
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Li-Gen Lin | + 2132 word(s) | 2132 | 2021-11-05 04:07:26 | | | |
| 2 | Peter Tang | Meta information modification | 2132 | 2021-11-17 03:10:46 | | |
Video Upload Options
Inflammation is the body’s self-protective response to multiple stimulus, from external harmful substances to internal danger signals released after trauma or cell dysfunction. Many diseases are considered to be related to inflammation, such as cancer, metabolic disorders, aging, and neurodegenerative diseases. Xanthones, a unique scaffold with a 9H-Xanthen-9-one core structure, widely exist in natural sources. Till now, over 250 xanthones were isolated and identified in plants from the families Gentianaceae and Hypericaceae. Many xanthones have been disclosed with anti-inflammatory properties on different models, either in vitro or in vivo.
1. Introduction
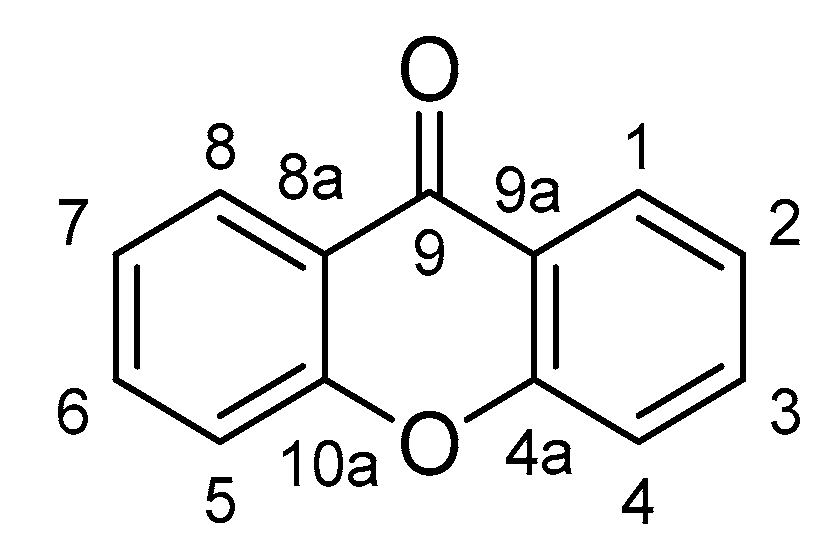
2. Xanthones with Anti-Inflammatory Properties
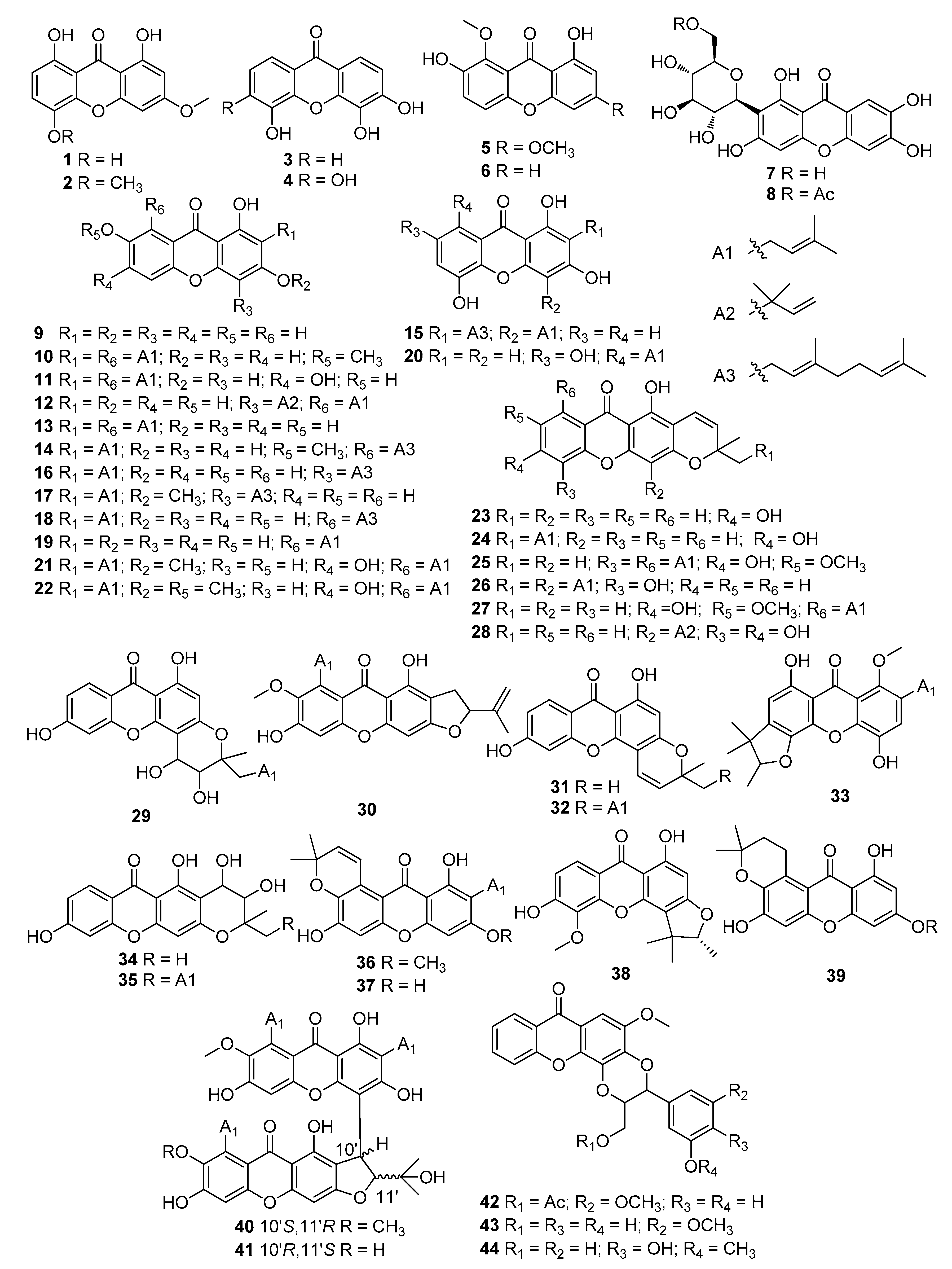
|
Model/Method |
No |
Dose |
Outcomes |
Ref. |
|---|---|---|---|---|
|
LPS-stimulated RAW264.7 macrophages |
1 |
10 μmol/L |
Suppressed the phosphorylation of IKK-β, Akt, and p65 |
[20] |
|
2 |
10 μmol/L |
Inhibited the production of IL-6 and TNF-α |
[20] |
|
|
4, 7 |
25, 50 μg/mL |
Suppressed the generation of TNF-α and ICAM-1 |
[21] |
|
|
6, 15−18, 21, 26−28, 37 |
3, 10, 30, 100 μmol/L |
Downregulated mRNA expressions of iNOS and COX-2 |
[22] |
|
|
9 |
50 μmol/L |
Suppressed iNOS, COX-2, inhibited TNF-α, IL-1β, IL-6, IκB-α |
[23] |
|
|
12 |
1, 2, 5, 10 μmol/L |
Induced HO-1 expression and increased HO-1 activity, inhibited TNF-α, IL-1β |
[24] |
|
|
19 |
5, 10, 20 μmol/L |
Inhibited NO production and IL-6 secretion |
[25] |
|
|
22 |
11.72 ± 1.16 μmol/L |
Inhibited NO production |
[26] |
|
|
30 |
20, 40, 60 μmol/L |
Inhibited the production of NO, iNOS, TNF-α, IL-6, and IL-1β |
[27] |
|
|
33 |
6.25 μmol/L |
Suppressed NO production |
[28] |
|
|
38 |
50 μg/mL |
Inhibited COX-1, COX-2 and 5-LOX-mediated LTB4 formation |
[29] |
|
|
40 |
11.3 ± 1.7 μmol/L |
Inhibited NO production |
[30] |
|
|
41 |
18.0 ± 1.8 μmol/L |
Inhibited NO production |
[30] |
|
|
LPS/IFN?-stimulated RAW264.7 macrophages |
20 |
3.125–25 ?mol/L |
Suppressed IL-6, IL-12, and TNF-? |
[31] |
|
39 |
10 μmol/L |
Decreased NO production |
[32] |
|
|
Human neutrophils |
3, 7, 42, 43 |
1000 μg/mL |
Inhibited WST-1 by NADPH oxidase |
[33] |
|
23, 24, 29, 31, 32, 34, 35 |
10 μg/mL |
Inhibited superoxide anion generation and elastase release |
[34] |
|
|
CD3− synovial cells |
7 |
100 μg/mL |
Downregulation of TNF-α, IL-1β, and IFN-γ |
[35] |
|
Lung of septic mice |
10, 30, 100 mg/kg |
Upregulated the expression and activity of HO-1 |
[36] |
|
|
Carrageenan-induced mechanical hyperalgesia Wistar rats |
100 μg/paw |
Inhibited TNF-α level through CINC-1/epinephrine/PKA pathway |
[37] |
|
|
MC 3T3-E1 cell line |
10, 20, 30, 40 μmol/L |
Alleviated oxidative stress by activating the BMP2/Smad-1 signaling pathway |
[38] |
|
|
HFLS-RA cells |
10 |
10 μg/mL |
Inhibited nuclear translocation of p65 |
[39] |
|
AA rats |
10 |
2.5−10 μg/mL |
Inhibited fibrous hyperplasia, synovial angiogenesis, cartilage |
[39] |
|
Peripheral LPS-induced neuroinflammation in C57BL/6J mice |
10 |
40 mg/kg |
Reduced brain levels of IL-6 and COX-2 |
[40] |
|
Established CIA in DBA/1J mice |
10 |
10, 40 mg/kg |
Reduced the levels of anti-collagen IgG2a and autoantibodies in serum and the production of LIX/CXCL5, IP-10/CXCL10, MIG/CXCL9, RANTES/CCL5, IL-6 and IL-33 in joints |
[41] |
|
Ovalbumin-induced allergic asthma mice |
9, 10 |
10, 30 mg/kg |
Increased Th2 cytokine |
[42] |
|
3T3-L1 cells |
10, 19 |
10 μmol/L |
Inhibited PPARγ and NFR2 through NF-κB |
[43] |
|
Acetic acid-induced mice |
5 |
10, 20 mg/kg |
Reduced paw edema |
[44] |
|
EPP-induced ear edema |
10, 13, 14, 25, 36 |
1 mg/kg |
Inhibited edema |
[45] |
|
LPS-induced adipose tissue inflammation mice |
10 |
10 mg/kg |
Reduced macrophage content and shifted pro-inflammatory macrophage polarization |
[12] |
|
19 |
20 mg/kg |
Reduced macrophage content through inhibiting MAPKs and NF-κB activation |
[25] |
3. Comparison of the Drug Likeness of Anti-Inflammatory Xanthones with Marketed Drugs
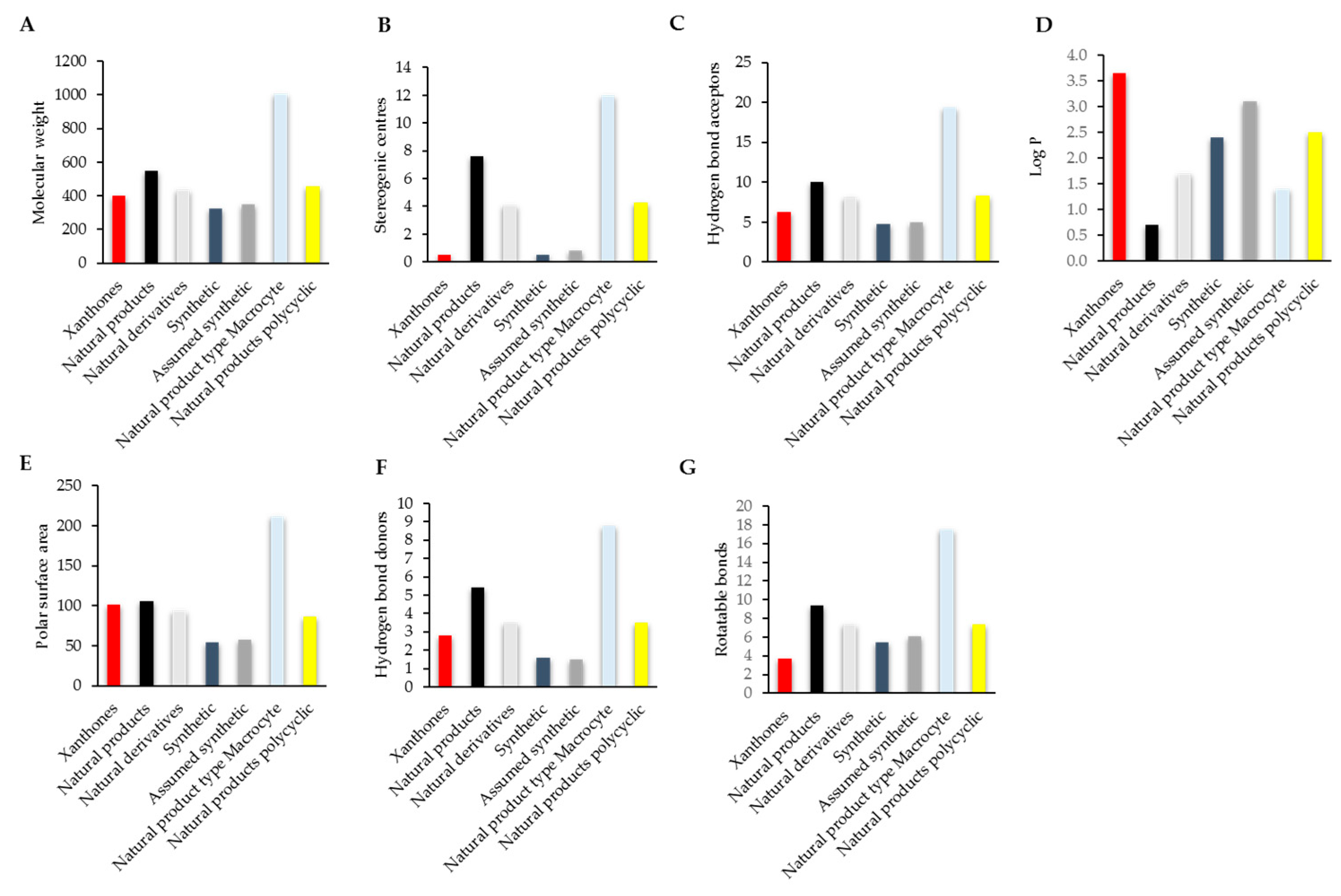
4. Compliance of Xanthones with the Rules of Drug Likeness
References
- Artis, D.; Spits, H. The biology of innate lymphoid cells. Nature 2015, 517, 293–301.
- Rock, K.L.; Lai, J.J.; Kono, H. Innate and adaptive immune responses to cell death. Immunol. Rev. 2011, 243, 191–205.
- Pedraza-Alva, G.; Pérez-Martínez, L.; Valdez-Hernández, L.; Meza-Sosa, K.F.; Ando-Kuri, M. Negative regulation of the inflammasome: Keeping inflammation under control. Immunol. Rev. 2015, 265, 231–257.
- Wentworth, J.M.; Naselli, G.; Brown, W.A.; Doyle, L.; Phipson, B.; Smyth, G.K.; Wabitsch, M.; O’Brien, P.E.; Harrison, L.C. Pro-inflammatory CD11c+ CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes 2010, 59, 1648–1656.
- Lumeng, C.N.; Bodzin, J.L.; Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 2007, 117, 175–184.
- Vegeto, E.; Benedusi, V.; Maggi, A. Estrogen anti-inflammatory activity in brain: A therapeutic opportunity for menopause and neurodegenerative diseases. Front. Neuroendocrin. 2008, 29, 507–519.
- Navab, M.; Gharavi, N.; Watson, A.D. Inflammation and metabolic disorders. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 459–464.
- Bally, M.; Dendukuri, N.; Rich, B.; Nadeau, L.; Helin-Salmivaara, A.; Garbe, E.; Brophy, J.M. Risk of acute myocardial infarction with NSAIDs in real world use: bayesian meta-analysis of individual patient data. Brit. Med. J. 2017, 357, j1909.
- Sriuttha, P.; Sirichanchuen, B.; Permsuwan, U. Hepatotoxicity of nonsteroidal anti-inflammatory drugs: A systematic review of randomized controlled trials. Int. J. Hepatol. 2018, 2018, 5253623.
- Pal, S.; Pal, P.B.; Das, J.; Sil, P.C. Involvement of both intrinsic and extrinsic pathways in hepatoprotection of arjunolic acid against cadmium induced acute damage in vitro. Toxicology 2011, 283, 129–139.
- Manna, P.; Das, J.; Ghosh, J.; Sil, P.C. Contribution of type 1 diabetes to rat liver dysfunction and cellular damage via activation of NOS, PARP, IκBα/NF-κB, MAPKs, and mitochondria-dependent pathways: Prophylactic role of arjunolic acid. Free Radic. Biol. Med. 2010, 48, 1465–1484.
- Masters, K.S.; Brase, S. Xanthones from fungi, lichens, and bacteria: The natural products and their synthesis. Chem. Rev. 2012, 112, 3717–3776.
- Cardona, M.L.; Fernández, I.; Pedro, J.R.; Serrano, A. Xanthones from Hypericum reflexum. Phytochemistry 1990, 29, 3003–3006.
- Peres, V.; Nagem, T.J. Trioxygenated naturally occurring xanthones. Phytochemistry 1997, 44, 191–214.
- Pant, N.; Jain, D.; Bhakuni, R. Phytochemicals from genus Swertia and their biological activities. Ind. J. Chem. 2000, 39, 565–586.
- Jantan, I.; Saputri, F.C. Benzophenones and xanthones from Garcinia cantleyana var. cantleyana and their inhibitory activities on human low-density lipoprotein oxidation and platelet aggregation. Phytochemistry 2012, 80, 58–63.
- Chin, Y.-W.; Jung, H.-A.; Chai, H.; Keller, W.J.; Kinghorn, A.D. Xanthones with quinone reductase-inducing activity from the fruits of Garcinia mangostana (Mangosteen). Phytochemistry 2008, 69, 754–758.
- Louh, G.N.; Lannang, A.M.; Mbazoa, C.D.; Tangmouo, J.G.; Komguem, J.; Castilho, P.; Ngninzeko, F.N.; Qamar, N.; Lontsi, D.; Choudhary, M.I. Polyanxanthone A, B and C, three xanthones from the wood trunk of Garcinia polyantha Oliv. Phytochemistry 2008, 69, 1013–1017.
- Chhetri, D.; Parajuli, P.; Subba, G. Antidiabetic plants used by Sikkim and Darjeeling Himalayan tribes, India. J. Ethnopharmacol. 2005, 99, 199–202.
- Langenfeld, E.; Kong, Y.; Langenfeld, J. Bone morphogenetic protein 2 stimulation of tumor growth involves the activation of Smad-1/5. Oncogene 2006, 25, 685.
- Chang, T.; Neelakandan, C.; Define, L.; Alexander, T.; Kyu, T. Effects of glucose on cell viability and antioxidant and anti-inflammatory properties of phytochemicals and phytochemically modified membranes. J. Phys. Chem. B 2014, 118, 11993–12001.
- Boonnak, N.; Chantrapromma, S.; Tewtrakul, S.; Sudsai, T. Inhibition of nitric oxide production in lipopolysaccharide-activated RAW264. 7 macrophages by isolated xanthones from the roots of Cratoxylum formosum ssp. pruniflorum. Arch. Pharm. Res. 2014, 37, 1329–1335.
- Jang, J.-H.; Lee, K.-H.; Jung, H.-K.; Sim, M.-O.; Kim, T.-M.; Woo, K.-W.; An, B.-K.; Cho, J.-H.; Cho, H.-W. Anti-inflammatory effects of 6′-O-acetyl mangiferin from Iris rossii Baker via NF-κb signal blocking in lipopolysaccharide-stimulated RAW 264.7 cells. Chem.-Biol. Interact. 2016, 257, 54–60.
- Jeong, G.; Lee, D. Yc Cudratricusxanthone A from Cudrania tricuspidata suppresses pro-inflammatory mediators through expression of anti-inflammatory heme oxygenase-1 in RAW264.7 macrophages. Int. Immunopharmacol. 2009, 9, 241–246.
- Li, D.; Liu, Q.; Sun, W.; Chen, X.; Wang, Y.; Sun, Y.; Lin, L. 1, 3, 6, 7-Tetrahydroxy-8-prenylxanthone ameliorates inflammatory responses resulting from the paracrine interaction of adipocytes and macrophages. Br. J. Pharmacol. 2018, 175, 1590–1606.
- Karunakaran, T.; Ee, G.C.L.; Ismail, I.S.; Mohd Nor, S.M.; Zamakshshari, N.H. Acetyl-and O-alkyl-derivatives of β-mangostin from Garcinia mangostana and their anti-inflammatory activities. Nat. Prod. Res. 2018, 32, 1390–1394.
- Cho, B.O.; Ryu, H.W.; So, Y.; Chang, W.L.; Chang, H.J.; Hong, S.Y.; Yong, W.J.; Park, J.C.; Jeong, I.Y. Anti-Inflammatory effect of mangostenone F in lipopolysaccharide-stimulated RAW264.7 macrophages by suppressing NF-κB and MAPK activation. Biomol. Ther. 2014, 22, 288–294.
- Ee, G.C.L.; Mah, S.H.; Rahmani, M.; Yun, T.Y.; Teh, S.S.; Yang, M.L. A new furanoxanthone from the stem bark of Calophyllum inophyllum. Lett. Org. Chem. 2011, 13, 956–960.
- Crockett, S.L.; Poller, B.; Tabanca, N.; Pferschywenzig, E.M.; Kunert, O.; Wedge, D.E.; Bucar, F. Bioactive xanthones from the roots of Hypericum perforatum (common St John’s wort). J. Sci. Food Agric. 2011, 91, 428–434.
- Liu, Q.; Li, D.; Wang, A.; Dong, Z.; Yin, S.; Zhang, Q.; Ye, Y.; Li, L.; Lin, L. Nitric oxide inhibitory xanthones from the pericarps of Garcinia mangostana. Phytochemistry 2016, 131, 115–123.
- Zhang, D.D.; Hong, Z.; Lao, Y.Z.; Rong, W.; Xu, J.W.; Ferid, M.; Bian, K.; Xu, H.X. Anti-inflammatory effect of 1,3,5,7-tetrahydroxy-8-isoprenylxanthone isolated from twigs of Garcinia esculentaon stimulated macrophage. Mediat. Inflamm. 2015, 2015, 11–24.
- Zhang, H.; Zhang, D.D.; Lao, Y.Z.; Fu, W.W.; Liang, S.; Yuan, Q.H.; Yang, L.; Xu, H.X. Cytotoxic and anti-inflammatory prenylated benzoylphloroglucinols and xanthones from the twigs of Garcinia esculenta. J. Nat. Prod. 2014, 77, 2148–2149.
- Ali, M.; Arfan, M.; Ahmad, M.; Singh, K.; Anis, I.; Ahmad, H.; Choudhary, M.I.; Shah, M.R. Anti-inflammatory xanthones from the twigs of Hypericum oblongifolium wall. Planta Med. 2011, 77, 2013–2018.
- Yen, C.T.; Nakagawagoto, K.; Hwang, T.; Morrisnatschke, S.L.; Bastow, K.F.; Wu, Y.C.; Lee, K.H. Design and synthesis of gambogic acid analogs as potent cytotoxic and anti-inflammatory agents. Bioorg. Med. Chem. Lett. 2012, 22, 4018–4022.
- Kokotkiewicz, A.; Luczkiewicz, M.; Pawlowska, J.; Luczkiewicz, P.; Sowinski, P.; Witkowski, J.M.; Bryl, E.; Bucinski, A. Isolation of xanthone and benzophenone derivatives from Cyclopia genistoides (L.) Vent. (honeybush) and their pro-apoptotic activity on synoviocytes from patients with rheumatoid arthritis. Fitoterapia 2013, 90, 199–208.
- Gong, X.; Zhang, L.; Jiang, R.; Ye, M.; Yin, X.; Wan, J. Anti-inflammatory effects of mangiferin on sepsis-induced lung injury in mice via up-regulation of heme oxygenase-1. J. Nutr. Biochem. 2013, 24, 1173–1181.
- Rocha, L.W.; Bonet, I.J.M.; Tambeli, C.H.; De-Faria, F.M.; Parada, C.A. Local administration of mangiferin prevents experimental inflammatory mechanical hyperalgesia through CINC-1/epinephrine/PKA pathway and TNF-α inhibition. Eur. J. Pharmacol. 2018, 830, 87–94.
- Ding, L.Z.; Teng, X.; Zhang, Z.B.; Zheng, C.J.; Chen, S.H. Mangiferin inhibits apoptosis and oxidative stress via BMP2/Smad-1 signaling in dexamethasone-induced MC3T3-E1 cells. Int. J. Mol. Med. 2018, 41, 2517–2526.
- Zuo, J.; Yin, Q.; Wang, Y.W.; Li, Y.; Lu, L.M.; Xiao, Z.G.; Wang, G.D.; Luan, J.J. Inhibition of NF-κB pathway in fibroblast-like synoviocytes by α-mangostin implicated in protective effects on joints in rats suffering from adjuvant-induced arthritis. Int. Immunopharmacol. 2018, 56, 78–89.
- Nava, C.M.; Acero, G.; Pedraza-Chaverri, J.; Fragoso, G.; Govezensky, T.; Gevorkian, G. Alpha-mangostin attenuates brain inflammation induced by peripheral lipopolysaccharide administration in C57BL/6J mice. J. Neuroimmunol. 2016, 297, 20–27.
- Herrera-Aco, D.R.; Medina-Campos, O.N.; Pedraza-Chaverri, J.; Sciutto-Conde, E.; Rosas-Salgado, G.; Fragoso-González, G. Alpha-mangostin: Anti-inflammatory and antioxidant effects on established collagen-induced arthritis in DBA/1J mice. Food Chem. Toxicol. 2019, 124, 300–315.
- Jang, H.Y.; Kwon, O.K.; Oh, S.R.; Lee, H.K.; Ahnab, K.S. Mangosteen xanthones mitigate ovalbumin-induced airway inflammation in a mouse model of asthma. Food Chem. Toxicol. 2012, 50, 4042–4051.
- Shen, Q.; Chitchumroonchokchai, C.; Thomas, J.L.; Gushchina, L.V.; Disilvestro, D.; Failla, M.L.; Ziouzenkova, O. Adipocyte reporter assays: Application for identification of anti-inflammatory and antioxidant properties of mangostin xanthones. Mol. Nutr. Food Res. 2014, 58, 239–247.
- Moreira, M.E.; Pereira, R.G.; Dias Silva, M.J.; Dias, D.F.; Gontijo, V.S.; Giustipaiva, A.; Veloso, M.P.; Doriguetto, A.C.; Nagem, T.J.; dos Santos, M.H. Analgesic and anti-inflammatory activities of the 2,8-dihydroxy-1,6-dimethoxyxanthone from Haploclathra paniculata (Mart) Benth (Guttiferae). J. Med. Food 2014, 17, 686–693.
- Panthong, K.; Hutadilok-Towatana, N.; Panthong, A. Cowaxanthone F, a new tetraoxygenated xanthone, and other antiinflammatory and antioxidant compounds from Garcinia cowa. Cheminform 2010, 41, 281–286.
- ujiwara, N.; Kobayashi, K. Macrophages in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 281–286.
- Poltorak, A.; He, X.; Smirnova, I.; Liu, M.-Y.; Van Huffel, C.; Du, X.; Birdwell, D.; Alejos, E.; Silva, M.; Galanos, C. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 1998, 282, 2085–2088.
- Gray, P.W.; Goeddel, D.V. Structure of the human immune interferon gene. Nature 1982, 298, 859–863.
- Witko-Sarsat, V.; Rieu, P.; Descamps-Latscha, B.; Lesavre, P.; Halbwachs-Mecarelli, L. Neutrophils: molecules, functions and pathophysiological aspects. Lab. Invest. 2000, 80, 617–624.
- Panaro, M.; Mitolo, V. Cellular responses to FMLP challenging: A mini-review. Immunopharmacol. Immunotoxicol. 1999, 21, 397–419.
- Faurschou, M.; Borregaard, N. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect. 2003, 5, 1317–1327.
- Lever, A.; Mackenzie, I. Sepsis: definition, epidemiology, and diagnosis. Brit. Med. J. 2007, 335, 879–883.
- Levy, L. Carrageenan paw edema in the mouse. Life Sci. 1969, 8, 601–606.
- Sammons, M.J.; Raval, P.; Davey, P.T.; Rogers, D.; Parsons, A.A.; Bingham, S. Carrageenan-induced thermal hyperalgesia in the mouse: role of nerve growth factor and the mitogen-activated protein kinase pathway. Brain Res. 2000, 876, 48–54.
- Kodama, H.-a.; Amagai, Y.; Sudo, H.; Kasai, S.; Yamamoto, S. Establishment of a clonal osteogenic cell line from newborn mouse calvaria. Japanese J. Oral Biol. 1981, 23, 899–901.
- Fujita, I.; Hirano, J.; Itoh, N.; Nakanishi, T.; Tanaka, K. Dexamethasone induces sodium-dependant vitamin C transporter in a mouse osteoblastic cell line MC3T3-E1. Br. J. Nutr. 2001, 86, 145–149.
- Liu, F.-L.; Chen, C.-H.; Chu, S.-J.; Chen, J.-H.; Lai, J.-H.; Sytwu, H.-K.; Chang, D.-M. Interleukin (IL)-23 p19 expression induced by IL-1 β in human fibroblast-like synoviocytes with rheumatoid arthritis via active nuclear factor-κ B and AP-1 dependent pathway. Rheumatology 2007, 46, 1266–1273.
- Pasqualetti, G.; Brooks, D.J.; Edison, P. The role of neuroinflammation in dementias. Curr. Neurol. Neurosci. Rep. 2015, 15, 17–24.
- Firestein, G.S. Evolving concepts of rheumatoid arthritis. Nature 2003, 423, 356–361.
- Choi, M.S.; Park, S.; Choi, T.; Lee, G.; Haam, K.-K.; Hong, M.-C.; Min, B.-I.; Bae, H. Bee venom ameliorates ovalbumin induced allergic asthma via modulating CD4+ CD25+ regulatory T cells in mice. Cytokine 2013, 61, 256–265.
- Loureiro, D.R.; Soares, J.X.; Costa, J.C.; Magalhães, Á.F.; Azevedo, C.M.; Pinto, M.M.; Afonso, C.M. Structures, activities and drug-likeness of anti-infective xanthone derivatives isolated from the marine environment: A review. Molecules 2019, 24, 243.
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686.
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623.





