe alternative, is also efficient, with a 98% rate of R0 resections and a 3.1% rate of conversion to open.
Resections of primary and metastatic lesions located within the caudate lobe are considered technically challenging, due to its deep intra-abdominal location and proximity to major vascular structures. The caudate lobe entails three distinct subsegments: (a) Spiegel’s lobe, located behind ligamentum venosum and on the left of the inferior vena cava (IVC); (b) the caudate process, extending to the left and useful in traction of the caudate lobe; and (c) the paracaval portion (Couinaud’s segment IX) corresponding to the dorsally located hepatic tissue in front of the inferior vena cava. Left and right portal veins, along with the hilar bifurcation area, provide the branches supplying the caudate lobe subregions, while short hepatic veins drain blood directly to the IVC [28][29].
Resection of the caudate lobe remains one of the most demanding resections, even through the open approach, while proper management of the short hepatic veins is of cardinal importance. Laparoscopy offers caudal-to-cephalad vision, which results in better exposure and control of the short hepatic veins. Other advantages include proper staging and determination of resectability since tumor seeding, and occult liver and lymph node metastases are identified intraoperatively [30][31]. However, laparoscopy poses a risk of massive intraoperative bleeding occurring from the anterior IVC, which may occur when dealing with tumors larger than 5cm, potentially invading the IVC and found close to the hilum or major hepatic veins [32]. In these situations, obtaining an adequate resection margin, despite laparoscopy’s aforementioned advantages, can be challenging.
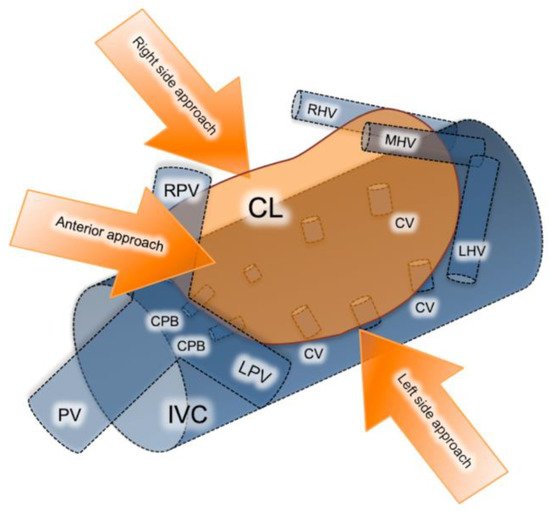
There are three different approaches for isolated laparoscopic excision of the caudate lobe described in the literature including the left, right and anterior trans parenchymal, each chosen depending on the tumor size and location (Figure 1) [33]. Decision on which approach is more suitable is made based on tumor localization and size. The right-sided approach is usually reserved for bulky tumors. Taking into consideration that this approach requires a complete rotation of the right lobe to the left in order to expose the caudate hepatic veins, it is quite difficult to perform laparoscopically. The left-sided approach on the other hand is preferred for smaller-size tumors located in the Spiegel process or paracaval portion. Lastly, the anterior trans parenchymal approach includes the performance of retrohepatictransparenchymal resection and suspension of the right lobe [33]. It is furthermore recommended by several authors as a preferred alternative for lesions exceeding 4cm in diameter, or lesions involving critical vascular structures such as the inferior vena cava IVC and short hepatic veins. From a technical standpoint, it prevents hepatic rotation and subsequent venous rupture, making it feasible to dissect the liver parenchyma along the IVC [30].
References
- Hilal, A.M.; Aldrighetti, L.; Dagher, I.; Edwin, B.; Troisi, R.I.; Alikhanov, R.; Aroori, S.; Belli, G.; Besselink, M.; Briceno, J. The Southampton Consensus Guidelines for Laparoscopic Liver Surgery: From Indication to Implementation. Ann. Surg. 2018, 268, 11–18.
- Machairas, N.; Sotiropoulos, G.C. Laparoscopic liver surgery: Yesterday, today and tomorrow. Hepatobiliary Surg. Nutr. 2019, 8, 324–326.
- Machairas, N.; Papaconstantinou, D.; Gaitanidis, A.; Hasemaki, N.; Paspala, A.; Stamopoulos, P.; Kykalos, S.; Sotiropoulos, G.C. Is Single-Incision Laparoscopic Liver Surgery Safe and Efficient for the Treatment of Malignant Hepatic Tumors? A Systematic Review. J. Gastrointest. Cancer 2019, 51, 425–432.
- Sotiropoulos, G.C.; Prodromidou, A.; Kostakis, I.D.; Machairas, N. Meta-analysis of laparoscopic vs open liver resection for hepatocellular carcinoma. Updates Surg. 2017, 69, 291–311.
- Machairas, N.; Kostakis, I.D.; Schizas, D.; Kykalos, S.; Nikiteas, N.; Sotiropoulos, G.C. Meta-analysis of laparoscopic versus open liver resection for intrahepatic cholangiocarcinoma. Updat. Surg. 2020, 73, 59–68.
- Ciria, R.; Ocaña, S.; Gomez-Luque, I.; Cipriani, F.; Halls, M.; Fretland, A.; Okuda, Y.; Aroori, S.; Briceño, J.; Aldrighetti, L.; et al. A systematic review and meta-analysis comparing the short- and long-term outcomes for laparoscopic and open liver resections for liver metastases from colorectal cancer. Surg. Endosc. 2019, 34, 349–360.
- Machairas, N.; Paspala, A.; Kostakis, I.; Prodromidou, A.; Sotiropoulos, G. Current Concepts in Laparoscopic Liver Surgery. Hell. J. Surg. 2018, 90, 261–266.
- Machairas, N.; Papakonstantinou, D.; Stamopoulos, P.; Prodromidou, A.; Garoufalia, Z.; Spartalis, E.; Kostakis, I.D.; Sotiropoulos, G.C. The Emerging Role of Laparoscopic Liver Resection in the Treatment of Recurrent Hepatocellular Carcinoma: A Systematic Review. Anticancer. Res. 2018, 38, 3181–3186.
- Machairas, N.; Prodromidou, A.; Kostakis, I.D.; Spartalis, E.; Sotiropoulos, G.C. Safety and Efficacy of Laparoscopic Liver Resection for Lesions Located on Posterosuperior Segments: A Meta-Analysis of Short-term Outcomes. Surg. Laparosc. Endosc. Percutaneous Tech. 2018, 28, 203–208.
- Ratti, F.; Rawashdeh, A.; Cipriani, F.; Primrose, J.; Fiorentini, G.; Hilal, A.M. Intrahepatic cholangiocarcinoma as the new field of implementation of laparoscopic liver resection programs. A comparative propensity score-based analysis of open and laparoscopic liver resections. Surg. Endosc. 2021, 35, 1851–1862.
- Moris, D.; Tsilimigras, D.I.; Machairas, N.; Merath, K.; Cerullo, M.; Hasemaki, N.; Prodromidou, A.; Cloyd, J.M.; Pawlik, T.M. Laparoscopic synchronous resection of colorectal cancer and liver metastases: A systematic review. J. Surg. Oncol. 2019, 119, 30–39.
- Cherqui, D.; Soubrane, O.; Husson, E.; Barshasz, E.; Vignaux, O.; Ghimouz, M.; Branchereau, S.; Chardot, C.; Gauthier, F.; Fagniez, P.L.; et al. Laparoscopic living donor hepatectomy for liver transplantation in children. Lancet 2002, 359, 392–396.
- Kumon, M. Anatomical Study of the Caudate Lobe with Special Reference to Portal Venous and Biliary Branches Using Corrosion Liver Casts and Clinical Application. Liver Cancer 2017, 6, 161–170.
- Machado, M.A.; Surjan, R.; Basseres, T.; Makdissi, F. Laparoscopic resection of caudate lobe. Technical strategies for a difficult liver segment―Video article. Surg. Oncol. 2018, 27, 674–675.
- Cappelle, M.; Aghayan, D.L.; van der Poel, M.J.; Besselink, M.G.; Sergeant, G.; Edwin, B.; Parmentier, I.; De Meyere, C.; Vansteenkiste, F.; D’Hondt, M. A multicenter cohort analysis of laparoscopic hepatic caudate lobe resection. Langenbecks Arch. Surg. 2020, 405, 181–189.
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196.
- Cheung, T.T. Technical notes on pure laparoscopic isolated caudate lobectomy for patient with liver cancer. Transl. Gastroenterol. Hepatol. 2016, 1, 56.
- Wan, H.-F.; Xie, K.-L.; Li, J.-X.; Ho, K.-M.; Wu, H.; Huang, J.-W. Laparoscopic Caudate Lobectomy for Cholangiocarcinoma of Caudate Lobe Invading Middle Hepatic Vein. Ann. Surg. Oncol. 2020, 27, 4181–4185.
- Dulucq, J.-L.; Wintringer, P.; Stabilini, C.; Mahajna, A. Isolated Laparoscopic Resection of the Hepatic Caudate Lobe: Surgical Technique and a Report of 2 Cases. Surg. Laparosc. Endosc. Percutaneous Tech. 2006, 16, 32–35.
- Ho, K.M.; Han, H.S.; Yoon, Y.S.; Cho, J.Y.; Choi, Y.R.; Jang, J.S.; Kwon, S.U.; Kim, S.; Choi, J.K. Laparoscopic Total Caudate Lobectomy for Hepatocellular Carcinoma. J. Laparoendosc. Adv. Surg. Tech. A 2017, 27, 1074–1078.
- Oh, D.; Kwon, C.H.D.; Na, B.G.; Lee, K.W.; Cho, W.T.; Lee, S.H.; Choi, J.Y.; Choi, G.S.; Kim, J.M.; Joh, J.-W. Surgical Techniques for Totally Laparoscopic Caudate Lobectomy. J. Laparoendosc. Adv. Surg. Tech. 2016, 26, 689–692.
- Li, Y.; Zeng, K.-N.; Ruan, D.-Y.; Yao, J.; Yang, Y.; Chen, G.-H.; Wang, G.-S. Feasibility of laparoscopic isolated caudate lobe resection for rare hepatic mesenchymal neoplasms. World J. Clin. Cases 2019, 7, 3194–3201.
- Chen, K.-H.; Jeng, K.-S.; Huang, S.-H.; Chu, S.-H. Laparoscopic Caudate Hepatectomy for Cancer—An Innovative Approach to the No-Man’s Land. J. Gastrointest. Surg. 2013, 17, 522–526.
- Kyriakides, C.; Panagiotopoulos, N.; Jiao, L.R. Isolated Laparoscopic Caudate Lobe Resection. Surg. Laparosc. Endosc. Percutaneous Tech. 2012, 22, e209.
- Parikh, M.; Han, H.S.; Cho, J.Y. Laparoscopic isolated caudate lobe resection. Sci. Rep. 2021, 11, 4328.
- Sun, T.-G.; Wang, X.-J.; Cao, L.; Li, J.-W.; Chen, J.; Li, X.-S.; Liao, K.-X.; Cao, Y.; Zheng, S.-G. Laparoscopic anterior hepatic transection for resecting lesions originating in the paracaval portion of the caudate lobe (with videos). Surg. Endosc. 2021, 1–7.
- Ruzzenente, A.; Ciangherotti, A.; Aldrighetti, L.; Ettorre, G.M.; De Carlis, L.; Ferrero, A.; Valle, R.D.; Tisone, G.; Guglielmi, A. Technical feasibility and short-term outcomes of laparoscopic isolated caudate lobe resection: An IgoMILS (Italian Group of Minimally Invasive Liver Surgery) registry-based study. Surg. Endosc. 2021, 1–10.
- Makuuchi, M.; Yamamoto, J.; Takayama, T.; Kosuge, T.; Gunvén, P.; Yamazaki, S.; Hasegawa, H. Extrahepatic division of the right hepatic vein in hepatectomy. Hepatogastroenterology 1991, 38.
- Murakami, G.; Hata, F. Human liver caudate lobe and liver segment. Anat. Sci. Int. 2002, 77, 211–224.
- Asahara, T.; Dohi, K.; Hino, H.; Nakahara, H.; Katayama, K.; Itamoto, T.; Ono, E.; Moriwaki, K.; Yuge, O.; Nakanishi, T.; et al. Isolated caudate lobectomy by anterior approach for hepatocellular carcinoma originating in the paracaval portion of the caudate lobe. J. Hepato-Biliary-Pancreatic Surg. 1998, 5, 416–421.
- D’Angelica, M.; Fong, Y.; Weber, S.; Gonen, M.; DeMatteo, R.P.; Conlon, K.; Blumgart, L.H.; Jarnagin, W.R. The Role of Staging Laparoscopy in Hepatobiliary Malignancy: Prospective Analysis of 401 Cases. Ann. Surg. Oncol. 2003, 10, 183–189.
- Yoon, Y.-S.; Han, H.-S.; Cho, J.Y.; Kim, J.H.; Kwon, Y. Laparoscopic liver resection for centrally located tumors close to the hilum, major hepatic veins, or inferior vena cava. Surgery 2013, 153, 502–509.
- Jin, B.; Jiang, Z.; Hu, S.; Du, G.; Shi, B.; Kong, D.; Yang, J. Surgical Technique and Clinical Analysis of Twelve Cases of Isolated Laparoscopic Resection of the Hepatic Caudate Lobe. Biomed. Res. Int. 2018, 2018, 5848309.




