
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rafael Cypriano Dutra | + 3539 word(s) | 3539 | 2021-11-12 05:13:02 | | | |
| 2 | Peter Tang | Meta information modification | 3539 | 2021-11-16 02:54:31 | | |
Video Upload Options
Cannabinoids is a vast term that defines several compounds that have been characterized in three categories: (i) endogenous, (ii) synthetic, and (iii) phytocannabinoids, and are able to modulate the CBR and ECS. Particularly, phytocannabinoids are natural terpenoids or phenolic compounds derived from Cannabis sativa. Cannabimimetic ligands, beyond the Cannabis plant, can act as CBR agonists or antagonists, or ECS enzyme inhibitors, besides being able of playing a role in immune-mediated inflammatory and infectious diseases, neuroinflammatory, neurological, and neurodegenerative diseases, as well as in cancer, and autoimmunity by itself.
1. The Era of Cannabis sativa, Cannabinoids, and the Endocannabinoid System: A Long Journey Traveled
|
Chemical Class |
Compounds |
|---|---|
|
Δ9-THC types |
23 |
|
Δ8-THC types |
5 |
|
CBG types |
16 |
|
CBC types |
9 |
|
CBD types |
7 |
|
CBND types |
2 |
|
CBE types |
5 |
|
CBL types |
3 |
|
CBN types |
11 |
|
CBT types |
9 |
|
Miscellaneous types |
30 |
|
Total cannabinoids |
120 |
|
Total non-cannabinoids |
445 |
|
Grand Total |
565 |
|
Research Themes |
Main Findings |
References |
|---|---|---|
|
Alzheimer’s disease (AD) |
CBD prevented expression of proteins involved with tau phosphorylation and AD progression. CBD showed therapeutic potential for AD-associated cognitive impairment. |
|
|
Anti-inflammatory properties |
CBD induced apoptosis and inhibited lipopolysaccharide-activated NF-κB and interferon-β/STAT inflammatory pathways in microglial cells; CBD protected oligodendrocytes progenitor cells from inflammatory-induced apoptosis. |
[24] |
|
Anxiety |
CBD modulated anxiety responses partially through 5-HT1A-mediated neurotransmission, and demonstrated anxiolytic effects during a stimulated public speaking test; CBD action on limbic and paralimbic regions contributed to reduced autonomic arousal and subjective anxiety; CBD blocked anxiety-induced REM sleep alteration through anxiolytic properties. |
|
|
Diabetes |
CBD showed beneficial effects on glycemic control and cardiovascular dysfunction during diabetes. |
[27] |
|
Immunomodulatory effects |
CBD modulated T-cell function and apoptotic signaling pathway. |
[28] |
|
Inflammatory bowel disease (IBD) |
CBD attenuated intestinal inflammation and normalized motility in patients with IBD. |
[29] |
|
Cognitive impairments |
CBD interacted with components of emotional memory processing and memory-rescuing, as well as attenuated THC-induced memory impairment effects. |
[30] |
|
Neuropathic pain |
CBD inhibited chemotherapy-induced neuropathic pain. |
|
|
Parkinson’s disease (PD) |
CBD administration showed neuroprotective effects during PD progression. |
[33] |
|
Schizophrenia |
CBD showed antipsychotic-like properties in schizophrenia, as well as prevented clinical social dysfunction, and inhibited psychomotor agitation. |
|
|
Seizure/Epilepsy |
CBD showed anticonvulsant effects in animal models of seizure and patients with refractory epilepsy. CBD was also described as safe and beneficial for the treatment of epileptic disorders. |
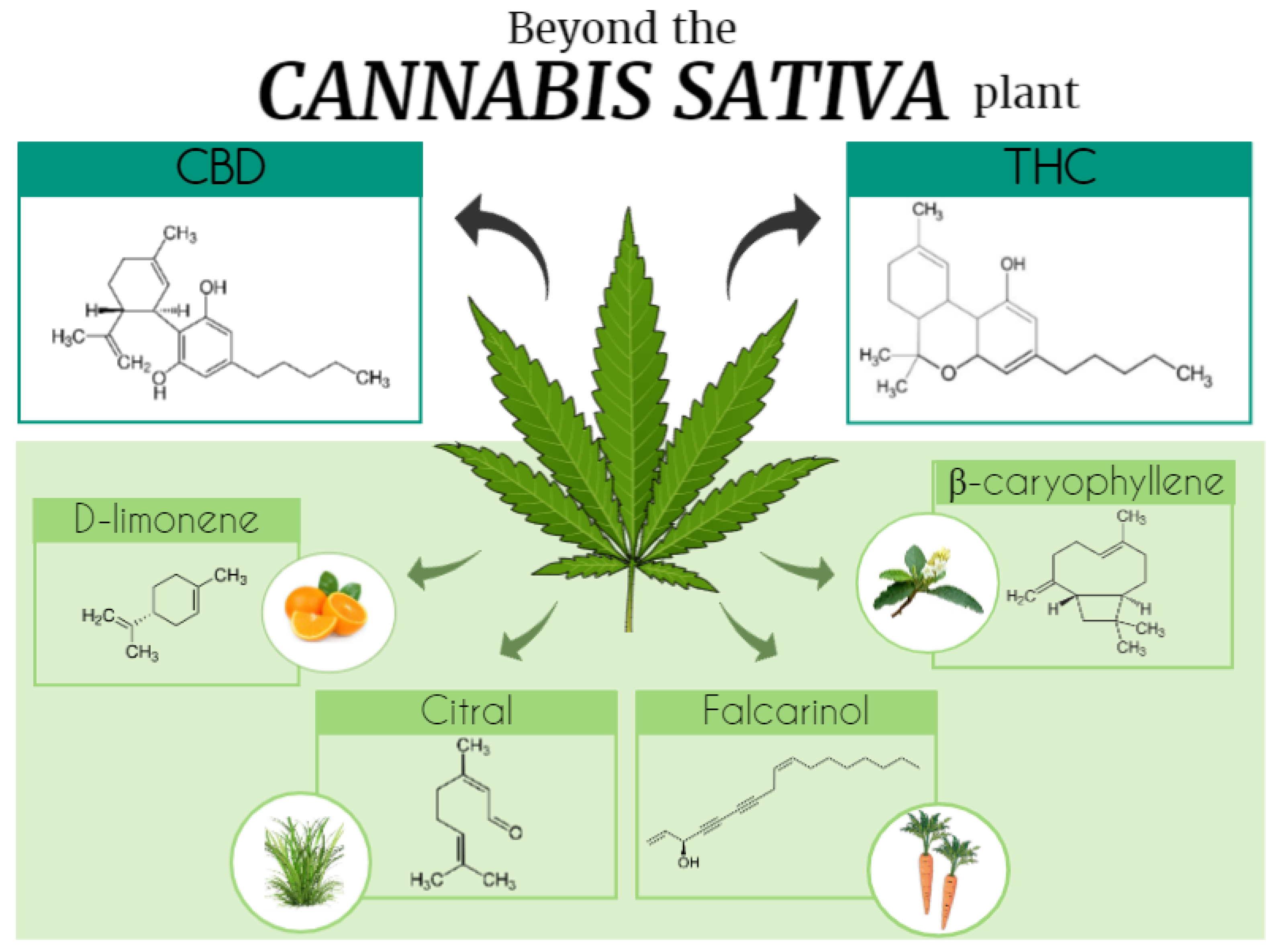
2. Cannabis Phytocannabinoids: Focus on Tetrahydrocannabinol and Cannabidiol
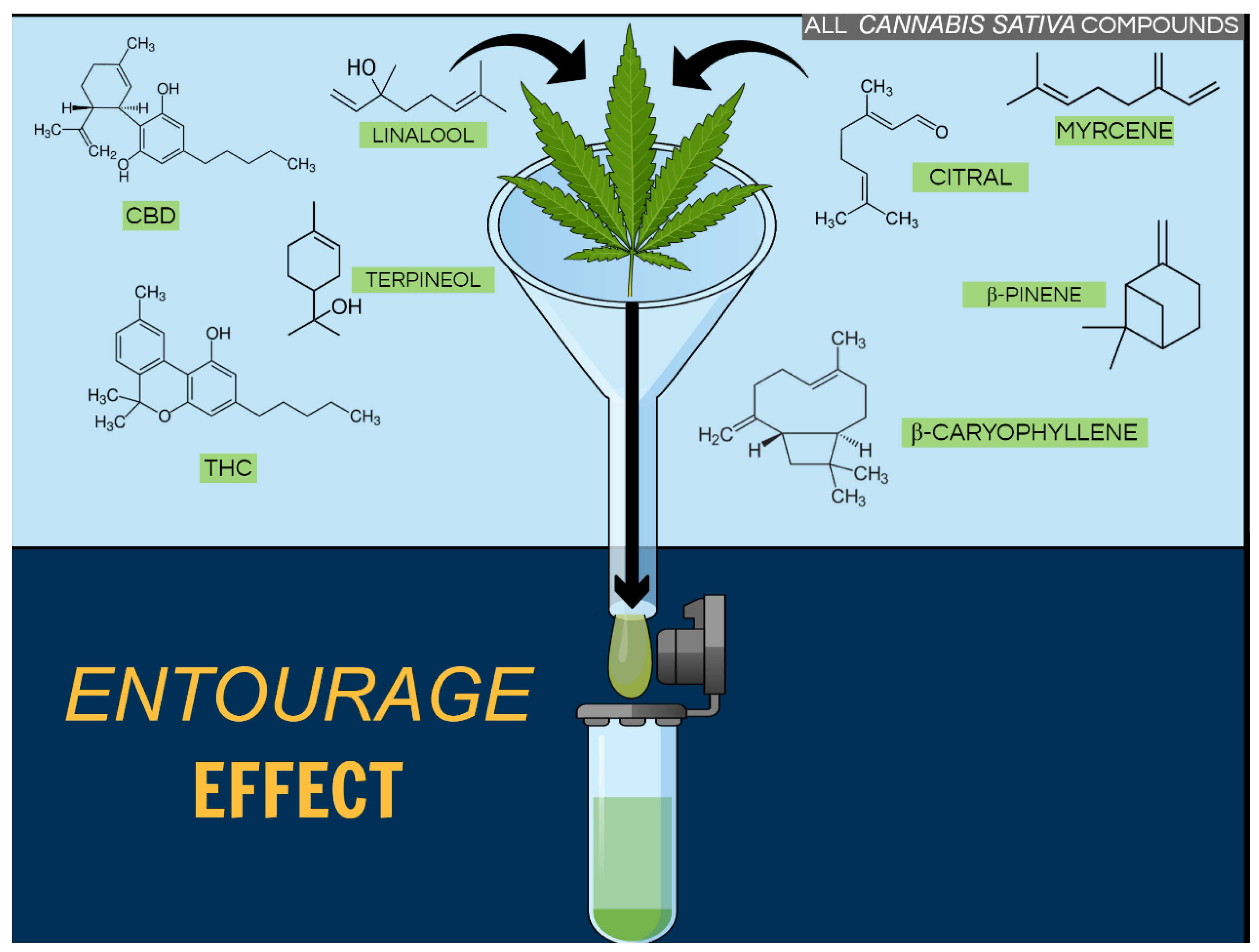
Cannabis Terpenoids
3. Terpenoids in and beyond the Cannabis Plant
References
- Di Marzo, V.; Bifulco, M.; De Petrocellis, L. The endocannabinoid system and its therapeutic exploitation. Nat. Reviews. Drug Discov. 2004, 3, 771–784.
- Rubin, V. Cannabis and Culture; Mouton: The Hague, The Netherlands, 1975.
- Guy, G.W.; Whittle, B.A.; Robson, P. The Medicinal Uses of Cannabis and Cannabinoids; Pharmaceutical Press: London, UK, 2004.
- Pacher, P.; Batkai, S.; Kunos, G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol. Rev. 2006, 58, 389–462.
- Russell, C.; Rueda, S.; Room, R.; Tyndall, M.; Fischer, B. Routes of administration for cannabis use—Basic prevalence and related health outcomes: A scoping review and synthesis. Int. J. Drug Policy 2018, 52, 87–96.
- Azofeifa, A.; Mattson, M.E.; Schauer, G.; McAfee, T.; Grant, A.; Lyerla, R. National Estimates of Marijuana Use and Related Indicators—National Survey on Drug Use and Health, United States, 2002–2014. Morb. Mortal. Wkly. Rep. Surveill. Summ. 2016, 65, 1–28.
- Degenhardt, L.; Ferrari, A.J.; Calabria, B.; Hall, W.D.; Norman, R.E.; McGrath, J.; Flaxman, A.D.; Engell, R.E.; Freedman, G.D.; Whiteford, H.A.; et al. The global epidemiology and contribution of cannabis use and dependence to the global burden of disease: Results from the GBD 2010 study. PLoS ONE 2013, 8, e76635.
- Hasin, D.S.; Saha, T.D.; Kerridge, B.T.; Goldstein, R.B.; Chou, S.P.; Zhang, H.; Jung, J.; Pickering, R.P.; Ruan, W.J.; Smith, S.M.; et al. Prevalence of Marijuana Use Disorders in the United States between 2001–2002 and 2012–2013. JAMA Psychiatry 2015, 72, 1235–1242.
- Hasin, D.S.; Sarvet, A.L.; Cerda, M.; Keyes, K.M.; Stohl, M.; Galea, S.; Wall, M.M. US Adult Illicit Cannabis Use, Cannabis Use Disorder, and Medical Marijuana Laws: 1991–1992 to 2012–2013. JAMA Psychiatry 2017, 74, 579–588.
- Han, B.; Compton, W.M.; Blanco, C.; Jones, C.M. Trends in and correlates of medical marijuana use among adults in the United States. Drug Alcohol Depend. 2018, 186, 120–129.
- Han, B.; Compton, W.M.; Jones, C.M.; Blanco, C. Cannabis Use and Cannabis Use Disorders among Youth in the United States, 2002–2014. J. Clin. Psychiatry 2017, 78, 1404–1413.
- Hill, K.P. Cannabis Use and Risk for Substance Use Disorders and Mood or Anxiety Disorders. JAMA 2017, 317, 1070–1071.
- Belackova, V.; Stefunkova, M. Interpreting the Czech drug decriminalization: The glass is half full—Response to Cerveny, J., Chomynova, P., Mravcik, V., & van Ours, J.C. (2017). Cannabis decriminalization and the age of onset of cannabis use. Int. J. Drug Policy 2018, 52, 102–105.
- Belackova, V.; Wilkins, C. Consumer agency in cannabis supply—Exploring auto-regulatory documents of the cannabis social clubs in Spain. Int. J. Drug Policy 2018, 54, 26–34.
- Room, R. Legalizing a market for cannabis for pleasure: Colorado, Washington, Uruguay and beyond. Addiction 2014, 109, 345–351.
- Room, R. Cannabis legalization and public health: Legal niceties, commercialization and countercultures. Addiction 2014, 109, 358–359.
- Wilkinson, S.T.; D’Souza, D.C. Problems with the medicalization of marijuana. JAMA 2014, 311, 2377–2378.
- Devane, W.A.; Dysarz, F.A., 3rd; Johnson, M.R.; Melvin, L.S.; Howlett, A.C. Determination and characterization of a cannabinoid receptor in rat brain. Mol. Pharmacol. 1988, 34, 605–613.
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990, 346, 561–564.
- ElSohly, M.A.; Radwan, M.M.; Gul, W.; Chandra, S.; Galal, A. Phytochemistry of Cannabis sativa L. Prog. Chem. Org. Nat. Prod. 2017, 103, 1–36.
- Russo, E.; Guy, G.W. A tale of two cannabinoids: The therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Med. Hypotheses 2006, 66, 234–246.
- Esposito, G.; Scuderi, C.; Valenza, M.; Togna, G.I.; Latina, V.; De Filippis, D.; Cipriano, M.; Carratu, M.R.; Iuvone, T.; Steardo, L. Cannabidiol reduces Abeta-induced neuroinflammation and promotes hippocampal neurogenesis through PPARgamma involvement. PLoS ONE 2011, 6, e28668.
- Martin-Moreno, A.M.; Reigada, D.; Ramirez, B.G.; Mechoulam, R.; Innamorato, N.; Cuadrado, A.; de Ceballos, M.L. Cannabidiol and other cannabinoids reduce microglial activation in vitro and in vivo: Relevance to Alzheimer’s disease. Mol. Pharmacol. 2011, 79, 964–973.
- Mecha, M.; Torrao, A.S.; Mestre, L.; Carrillo-Salinas, F.J.; Mechoulam, R.; Guaza, C. Cannabidiol protects oligodendrocyte progenitor cells from inflammation-induced apoptosis by attenuating endoplasmic reticulum stress. Cell Death Dis. 2012, 3, e331.
- Shannon, S.; Opila-Lehman, J. Effectiveness of Cannabidiol Oil for Pediatric Anxiety and Insomnia as Part of Posttraumatic Stress Disorder: A Case Report. Perm. J. 2016, 20, 16-005.
- Marinho, A.L.; Vila-Verde, C.; Fogaca, M.V.; Guimaraes, F.S. Effects of intra-infralimbic prefrontal cortex injections of cannabidiol in the modulation of emotional behaviors in rats: Contribution of 5HT(1)A receptors and stressful experiences. Behav. Brain Res. 2015, 286, 49–56.
- Jadoon, K.A.; Ratcliffe, S.H.; Barrett, D.A.; Thomas, E.L.; Stott, C.; Bell, J.D.; O’Sullivan, S.E.; Tan, G.D. Efficacy and Safety of Cannabidiol and Tetrahydrocannabivarin on Glycemic and Lipid Parameters in Patients With Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled, Parallel Group Pilot Study. Diabetes Care 2016, 39, 1777–1786.
- Dhital, S.; Stokes, J.V.; Park, N.; Seo, K.S.; Kaplan, B.L. Cannabidiol (CBD) induces functional Tregs in response to low-level T cell activation. Cell. Immunol. 2017, 312, 25–34.
- Krohn, R.M.; Parsons, S.A.; Fichna, J.; Patel, K.D.; Yates, R.M.; Sharkey, K.A.; Storr, M.A. Abnormal cannabidiol attenuates experimental colitis in mice, promotes wound healing and inhibits neutrophil recruitment. J. Inflamm. 2016, 13, 21.
- Norris, C.; Loureiro, M.; Kramar, C.; Zunder, J.; Renard, J.; Rushlow, W.; Laviolette, S.R. Cannabidiol Modulates Fear Memory Formation through Interactions with Serotonergic Transmission in the Mesolimbic System. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2016, 41, 2839–2850.
- Genaro, K.; Fabris, D.; Arantes, A.L.F.; Zuardi, A.W.; Crippa, J.A.S.; Prado, W.A. Cannabidiol Is a Potential Therapeutic for the Affective-Motivational Dimension of Incision Pain in Rats. Front. Pharmacol. 2017, 8, 391.
- Harris, H.M.; Sufka, K.J.; Gul, W.; ElSohly, M.A. Effects of Delta-9-Tetrahydrocannabinol and Cannabidiol on Cisplatin-Induced Neuropathy in Mice. Planta Med. 2016, 82, 1169–1172.
- Peres, F.F.; Levin, R.; Suiama, M.A.; Diana, M.C.; Gouvea, D.A.; Almeida, V.; Santos, C.M.; Lungato, L.; Zuardi, A.W.; Hallak, J.E.; et al. Cannabidiol Prevents Motor and Cognitive Impairments Induced by Reserpine in Rats. Front. Pharmacol. 2016, 7, 343.
- Peres, F.F.; Diana, M.C.; Suiama, M.A.; Justi, V.; Almeida, V.; Bressan, R.A.; Zuardi, A.W.; Hallak, J.E.; Crippa, J.A.; Abilio, V.C. Peripubertal treatment with cannabidiol prevents the emergence of psychosis in an animal model of schizophrenia. Schizophr. Res. 2016, 172, 220–221.
- Renard, J.; Loureiro, M.; Rosen, L.G.; Zunder, J.; de Oliveira, C.; Schmid, S.; Rushlow, W.J.; Laviolette, S.R. Cannabidiol Counteracts Amphetamine-Induced Neuronal and Behavioral Sensitization of the Mesolimbic Dopamine Pathway through a Novel mTOR/p70S6 Kinase Signaling Pathway. J. Neurosci. Off. J. Soc. Neurosci. 2016, 36, 5160–5169.
- Devinsky, O.; Cross, J.H.; Wright, S. Trial of Cannabidiol for Drug-Resistant Seizures in the Dravet Syndrome. N. Engl. J. Med. 2017, 377, 699–700.
- Hosseinzadeh, M.; Nikseresht, S.; Khodagholi, F.; Naderi, N.; Maghsoudi, N. Cannabidiol Post-Treatment Alleviates Rat Epileptic-Related Behaviors and Activates Hippocampal Cell Autophagy Pathway Along with Antioxidant Defense in Chronic Phase of Pilocarpine-Induced Seizure. J. Mol. Neurosci. MN 2016, 58, 432–440.
- Kaplan, E.H.; Offermann, E.A.; Sievers, J.W.; Comi, A.M. Cannabidiol Treatment for Refractory Seizures in Sturge-Weber Syndrome. Pediatr. Neurol. 2017, 71, 18–23 e2.
- Patel, R.R.; Barbosa, C.; Brustovetsky, T.; Brustovetsky, N.; Cummins, T.R. Aberrant epilepsy-associated mutant Nav1.6 sodium channel activity can be targeted with cannabidiol. Brain J. Neurol. 2016, 139 Pt 8, 2164–2181.
- Ibsen, M.S.; Connor, M.; Glass, M. Cannabinoid CB1 and CB2 Receptor Signaling and Bias. Cannabis Cannabinoid Res. 2017, 2, 48–60.
- McHugh, D.; Page, J.; Dunn, E.; Bradshaw, H.B. Delta(9) -Tetrahydrocannabinol and N-arachidonyl glycine are full agonists at GPR18 receptors and induce migration in human endometrial HEC-1B cells. Br. J. Pharmacol. 2012, 165, 2414–2424.
- Pertwee, R.G.; Howlett, A.C.; Abood, M.E.; Alexander, S.P.; Di Marzo, V.; Elphick, M.R.; Greasley, P.J.; Hansen, H.S.; Kunos, G.; Mackie, K.; et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: Beyond CB(1) and CB(2). Pharmacol. Rev. 2010, 62, 588–631.
- Davis, K.D.; Flor, H.; Greely, H.T.; Iannetti, G.D.; Mackey, S.; Ploner, M.; Pustilnik, A.; Tracey, I.; Treede, R.D.; Wager, T.D. Brain imaging tests for chronic pain: Medical, legal and ethical issues and recommendations. Nat. Rev. Neurol. 2017, 13, 624–638.
- Howlett, A.C.; Barth, F.; Bonner, T.I.; Cabral, G.; Casellas, P.; Devane, W.A.; Felder, C.C.; Herkenham, M.; Mackie, K.; Martin, B.R.; et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 2002, 54, 161–202.
- Ashton, J.C.; Glass, M. The cannabinoid CB2 receptor as a target for inflammation-dependent neurodegeneration. Curr. Neuropharmacol. 2007, 5, 73–80.
- Rom, S.; Persidsky, Y. Cannabinoid receptor 2: Potential role in immunomodulation and neuroinflammation. J. Neuroimmune Pharmacol. Off. J. Soc. Neuroimmune Pharmacol. 2013, 8, 608–620.
- Pertwee, R.G. The pharmacology of cannabinoid receptors and their ligands: An overview. Int. J. Obes. 2006, 30 (Suppl. 1), S13–S18.
- Pertwee, R.G. Pharmacological actions of cannabinoids. Handb. Exp. Pharmacol. 2005, 168, 1–51.
- Devane, W.A.; Hanus, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992, 258, 1946–1949.
- Mechoulam, R.; Ben-Shabat, S.; Hanus, L.; Ligumsky, M.; Kaminski, N.E.; Schatz, A.R.; Gopher, A.; Almog, S.; Martin, B.R.; Compton, D.R.; et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 1995, 50, 83–90.
- Sugiura, T.; Kondo, S.; Sukagawa, A.; Nakane, S.; Shinoda, A.; Itoh, K.; Yamashita, A.; Waku, K. 2-Arachidonoylglycerol: A possible endogenous cannabinoid receptor ligand in brain. Biochem. Biophys. Res. Commun. 1995, 215, 89–97.
- Pertwee, R.G. Receptors and channels targeted by synthetic cannabinoid receptor agonists and antagonists. Curr. Med. Chem. 2010, 17, 1360–1381.
- Goncalves, E.D.; Dutra, R.C. Cannabinoid receptors as therapeutic targets for autoimmune diseases: Where do we stand? Drug Discov. Today 2019, 24, 1845–1853.
- Di Iorio, G.; Lupi, M.; Sarchione, F.; Matarazzo, I.; Santacroce, R.; Petruccelli, F.; Martinotti, G.; Di Giannantonio, M. The endocannabinoid system: A putative role in neurodegenerative diseases. Int. J. High Risk Behav. Addict. 2013, 2, 100–106.
- Wolf, S.A.; Tauber, S.; Ullrich, O. CNS immune surveillance and neuroinflammation: Endocannabinoids keep control. Curr. Pharm. Des. 2008, 14, 2266–2278.
- Cabral, G.A.; Ferreira, G.A.; Jamerson, M.J. Endocannabinoids and the Immune System in Health and Disease. Handb. Exp. Pharmacol. 2015, 231, 185–211.
- De Laurentiis, A.; Araujo, H.A.; Rettori, V. Role of the endocannabinoid system in the neuroendocrine responses to inflammation. Curr. Pharm. Des. 2014, 20, 4697–4706.
- Pacher, P.; Steffens, S.; Hasko, G.; Schindler, T.H.; Kunos, G. Cardiovascular effects of marijuana and synthetic cannabinoids: The good, the bad, and the ugly. Nat. Re. Cardiol. 2018, 15, 151–166.
- Ashton, J.C.; Smith, P.F. Cannabinoids and cardiovascular disease: The outlook for clinical treatments. Curr. Vasc. Pharmacol. 2007, 5, 175–185.
- Ho, W.S.V.; Kelly, M.E.M. Cannabinoids in the Cardiovascular System. Adv. Pharmacol. 2017, 80, 329–366.
- Wolff, V.; Jouanjus, E. Strokes are possible complications of cannabinoids use. Epilepsy Behav. EB 2017, 70 Pt B, 355–363.
- Singh, A.; Saluja, S.; Kumar, A.; Agrawal, S.; Thind, M.; Nanda, S.; Shirani, J. Cardiovascular Complications of Marijuana and Related Substances: A Review. Cardiol. Ther. 2018, 7, 45–59.
- Lu, Y.; Anderson, H.D. Cannabinoid signaling in health and disease. Can. J. Physiol. Pharmacol. 2017, 95, 311–327.
- Huestis, M.A.; Boyd, S.J.; Heishman, S.J.; Preston, K.L.; Bonnet, D.; Le Fur, G.; Gorelick, D.A. Single and multiple doses of rimonabant antagonize acute effects of smoked cannabis in male cannabis users. Psychopharmacology 2007, 194, 505–515.
- Kumar, A.; Premoli, M.; Aria, F.; Bonini, S.A.; Maccarinelli, G.; Gianoncelli, A.; Memo, M.; Mastinu, A. Cannabimimetic plants: Are they new cannabinoidergic modulators? Planta 2019, 249, 1681–1694.
- Solymosi, K.; Kofalvi, A. Cannabis: A Treasure Trove or Pandora’s Box? Mini Rev. Med. Chem. 2017, 17, 1223–1291.
- Morales, P.; Hurst, D.P.; Reggio, P.H. Molecular Targets of the Phytocannabinoids: A Complex Picture. Prog. Chem. Org. Nat. Prod. 2017, 103, 103–131.
- Campos, A.C.; Fogaca, M.V.; Sonego, A.B.; Guimaraes, F.S. Cannabidiol, neuroprotection and neuropsychiatric disorders. Pharmacol. Res. 2016, 112, 119–127.
- Crivelaro do Nascimento, G.; Ferrari, D.P.; Guimaraes, F.S.; Del Bel, E.A.; Bortolanza, M.; Ferreira-Junior, N.C. Cannabidiol increases the nociceptive threshold in a preclinical model of Parkinson’s disease. Neuropharmacology 2020, 163, 107808.
- Mammana, S.; Cavalli, E.; Gugliandolo, A.; Silvestro, S.; Pollastro, F.; Bramanti, P.; Mazzon, E. Could the Combination of Two Non-Psychotropic Cannabinoids Counteract Neuroinflammation? Effectiveness of Cannabidiol Associated with Cannabigerol. Medicina 2019, 55, 747.
- Russo, E.B.; Marcu, J. Cannabis Pharmacology: The Usual Suspects and a Few Promising Leads. Adv. Pharmacol. 2017, 80, 67–134.
- Borrelli, F.; Pagano, E.; Romano, B.; Panzera, S.; Maiello, F.; Coppola, D.; De Petrocellis, L.; Buono, L.; Orlando, P.; Izzo, A.A. Colon carcinogenesis is inhibited by the TRPM8 antagonist cannabigerol, a Cannabis-derived non-psychotropic cannabinoid. Carcinogenesis 2014, 35, 2787–2797.
- Udoh, M.; Santiago, M.; Devenish, S.; McGregor, I.S.; Connor, M. Cannabichromene is a cannabinoid CB2 receptor agonist. Br. J. Pharmacol. 2019, 176, 4537–4547.
- Wilkinson, J.D.; Williamson, E.M. Cannabinoids inhibit human keratinocyte proliferation through a non-CB1/CB2 mechanism and have a potential therapeutic value in the treatment of psoriasis. J. Dermatol. Sci. 2007, 45, 87–92.
- Al-Ghezi, Z.Z.; Miranda, K.; Nagarkatti, M.; Nagarkatti, P.S. Combination of Cannabinoids, Delta9- Tetrahydrocannabinol and Cannabidiol, Ameliorates Experimental Multiple Sclerosis by Suppressing Neuroinflammation Through Regulation of miRNA-Mediated Signaling Pathways. Front. Immunol. 2019, 10, 1921.
- Haupts, M.; Vila, C.; Jonas, A.; Witte, K.; Alvarez-Ossorio, L. Influence of Previous Failed Antispasticity Therapy on the Efficacy and Tolerability of THC:CBD Oromucosal Spray for Multiple Sclerosis Spasticity. Eur. Neurol. 2016, 75, 236–243.
- Laprairie, R.B.; Bagher, A.M.; Kelly, M.E.; Denovan-Wright, E.M. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br. J. Pharmacol. 2015, 172, 4790–4805.
- Blasco-Benito, S.; Seijo-Vila, M.; Caro-Villalobos, M.; Tundidor, I.; Andradas, C.; Garcia-Taboada, E.; Wade, J.; Smith, S.; Guzman, M.; Perez-Gomez, E.; et al. Appraising the “entourage effect”: Antitumor action of a pure cannabinoid versus a botanical drug preparation in preclinical models of breast cancer. Biochem. Pharmacol. 2018, 157, 285–293.
- Russo, E.B. The Case for the Entourage Effect and Conventional Breeding of Clinical Cannabis: No “Strain,” No Gain. Front. Plant Sci. 2018, 9, 1969.
- Felipe, C.F.B.; Albuquerque, A.M.S.; de Pontes, J.L.X.; de Melo, J.I.V.; Rodrigues, T.; de Sousa, A.M.P.; Monteiro, A.B.; Ribeiro, A.; Lopes, J.P.; de Menezes, I.R.A.; et al. Comparative study of alpha- and beta-pinene effect on PTZ-induced convulsions in mice. Fundam. Clin. Pharmacol. 2019, 33, 181–190.
- Zamyad, M.; Abbasnejad, M.; Esmaeili-Mahani, S.; Mostafavi, A.; Sheibani, V. The anticonvulsant effects of Ducrosia anethifolia (Boiss) essential oil are produced by its main component alpha-pinene in rats. Arq. De Neuro-Psiquiatr. 2019, 77, 106–114.
- Khoshnazar, M.; Bigdeli, M.R.; Parvardeh, S.; Pouriran, R. Attenuating effect of alpha-pinene on neurobehavioural deficit, oxidative damage and inflammatory response following focal ischaemic stroke in rat. J. Pharm. Pharmacol. 2019, 71, 1725–1733.
- Zhao, Y.; Chen, R.; Wang, Y.; Yang, Y. alpha-Pinene Inhibits Human Prostate Cancer Growth in a Mouse Xenograft Model. Chemotherapy 2018, 63, 1–7.
- Araujo-Filho, H.G.; Pereira, E.W.M.; Rezende, M.M.; Menezes, P.P.; Araujo, A.A.S.; Barreto, R.S.S.; Martins, A.; Albuquerque, T.R.; Silva, B.A.F.; Alcantara, I.S.; et al. D-limonene exhibits superior antihyperalgesic effects in a beta-cyclodextrin-complexed form in chronic musculoskeletal pain reducing Fos protein expression on spinal cord in mice. Neuroscience 2017, 358, 158–169.




