
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Omar Hahad | + 3856 word(s) | 3856 | 2021-11-16 04:47:41 | | | |
| 2 | Peter Tang | Meta information modification | 3856 | 2021-11-17 03:07:24 | | |
Video Upload Options
Both exposure to higher levels of polluted air and physical inactivity are crucial risk factors for the development and progression of major noncommunicable diseases and, in particular, of cardiovascular disease. While regular physical activity is well known to improve general health, it may also increase the uptake and deposit of air pollutants in the lungs/airways and circulation, due to increased breathing frequency and minute ventilation, thus increasing the risk of cardiovascular disease.
1. Introduction
2. Pathomechanisms of Air Pollution with Focus on Oxidative Stress and Inflammation
|
First Author/Year |
Population/Cohort |
Air Pollutants |
Major Outcomes |
Ref. |
|---|---|---|---|---|
|
Liu, 2021 |
40 chronic obstructive pulmonary disease patients and 75 controls |
PAHs |
A one fold increase in hydroxylated PAHs was associated with a 4.1–15.1% elevation of malondialdehyde, which was stronger in subjects with impaired lung function. |
[18] |
|
Abohashem, 2021 |
503 subjects without cardiovascular disease |
PM2.5 |
Higher PM2.5 was associated with increased risk for major adverse cardiovascular events, mediated by an increase in leucopoietic activity and arterial inflammation. |
[21] |
|
Ni, 2021 |
740 subjects |
PM2.5 |
Acute increases in PM2.5 were associated with increased soluble lectin like oxidized LDL receptor-1, but not with nitrite. |
[46] |
|
Nassan, 2021 |
456 men |
PM2.5 species |
Acute increases in PM2.5 species were associated with metabolic pathways involved in inflammation, oxidative stress, immunity, and nucleic acid damage and repair. |
[19] |
|
Mann, 2021 |
299 children |
Traffic related air pollutants (sum of PAH456, NO2, elemental carbon, PM2.5) |
Acute increases in traffic related air pollutants were associated with 8-isoprostane. |
[22] |
|
Prunicki, 2020 |
100 subjects |
PM2.5, NO, NO2, CO, PAHs |
Air pollutants were associated with oxidative stress, acute inflammation, altered hemostasis, endothelial dysfunction, monocyte enrichment, and diastolic blood pressure. |
[23] |
|
Riggs, 2020 |
100 subjects |
PM2.5 |
A 10 μg/m3 increase in PM2.5 was associated with a 12.4% decrease in reactive hyperemia index (95% CI −21.0–−2.7). Increased PM2.5 was associated with elevated F-2 isoprostane metabolite, angiopoietin 1, vascular endothelial growth factor, placental growth factor, intracellular adhesion molecule-1, and matrix metalloproteinase-9 as well as reduced vascular adhesion molecule-1. |
[47] |
|
Li, 2019 |
73 subjects |
PM2.5, BC, NO2, CO |
Increases in air pollutants were associated with reductions in circulating high density lipoprotein cholesterol and apolipoprotein A-I, as well as elevations in HDL oxidation index, oxidized LDL, malondialdehyde, and C-reactive protein. |
[48] |
|
Lin, 2019 |
26 subjects |
PAHs |
Increases in 5-, 12-, and 15-hydroxyeicosatetraenoic acid, as well as 9- and 13-hydroxyoctadecadienoic acid, were observed. Decreases in paraoxonase and arylesterase, as well increases in C-reactive protein and fibrinogen, were observed. |
[49] |
|
Balmes, 2019 |
87 subjects |
O3 |
Acute O3 exposure did not alter C-reactive protein, monocyte–platelet conjugates, and microparticle associated tissue factor activity, whereas increases in endothelin-1 and decreases in nitrotyrosine were observed. |
[50] |
|
Han, 2019 |
60 subjects with prediabetes and 60 healthy subjects |
PM2.5 |
Acute exposure to PM2.5 resulted in increased exhaled NO, white blood cells, neutrophils, interleukin-1α, and glycated hemoglobin. Compared to healthy subjects, prediabetic subjects displayed pronounced PM2.5 associated systemic inflammation, elevated systolic and diastolic blood pressure, impaired endothelial function, and elevated fasting glucose. |
[51] |
|
Xia, 2019 |
215 pregnant women |
PM2.5 |
Acute increases in PM2.5 and lead constituent were associated with endothelial dysfunction (increased endothelin-1, E-selectin, and intracellular adhesion molecule-1) and inflammation (increased interleukin-1β, interleukin-6, tumor necrosis factor-α). Endothelial dysfunction and elevated inflammation were partially mediated by the effect of PM2.5 and lead constituent on blood pressure. |
[52] |
|
Li, 2019 |
3820 subjects |
PM2.5, BC, O3, sulfate, NOX |
Negative associations of acute PM2.5 and BC with P-selectin, of O3 with monocyte chemoattractant protein 1, and of sulfate and NOx with osteoprotegerin were found. |
[53] |
|
Li, 2017 |
3996 subjects |
PM2.5, sulfate, NOx, BC, O3 |
Acute increases in PM2.5 and sulfate were associated with increased C-reactive protein, which was also true for NOx in case of interleukin-6 and for BC, sulfate, and O3 in case of tumor necrosis factor receptor 2. Conversely, BC, sulfate, and NOx were negatively associated with fibrinogen, and sulfate was negatively associated with tumor necrosis factor α. |
[24] |
|
Mirowsky, 2017 |
13 subjects with coronary artery disease |
O3 |
Per acute IQR increase in O3, changes in tissue plasminogen factor (6.6%, 95% CI 0.4–13.2), plasminogen activator inhibitor-1 (40.5%, 95% CI 8.7–81.6), neutrophils (8.7%, 95% CI 1.5–16.4), monocytes (10.2%, 95% CI 1.0–20.1), interleukin-6 (15.9%, 95% CI 3.6–29.6), large artery elasticity index (−19.5%, 95% CI −34.0–−1.7), and the baseline diameter of the brachial artery (−2.5%, 95% CI −5.0–0.1) were observed. |
[54] |
|
Pope 3rd, 2016 |
24 subjects |
PM2.5 |
Episodic increases in PM2.5 were associated with increased endothelial cell apoptosis, an anti-angiogenic plasma profile, and elevated circulating monocytes, and T, but not B, lymphocytes. |
[55] |
|
Wu, 2016 |
89 subjects |
PM2.5, NO2 |
Acute increases in PM2.5 were associated with brachial–ankle pulse wave velocity, whereas no association was found for NO2. NO2 was associated with increased C-reactive protein. |
[56] |
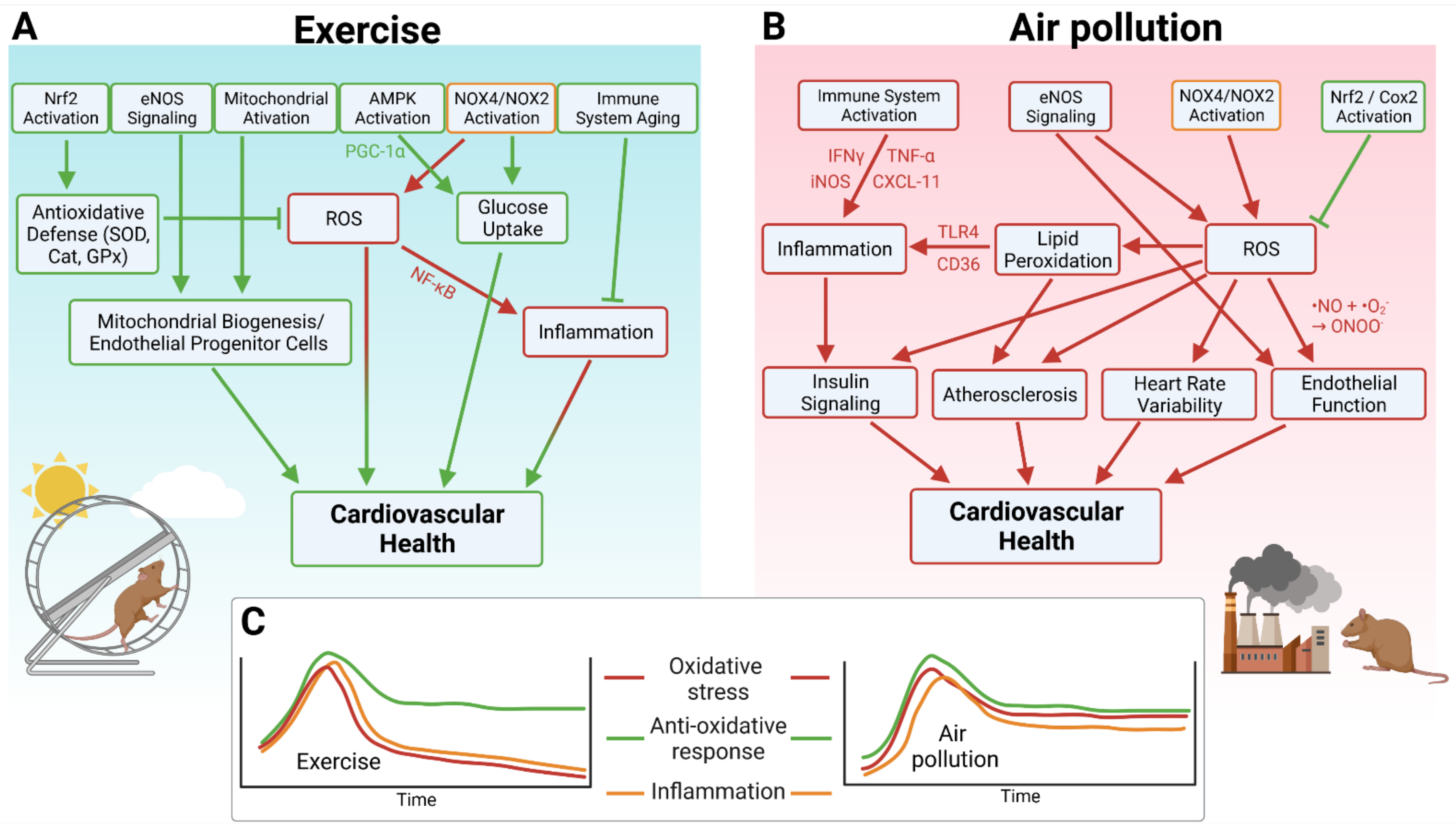
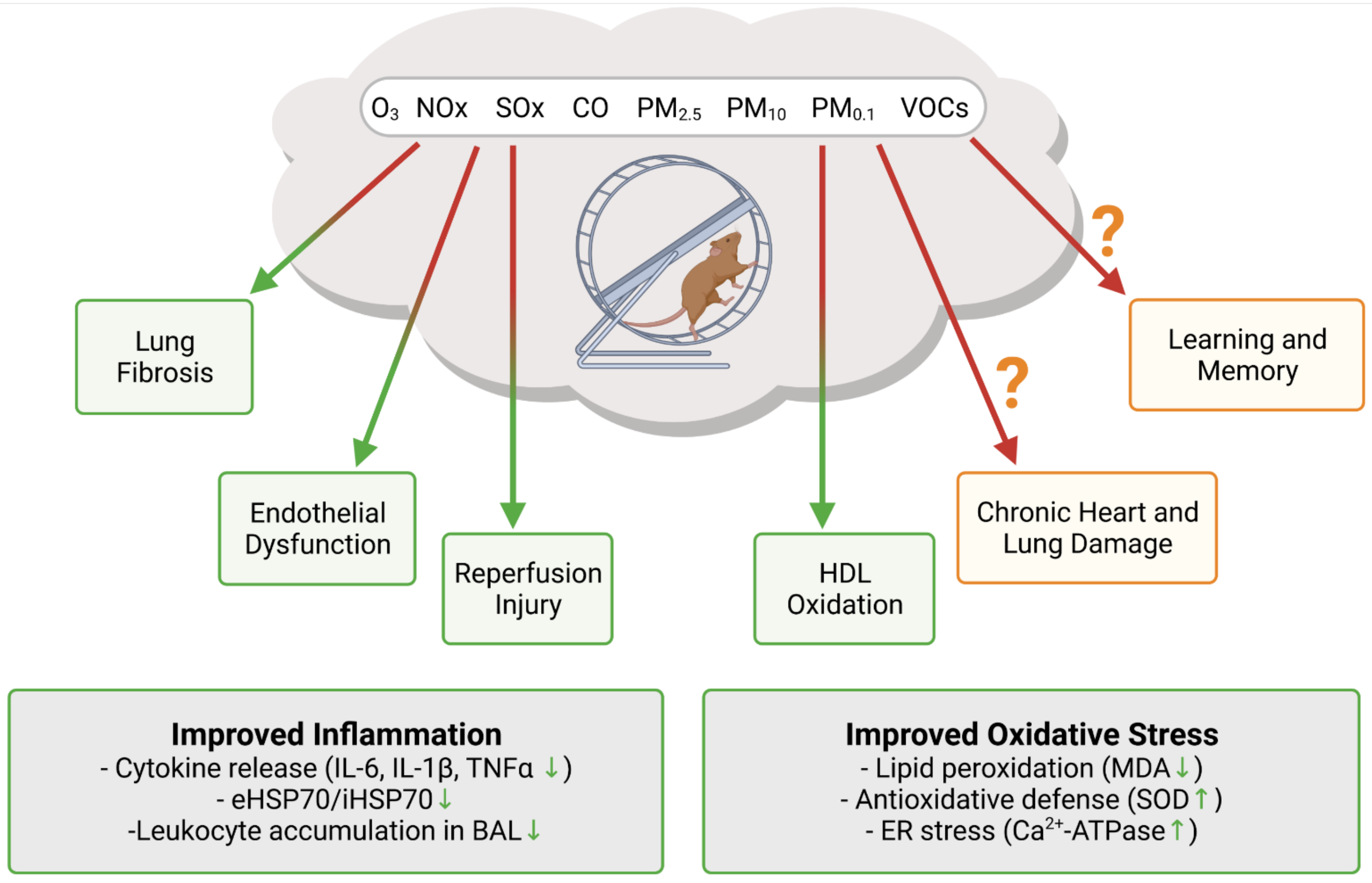
3. Key Mechanisms of Antioxidant and Anti-Inflammatory Effects of Physical Activity
References
- Hallal, P.C.; Victora, C.G.; Azevedo, M.R.; Wells, J.C. Adolescent physical activity and health: A systematic review. Sports Med. 2006, 36, 1019–1030.
- Guthold, R.; Stevens, G.A.; Riley, L.M.; Bull, F.C. Worldwide trends in insufficient physical activity from 2001 to 2016: A pooled analysis of 358 population-based surveys with 1.9 million participants. Lancet Glob. Health 2018, 6, e1077–e1086.
- Hou, J.; Liu, X.; Tu, R.; Dong, X.; Zhai, Z.; Mao, Z.; Huo, W.; Chen, G.; Xiang, H.; Guo, Y.; et al. Long-term exposure to ambient air pollution attenuated the association of physical activity with metabolic syndrome in rural Chinese adults: A cross-sectional study. Environ. Int. 2020, 136, 105459.
- Yu, Y.; Paul, K.; Arah, O.A.; Mayeda, E.R.; Wu, J.; Lee, E.; Shih, I.F.; Su, J.; Jerrett, M.; Haan, M.; et al. Air pollution, noise exposure, and metabolic syndrome—A cohort study in elderly Mexican-Americans in Sacramento area. Environ. Int. 2020, 134, 105269.
- Chevalier, N.; Fenichel, P. Endocrine disruptors: New players in the pathophysiology of type 2 diabetes? Diabetes Metab. 2015, 41, 107–115.
- Huang, T.; Chan, T.C.; Huang, Y.J.; Pan, W.C. The Association between Noise Exposure and Metabolic Syndrome: A Longitudinal Cohort Study in Taiwan. Int. J. Environ. Res. Public Health 2020, 17, 4236.
- World Health Organization. 9 out of 10 People Worldwide Breathe Polluted Air, but More Countries Are Taking Action. Available online: https://www.who.int/news-room/detail/02-05-2018-9-out-of-10-peopleworldwide-breathe-polluted-air-but-more-countries-are-taking-action (accessed on 6 August 2021).
- Tainio, M.; Jovanovic Andersen, Z.; Nieuwenhuijsen, M.J.; Hu, L.; de Nazelle, A.; An, R.; Garcia, L.M.T.; Goenka, S.; Zapata-Diomedi, B.; Bull, F.; et al. Air pollution, physical activity and health: A mapping review of the evidence. Environ. Int. 2021, 147, 105954.
- Clark, C.; Sbihi, H.; Tamburic, L.; Brauer, M.; Frank, L.D.; Davies, H.W. Association of Long-Term Exposure to Transportation Noise and Traffic-Related Air Pollution with the Incidence of Diabetes: A Prospective Cohort Study. Environ. Health Perspect. 2017, 125, 087025.
- Liu, C.; Ying, Z.; Harkema, J.; Sun, Q.; Rajagopalan, S. Epidemiological and experimental links between air pollution and type 2 diabetes. Toxicol. Pathol. 2013, 41, 361–373.
- Rajagopalan, S.; Brook, R.D. Air pollution and type 2 diabetes: Mechanistic insights. Diabetes 2012, 61, 3037–3045.
- World Health Organization. Air Pollution. Available online: https://www.who.int/health-topics/air-pollution#tab=tab_1 (accessed on 6 August 2021).
- World Health Organization. Physical Activity. Available online: https://www.who.int/westernpacific/health-topics/physical-activity (accessed on 6 August 2021).
- Lelieveld, J.; Klingmuller, K.; Pozzer, A.; Poschl, U.; Fnais, M.; Daiber, A.; Munzel, T. Cardiovascular disease burden from ambient air pollution in Europe reassessed using novel hazard ratio functions. Eur. Heart J. 2019, 40, 1590–1596.
- World Health Organization. Personal Interventions and Risk Communication on air Pollution. Available online: https://www.who.int/publications/i/item/9789240000278 (accessed on 6 August 2021).
- Hahad, O.; Lelieveld, J.; Birklein, F.; Lieb, K.; Daiber, A.; Munzel, T. Ambient Air Pollution Increases the Risk of Cerebrovascular and Neuropsychiatric Disorders through Induction of Inflammation and Oxidative Stress. Int. J. Mol. Sci. 2020, 21, 4306.
- Hahad, O.; Frenis, K.; Kuntic, M.; Daiber, A.; Munzel, T. Accelerated Aging and Age-Related Diseases (CVD and Neurological) Due to Air Pollution and Traffic Noise Exposure. Int. J. Mol. Sci. 2021, 22, 2419.
- Liu, J.; Chen, X.; Qiu, X.; Zhang, H.; Lu, X.; Li, H.; Chen, W.; Zhang, L.; Que, C.; Zhu, T. Association between exposure to polycyclic aromatic hydrocarbons and lipid peroxidation in patients with chronic obstructive pulmonary disease. Sci. Total Environ. 2021, 780, 146660.
- Nassan, F.L.; Wang, C.; Kelly, R.S.; Lasky-Su, J.A.; Vokonas, P.S.; Koutrakis, P.; Schwartz, J.D. Ambient PM2.5 species and ultrafine particle exposure and their differential metabolomic signatures. Environ. Int. 2021, 151, 106447.
- Daiber, A.; Kuntic, M.; Hahad, O.; Delogu, L.G.; Rohrbach, S.; Di Lisa, F.; Schulz, R.; Munzel, T. Effects of air pollution particles (ultrafine and fine particulate matter) on mitochondrial function and oxidative stress—Implications for cardiovascular and neurodegenerative diseases. Arch. Biochem. Biophys. 2020, 696, 108662.
- Abohashem, S.; Osborne, M.T.; Dar, T.; Naddaf, N.; Abbasi, T.; Ghoneem, A.; Radfar, A.; Patrich, T.; Oberfeld, B.; Tung, B.; et al. A leucopoietic-arterial axis underlying the link between ambient air pollution and cardiovascular disease in humans. Eur. Heart J. 2021, 42, 761–772.
- Mann, J.K.; Lutzker, L.; Holm, S.M.; Margolis, H.G.; Neophytou, A.M.; Eisen, E.A.; Costello, S.; Tyner, T.; Holland, N.; Tindula, G.; et al. Traffic-related air pollution is associated with glucose dysregulation, blood pressure, and oxidative stress in children. Environ. Res. 2021, 195, 110870.
- Prunicki, M.; Cauwenberghs, N.; Ataam, J.A.; Movassagh, H.; Kim, J.B.; Kuznetsova, T.; Wu, J.C.; Maecker, H.; Haddad, F.; Nadeau, K. Immune biomarkers link air pollution exposure to blood pressure in adolescents. Environ. Health 2020, 19, 108.
- Li, W.; Dorans, K.S.; Wilker, E.H.; Rice, M.B.; Ljungman, P.L.; Schwartz, J.D.; Coull, B.A.; Koutrakis, P.; Gold, D.R.; Keaney, J.F., Jr.; et al. Short-Term Exposure to Ambient Air Pollution and Biomarkers of Systemic Inflammation: The Framingham Heart Study. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1793–1800.
- Prows, D.R.; Shertzer, H.G.; Daly, M.J.; Sidman, C.L.; Leikauf, G.D. Genetic analysis of ozone-induced acute lung injury in sensitive and resistant strains of mice. Nat. Genet. 1997, 17, 471–474.
- Marchini, T.; Zirlik, A.; Wolf, D. Pathogenic Role of Air Pollution Particulate Matter in Cardiometabolic Disease: Evidence from Mice and Humans. Antioxid. Redox Signal. 2020, 33, 263–279.
- Sunyer, J.; Ballester, F.; Tertre, A.L.; Atkinson, R.; Ayres, J.G.; Forastiere, F.; Forsberg, B.; Vonk, J.M.; Bisanti, L.; Tenias, J.M.; et al. The association of daily sulfur dioxide air pollution levels with hospital admissions for cardiovascular diseases in Europe (The Aphea-II study). Eur. Heart J. 2003, 24, 752–760.
- Chuang, G.C.; Yang, Z.; Westbrook, D.G.; Pompilius, M.; Ballinger, C.A.; White, C.R.; Krzywanski, D.M.; Postlethwait, E.M.; Ballinger, S.W. Pulmonary ozone exposure induces vascular dysfunction, mitochondrial damage, and atherogenesis. Am. J. Physiol. Lung Cell Mol. Physiol. 2009, 297, L209–L216.
- Robertson, S.; Colombo, E.S.; Lucas, S.N.; Hall, P.R.; Febbraio, M.; Paffett, M.L.; Campen, M.J. CD36 mediates endothelial dysfunction downstream of circulating factors induced by O3 exposure. Toxicol. Sci. 2013, 134, 304–311.
- Zhong, J.; Allen, K.; Rao, X.; Ying, Z.; Braunstein, Z.; Kankanala, S.R.; Xia, C.; Wang, X.; Bramble, L.A.; Wagner, J.G.; et al. Repeated ozone exposure exacerbates insulin resistance and activates innate immune response in genetically susceptible mice. Inhal. Toxicol. 2016, 28, 383–392.
- Hamade, A.K.; Rabold, R.; Tankersley, C.G. Adverse cardiovascular effects with acute particulate matter and ozone exposures: Interstrain variation in mice. Environ. Health Perspect. 2008, 116, 1033–1039.
- Munzel, T.; Gori, T.; Al-Kindi, S.; Deanfield, J.; Lelieveld, J.; Daiber, A.; Rajagopalan, S. Effects of gaseous and solid constituents of air pollution on endothelial function. Eur. Heart J. 2018, 39, 3543–3550.
- Kampfrath, T.; Maiseyeu, A.; Ying, Z.; Shah, Z.; Deiuliis, J.A.; Xu, X.; Kherada, N.; Brook, R.D.; Reddy, K.M.; Padture, N.P.; et al. Chronic fine particulate matter exposure induces systemic vascular dysfunction via NADPH oxidase and TLR4 pathways. Circ. Res. 2011, 108, 716–726.
- Sun, Q.; Yue, P.; Ying, Z.; Cardounel, A.J.; Brook, R.D.; Devlin, R.; Hwang, J.S.; Zweier, J.L.; Chen, L.C.; Rajagopalan, S. Air pollution exposure potentiates hypertension through reactive oxygen species-mediated activation of Rho/ROCK. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1760–1766.
- Xu, Y.; Arora, R.C.; Hiebert, B.M.; Lerner, B.; Szwajcer, A.; McDonald, K.; Rigatto, C.; Komenda, P.; Sood, M.M.; Tangri, N. Non-invasive endothelial function testing and the risk of adverse outcomes: A systematic review and meta-analysis. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 736–746.
- Daiber, A.; Steven, S.; Weber, A.; Shuvaev, V.V.; Muzykantov, V.R.; Laher, I.; Li, H.; Lamas, S.; Munzel, T. Targeting vascular (endothelial) dysfunction. Br. J. Pharmacol. 2017, 174, 1591–1619.
- Matsuzawa, Y.; Kwon, T.G.; Lennon, R.J.; Lerman, L.O.; Lerman, A. Prognostic Value of Flow-Mediated Vasodilation in Brachial Artery and Fingertip Artery for Cardiovascular Events: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2015, 4, e002270.
- Beckman, J.S.; Koppenol, W.H. Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and ugly. Am. J. Physiol. 1996, 271, C1424–C1437.
- Crow, J.P.; Beckman, J.S. Reaction between Nitric Oxide, Superoxide, and Peroxynitrite: Footprints of Peroxynitrite in Vivo. Adv. Pharmacol. 1995, 35, 17–43.
- Sun, Q.; Wang, A.; Jin, X.; Natanzon, A.; Duquaine, D.; Brook, R.D.; Aguinaldo, J.G.; Fayad, Z.A.; Fuster, V.; Lippmann, M.; et al. Long-term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model. JAMA 2005, 294, 3003–3010.
- Cherng, T.W.; Paffett, M.L.; Jackson-Weaver, O.; Campen, M.J.; Walker, B.R.; Kanagy, N.L. Mechanisms of diesel-induced endothelial nitric oxide synthase dysfunction in coronary arterioles. Environ. Health Perspect. 2011, 119, 98–103.
- Davel, A.P.; Lemos, M.; Pastro, L.M.; Pedro, S.C.; de Andre, P.A.; Hebeda, C.; Farsky, S.H.; Saldiva, P.H.; Rossoni, L.V. Endothelial dysfunction in the pulmonary artery induced by concentrated fine particulate matter exposure is associated with local but not systemic inflammation. Toxicology 2012, 295, 39–46.
- Ying, Z.; Xu, X.; Chen, M.; Liu, D.; Zhong, M.; Chen, L.C.; Sun, Q.; Rajagopalan, S. A synergistic vascular effect of airborne particulate matter and nickel in a mouse model. Toxicol. Sci. 2013, 135, 72–80.
- Rao, X.; Zhong, J.; Maiseyeu, A.; Gopalakrishnan, B.; Villamena, F.A.; Chen, L.C.; Harkema, J.R.; Sun, Q.; Rajagopalan, S. CD36-dependent 7-ketocholesterol accumulation in macrophages mediates progression of atherosclerosis in response to chronic air pollution exposure. Circ. Res. 2014, 115, 770–780.
- Courtois, A.; Andujar, P.; Ladeiro, Y.; Baudrimont, I.; Delannoy, E.; Leblais, V.; Begueret, H.; Galland, M.A.; Brochard, P.; Marano, F.; et al. Impairment of NO-dependent relaxation in intralobar pulmonary arteries: Comparison of urban particulate matter and manufactured nanoparticles. Environ. Health Perspect. 2008, 116, 1294–1299.
- Ni, Y.; Tracy, R.P.; Cornell, E.; Kaufman, J.D.; Szpiro, A.A.; Campen, M.J.; Vedal, S. Short-term exposure to air pollution and biomarkers of cardiovascular effect: A repeated measures study. Environ. Pollut. 2021, 279, 116893.
- Riggs, D.W.; Zafar, N.; Krishnasamy, S.; Yeager, R.; Rai, S.N.; Bhatnagar, A.; O’Toole, T.E. Exposure to airborne fine particulate matter is associated with impaired endothelial function and biomarkers of oxidative stress and inflammation. Environ. Res. 2020, 180, 108890.
- Li, J.; Zhou, C.; Xu, H.; Brook, R.D.; Liu, S.; Yi, T.; Wang, Y.; Feng, B.; Zhao, M.; Wang, X.; et al. Ambient Air Pollution Is Associated With HDL (High-Density Lipoprotein) Dysfunction in Healthy Adults. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 513–522.
- Lin, Y.; Ramanathan, G.; Zhu, Y.; Yin, F.; Rea, N.D.; Lu, X.; Tseng, C.H.; Faull, K.F.; Yoon, A.J.; Jerrett, M.; et al. Pro-Oxidative and Proinflammatory Effects After Traveling from Los Angeles to Beijing: A Biomarker-Based Natural Experiment. Circulation 2019, 140, 1995–2004.
- Balmes, J.R.; Arjomandi, M.; Bromberg, P.A.; Costantini, M.G.; Dagincourt, N.; Hazucha, M.J.; Hollenbeck-Pringle, D.; Rich, D.Q.; Stark, P.; Frampton, M.W. Ozone effects on blood biomarkers of systemic inflammation, oxidative stress, endothelial function, and thrombosis: The Multicenter Ozone Study in oldEr Subjects (MOSES). PLoS ONE 2019, 14, e0222601.
- Han, Y.; Wang, Y.; Li, W.; Chen, X.; Xue, T.; Chen, W.; Fan, Y.; Qiu, X.; Zhu, T. Susceptibility of prediabetes to the health effect of air pollution: A community-based panel study with a nested case-control design. Environ. Health 2019, 18, 65.
- Xia, B.; Zhou, Y.; Zhu, Q.; Zhao, Y.; Wang, Y.; Ge, W.; Yang, Q.; Zhao, Y.; Wang, P.; Si, J.; et al. Personal exposure to PM2.5 constituents associated with gestational blood pressure and endothelial dysfunction. Environ. Pollut. 2019, 250, 346–356.
- Li, W.; Dorans, K.S.; Wilker, E.H.; Rice, M.B.; Ljungman, P.L.; Schwartz, J.D.; Coull, B.A.; Koutrakis, P.; Gold, D.R.; Keaney, J.F., Jr.; et al. Short-term exposure to ambient air pollution and circulating biomarkers of endothelial cell activation: The Framingham Heart Study. Environ. Res. 2019, 171, 36–43.
- Mirowsky, J.E.; Carraway, M.S.; Dhingra, R.; Tong, H.; Neas, L.; Diaz-Sanchez, D.; Cascio, W.; Case, M.; Crooks, J.; Hauser, E.R.; et al. Ozone exposure is associated with acute changes in inflammation, fibrinolysis, and endothelial cell function in coronary artery disease patients. Environ. Health 2017, 16, 126.
- Pope, C.A., 3rd; Bhatnagar, A.; McCracken, J.P.; Abplanalp, W.; Conklin, D.J.; O’Toole, T. Exposure to Fine Particulate Air Pollution Is Associated With Endothelial Injury and Systemic Inflammation. Circ. Res. 2016, 119, 1204–1214.
- Wu, C.F.; Shen, F.H.; Li, Y.R.; Tsao, T.M.; Tsai, M.J.; Chen, C.C.; Hwang, J.S.; Hsu, S.H.; Chao, H.; Chuang, K.J.; et al. Association of short-term exposure to fine particulate matter and nitrogen dioxide with acute cardiovascular effects. Sci. Total Environ. 2016, 569–570, 300–305.
- Schmidt, S.C.E.; Tittlbach, S.; Bos, K.; Woll, A. Different Types of Physical Activity and Fitness and Health in Adults: An 18-Year Longitudinal Study. Biomed. Res. Int. 2017, 2017, 1785217.
- Arnold, N.; Deiseroth, A.; Hahad, O.; Diestelmeier, S.; Schulz, A.; Daubenbuchel, A.; Gori, T.; Binder, H.; Pfeiffer, N.; Prochaska, J.; et al. Domains of Physical Activity in Relation to Stiffness Index in the General Population. J. Am. Heart Assoc. 2021, 10, e020930.
- Mury, P.; Chirico, E.N.; Mura, M.; Millon, A.; Canet-Soulas, E.; Pialoux, V. Oxidative Stress and Inflammation, Key Targets of Atherosclerotic Plaque Progression and Vulnerability: Potential Impact of Physical Activity. Sports Med. 2018, 48, 2725–2741.
- Gardner, A.W.; Montgomery, P.S.; Casanegra, A.I.; Silva-Palacios, F.; Ungvari, Z.; Csiszar, A. Association between gait characteristics and endothelial oxidative stress and inflammation in patients with symptomatic peripheral artery disease. Age 2016, 38, 64.
- Gardner, A.W.; Montgomery, P.S.; Zhao, Y.D.; Silva-Palacios, F.; Ungvari, Z.; Csiszar, A.; Sonntag, W.E. Association between daily walking and antioxidant capacity in patients with symptomatic peripheral artery disease. J. Vasc. Surg. 2017, 65, 1762–1768.
- Ford, E.S. Does exercise reduce inflammation? Physical activity and C-reactive protein among U.S. adults. Epidemiology 2002, 13, 561–568.
- Mora, S.; Lee, I.M.; Buring, J.E.; Ridker, P.M. Association of physical activity and body mass index with novel and traditional cardiovascular biomarkers in women. JAMA 2006, 295, 1412–1419.
- Yu, Z.; Ye, X.; Wang, J.; Qi, Q.; Franco, O.H.; Rennie, K.L.; Pan, A.; Li, H.; Liu, Y.; Hu, F.B.; et al. Associations of physical activity with inflammatory factors, adipocytokines, and metabolic syndrome in middle-aged and older chinese people. Circulation 2009, 119, 2969–2977.
- Palmefors, H.; DuttaRoy, S.; Rundqvist, B.; Borjesson, M. The effect of physical activity or exercise on key biomarkers in atherosclerosis—A systematic review. Atherosclerosis 2014, 235, 150–161.
- Ammar, A.; Trabelsi, K.; Boukhris, O.; Glenn, J.M.; Bott, N.; Masmoudi, L.; Hakim, A.; Chtourou, H.; Driss, T.; Hoekelmann, A.; et al. Effects of Aerobic-, Anaerobic- and Combined-Based Exercises on Plasma Oxidative Stress Biomarkers in Healthy Untrained Young Adults. Int. J. Environ. Res. Public Health 2020, 17, 2601.
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619.
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748.
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183.
- Andreadou, I.; Efentakis, P.; Frenis, K.; Daiber, A.; Schulz, R. Thiol-based redox-active proteins as cardioprotective therapeutic agents in cardiovascular diseases. Basic Res. Cardiol. 2021, 116, 44.
- Dillard, C.J.; Litov, R.E.; Savin, W.M.; Dumelin, E.E.; Tappel, A.L. Effects of exercise, vitamin E, and ozone on pulmonary function and lipid peroxidation. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1978, 45, 927–932.
- Brady, P.S.; Brady, L.J.; Ullrey, D.E. Selenium, vitamin E and the response to swimming stress in the rat. J. Nutr. 1979, 109, 1103–1109.
- Jackson, M.J.; Jones, D.A.; Edwards, R.H. Vitamin E and skeletal muscle. In Biology of Vitamin E; Pitman Books: London, UK, 1983; pp. 224–239.
- Quintanilha, A.T.; Packer, L. Vitamin E, physical exercise and tissue oxidative damage. In Biology of Vitamin E; Pitman Books: London, UK, 1983; pp. 56–69.
- Sakellariou, G.K.; Vasilaki, A.; Palomero, J.; Kayani, A.; Zibrik, L.; McArdle, A.; Jackson, M.J. Studies of mitochondrial and nonmitochondrial sources implicate nicotinamide adenine dinucleotide phosphate oxidase(s) in the increased skeletal muscle superoxide generation that occurs during contractile activity. Antioxid. Redox Signal. 2013, 18, 603–621.
- Hancock, M.; Hafstad, A.D.; Nabeebaccus, A.A.; Catibog, N.; Logan, A.; Smyrnias, I.; Hansen, S.S.; Lanner, J.; Schroder, K.; Murphy, M.P.; et al. Myocardial NADPH oxidase-4 regulates the physiological response to acute exercise. eLife 2018, 7, e41044.
- Henriquez-Olguin, C.; Knudsen, J.R.; Raun, S.H.; Li, Z.; Dalbram, E.; Treebak, J.T.; Sylow, L.; Holmdahl, R.; Richter, E.A.; Jaimovich, E.; et al. Cytosolic ROS production by NADPH oxidase 2 regulates muscle glucose uptake during exercise. Nat. Commun. 2019, 10, 4623.
- Osorio Alves, J.; Matta Pereira, L.; Cabral Coutinho do Rego Monteiro, I.; Pontes Dos Santos, L.H.; Soares Marreiros Ferraz, A.; Carneiro Loureiro, A.C.; Calado Lima, C.; Leal-Cardoso, J.H.; Pires Carvalho, D.; Soares Fortunato, R.; et al. Strenuous Acute Exercise Induces Slow and Fast Twitch-Dependent NADPH Oxidase Expression in Rat Skeletal Muscle. Antioxidants 2020, 9, 57.
- Vina, J.; Gimeno, A.; Sastre, J.; Desco, C.; Asensi, M.; Pallardo, F.V.; Cuesta, A.; Ferrero, J.A.; Terada, L.S.; Repine, J.E. Mechanism of free radical production in exhaustive exercise in humans and rats; role of xanthine oxidase and protection by allopurinol. IUBMB Life 2000, 49, 539–544.
- Powers, S.K.; Deminice, R.; Ozdemir, M.; Yoshihara, T.; Bomkamp, M.P.; Hyatt, H. Exercise-induced oxidative stress: Friend or foe? J. Sport Health Sci. 2020, 9, 415–425.
- Daiber, A.; Di Lisa, F.; Oelze, M.; Kroller-Schon, S.; Steven, S.; Schulz, E.; Munzel, T. Crosstalk of mitochondria with NADPH oxidase via reactive oxygen and nitrogen species signalling and its role for vascular function. Br. J. Pharmacol. 2017, 174, 1670–1689.
- Daiber, A. Redox signaling (cross-talk) from and to mitochondria involves mitochondrial pores and reactive oxygen species. Biochim. Biophys. Acta 2010, 1797, 897–906.
- Schulz, E.; Wenzel, P.; Munzel, T.; Daiber, A. Mitochondrial redox signaling: Interaction of mitochondrial reactive oxygen species with other sources of oxidative stress. Antioxid. Redox Signal. 2014, 20, 308–324.
- Dikalov, S. Cross talk between mitochondria and NADPH oxidases. Free Radic. Biol. Med. 2011, 51, 1289–1301.
- Jackson, M.J.; Vasilaki, A.; McArdle, A. Cellular mechanisms underlying oxidative stress in human exercise. Free Radic. Biol. Med. 2016, 98, 13–17.
- Bouviere, J.; Fortunato, R.S.; Dupuy, C.; Werneck-de-Castro, J.P.; Carvalho, D.P.; Louzada, R.A. Exercise-Stimulated ROS Sensitive Signaling Pathways in Skeletal Muscle. Antioxidants 2021, 10, 537.
- Higuchi, M.; Cartier, L.J.; Chen, M.; Holloszy, J.O. Superoxide dismutase and catalase in skeletal muscle: Adaptive response to exercise. J. Gerontol. 1985, 40, 281–286.
- Kanter, M.M.; Hamlin, R.L.; Unverferth, D.V.; Davis, H.W.; Merola, A.J. Effect of exercise training on antioxidant enzymes and cardiotoxicity of doxorubicin. J. Appl. Physiol. 1985, 59, 1298–1303.
- Kawamura, T.; Muraoka, I. Exercise-Induced Oxidative Stress and the Effects of Antioxidant Intake from a Physiological Viewpoint. Antioxidants 2018, 7, 119.
- Criswell, D.; Powers, S.; Dodd, S.; Lawler, J.; Edwards, W.; Renshler, K.; Grinton, S. High intensity training-induced changes in skeletal muscle antioxidant enzyme activity. Med. Sci. Sports Exerc. 1993, 25, 1135–1140.
- Ji, L.L.; Fu, R.; Mitchell, E.W. Glutathione and antioxidant enzymes in skeletal muscle: Effects of fiber type and exercise intensity. J. Appl. Physiol. 1992, 73, 1854–1859.
- Ji, L.L.; Stratman, F.W.; Lardy, H.A. Antioxidant enzyme systems in rat liver and skeletal muscle. Influences of selenium deficiency, chronic training, and acute exercise. Arch. Biochem. Biophys. 1988, 263, 150–160.
- Laughlin, M.H.; Simpson, T.; Sexton, W.L.; Brown, O.R.; Smith, J.K.; Korthuis, R.J. Skeletal muscle oxidative capacity, antioxidant enzymes, and exercise training. J. Appl. Physiol. 1990, 68, 2337–2343.
- Jansen, T.; Kvandova, M.; Daiber, A.; Stamm, P.; Frenis, K.; Schulz, E.; Munzel, T.; Kroller-Schon, S. The AMP-Activated Protein Kinase Plays a Role in Antioxidant Defense and Regulation of Vascular Inflammation. Antioxidants 2020, 9, 525.
- Kroller-Schon, S.; Jansen, T.; Hauptmann, F.; Schuler, A.; Heeren, T.; Hausding, M.; Oelze, M.; Viollet, B.; Keaney, J.F., Jr.; Wenzel, P.; et al. alpha1AMP-activated protein kinase mediates vascular protective effects of exercise. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1632–1641.
- Campbell, J.P.; Turner, J.E. Debunking the Myth of Exercise-Induced Immune Suppression: Redefining the Impact of Exercise on Immunological Health Across the Lifespan. Front. Immunol. 2018, 9, 648.
- Wenzel, P.; Kossmann, S.; Munzel, T.; Daiber, A. Redox regulation of cardiovascular inflammation—Immunomodulatory function of mitochondrial and Nox-derived reactive oxygen and nitrogen species. Free Radic. Biol. Med. 2017, 109, 48–60.
- Sen, C.K. Glutathione homeostasis in response to exercise training and nutritional supplements. Mol. Cell Biochem. 1999, 196, 31–42.
- Golbidi, S.; Li, H.; Laher, I. Oxidative Stress: A Unifying Mechanism for Cell Damage Induced by Noise, (Water-Pipe) Smoking, and Emotional Stress-Therapeutic Strategies Targeting Redox Imbalance. Antioxid. Redox Signal. 2018, 28, 741–759.
- Sallam, N.; Laher, I. Exercise Modulates Oxidative Stress and Inflammation in Aging and Cardiovascular Diseases. Oxid. Med. Cell Longev. 2016, 2016, 7239639.
- Golbidi, S.; Daiber, A.; Korac, B.; Li, H.; Essop, M.F.; Laher, I. Health Benefits of Fasting and Caloric Restriction. Curr. Diab. Rep. 2017, 17, 123.




