Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kumar Ganesan | + 8594 word(s) | 8594 | 2021-11-10 02:30:54 | | | |
| 2 | Bruce Ren | -4150 word(s) | 4444 | 2021-11-12 09:40:41 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ganesan, K.; Gao, F.; Li, P.; Ye, Q.; Chen, J. Targeting Engineered Nanoparticles for Breast Cancer Therapy. Encyclopedia. Available online: https://encyclopedia.pub/entry/15932 (accessed on 08 February 2026).
Ganesan K, Gao F, Li P, Ye Q, Chen J. Targeting Engineered Nanoparticles for Breast Cancer Therapy. Encyclopedia. Available at: https://encyclopedia.pub/entry/15932. Accessed February 08, 2026.
Ganesan, Kumar, Fei Gao, Peng Li, Qiaobo Ye, Jiianping Chen. "Targeting Engineered Nanoparticles for Breast Cancer Therapy" Encyclopedia, https://encyclopedia.pub/entry/15932 (accessed February 08, 2026).
Ganesan, K., Gao, F., Li, P., Ye, Q., & Chen, J. (2021, November 12). Targeting Engineered Nanoparticles for Breast Cancer Therapy. In Encyclopedia. https://encyclopedia.pub/entry/15932
Ganesan, Kumar, et al. "Targeting Engineered Nanoparticles for Breast Cancer Therapy." Encyclopedia. Web. 12 November, 2021.
Copy Citation
Breast cancer (BC) is the second most common cancer in women globally after lung cancer. Presently, the most important approach for BC treatment consists of surgery, followed by radiotherapy and chemotherapy. Therapeutic drugs or natural bioactive compounds generally incorporate engineered NPs of ideal sizes and shapes to enhance their solubility, circulatory half-life, and biodistribution, while reducing their side effects and immunogenicity. Furthermore, ligands such as peptides, antibodies, and nucleic acids on the surface of NPs precisely target BC cells. Engineered NPs and their ideal methodology can be validated in the next-generation platform for preventive and therapeutic effects against BC.
nanoparticles
ligands
engineering
therapeutic effects
breast cancer
1. Introduction
Breast cancer (BC) is the outcome of aberrant and uncontrolled cell proliferation of cancerous cells in the breast tissue. BC is the second most common cancer in females and the third-leading cause of death globally [1]. BC therapy involves a multidisciplinary approach comprising surgery as well as radiotherapy and chemotherapy as adjuvant and neoadjuvant therapies [2]. Chemotherapy is a technique that kills cancer cells using chemical agents. Although it is the most effective approach for cancer therapy, the cytotoxic effects of these chemotherapy agents generate various side effects [3]. Radiotherapy also decreases the risk of cancer recurrence and mortality. Nevertheless, it typically involves radiation exposure to adjacent organs, increasing the risk of cardiac and lung diseases. Such therapies may increase the risk of leukemia, especially in association with certain classes of adjuvant chemotherapy [4]. Conversely, these therapeutic methods are often unsuccessful in treating BC because of their adverse effects on healthy tissues and organs [5][6].
The main reason for these adverse effects and the mortality rate is the failure of therapeutic agents, which act not only on the tumor sites but also induce severe adverse effects on healthy tissues and organs, causing toxicity to the individual. BC is a highly multifaceted and heterogeneous disease and is categorized based on histopathological types. The most predominant BC cases are those of invasive ductal carcinoma, although other less-common subtypes are noteworthy due to their ferociousness and clinical manifestations [7]. The next major concern is the stage of the tumor. During cancer development, the primary tumor occurs within the breast tissue (stage 1), and then rapidly spreads to the adjacent tissues and lymph nodes (stage 2–3) or distant organs such as the lung, bone, liver, or brain (metastasis, i.e., stage 4) [7][8]. Staging of the disease is clinically important. The death rate increases as the tumor metastasizes. Moreover, BC is also categorized based on the grade and molecular subtype, viz., luminal A and B, human epidermal growth factor receptor 2 (HER2), and triple-negative BC (TNBC) [8]. Once the cancer metastasizes, the effectiveness of most standard drugs is significantly low. Finding novel, effective, and safe forms of therapy for this fatal malicious disease is thus critical. It is necessary to discover highly efficient therapeutics (the so-called “magic bullets”) which can pass through natural barriers and differentiate between benign and malignant cells in order to target malignant tissues. These agents “wisely” react to the complex tumor microenvironment for an on-demand discharge of an optimum dose range [9][10].
Tumor nanotechnology has the potential to modernize cancer diagnosis and treatment. Developments in protein engineering and material science have contributed to the development of innovative nanoscale targeting strategies, providing new optimism for BC patients. Nanoparticles (NPs), identified as pharmaceutical carriers, provide a new juncture for drug delivery to cancer cells by infiltrating tumors deeply, resulting in a high level of specificity to the targeted cancer cells [11][12][13][14][15]. Furthermore, NP treatment minimizes destructive effects on healthy tissues and organs [16][17]. Nanotechnology has been approved by the National Cancer Institute, which recognizes this technology as an outstanding paradigm-shifting approach for improving the diagnosis and treatment of BC [16].
Several therapeutic NPs, viz., Doxil®, Lipoplatin®, Onivyde®, Genexol-PM, and Abraxane®, have already been approved and are extensively employed for BC adjuvant therapy, with promising clinical outcomes [18][19][20][21]. NP-based drug delivery systems (DDSs) include several valid designs with regard to the size, shape, and nature of the biomaterials loaded with drugs, enhancing the solubility, drug stability, circulatory half-life, biodistribution, and drug release rate and reducing side effects, toxicity, and immunogenicity [22].
2. Designing of Engineered NP Carriers
Nanomedicine has the potential to evade various issues in the treatment of traditional formulations. Noteworthy strides have been made towards the application of engineered NPs for BC therapy with high sensitivity, specificity, and efficiency. The engineering of NPs primarily requires various classes of chemicals with extensive structures, sizes, and compositions [23][24]. In recent years, many techniques in nanotechnology have been developed using novel biomaterials and ligands to achieve treatments with little or no toxicity. For instance, physicochemical properties of NPs such as size, geometry or shape, composition, physical and chemical structure, charges on the surface, ligand binding, and mechanical effects can be engineered to advance their performance in vivo [25]. One fascinating example was established through the use of PEGylation, conjugation, and NP-loaded liposomes for BC diagnostics and therapeutics [26]. These techniques are feasible for reducing their deposition and toxicity in major organs to a satisfactory level by elevating their half-life in the circulation [27].
2.1. Organic/Inorganic Nanocarriers
Based on chemical requirements, NPs are classified as organic or inorganic. Organic NPs (macromolecular and lipid-based nanocarriers) are characterized by higher biocompatibility and biodegradability, with manifold activation and function of the drug on their surface. Inorganic nanoparticles (carbon, silica, quantum dots, and metallic NPs) exhibit high stability, with intrinsic and visual properties appropriate for theranostics. The most investigated types are metallic NPs (gold, silver, and iron oxide) that show distinctive properties (optical and electronic), and aid in cancer imaging [28]. Based on the stability pattern, therapeutic BC drugs are mostly conjugated on their surface. They can be degraded and exchange dynamics rapidly in vivo. Hybrid NPs occupy both organic and inorganic classes, improving the biocompatibility, biodegradability, and stability of the NPs (Figure 1). The application of inorganic NPs in therapy is inadequate due to their low biodegradability. Mesoporous inorganic NPs are typically biodegradable, and silica-based NPs enable us to preserve drugs within a porous morphology with physicochemical properties [29].
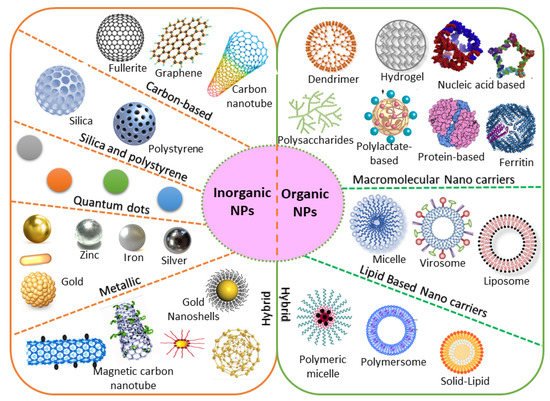
Figure 1. Chemical requirements for engineered NPs in BC therapy.
The large organic subfamily comprises macromolecular nanocarriers, both synthetic (polylactate derivatives, dendrimers, fluorescent organic NPs) and natural (protein, nucleic acid, ferritin, and polysaccharide-based NPs), with greater stability and several free functional groups, resulting in greater drug-loading capability [23]. Due to these functional characteristics, increasing attention is being paid to nanocarriers in BC therapy. Lipid-based NPs are also an important class in clinical investigation due to their noteworthy biocompatibility [30]. Lipid-based NPs comprise monolayer (micelles) or bilayer (liposomes) nanocarriers that can carry a wide range of materials with diverse physicochemical functions. The lipid bilayer of liposomes can be implanted with hydrophobic drugs, while hydrophilic drugs can be captured either in the aqueous core of liposomes or are exhibited on the surface [31]. Nevertheless, lipid-based NPs still have numerous issues, including instability and poor loading capacity, which lead to drug leakage. Novel hybrid NPs have been established to conjugate with subclasses. Examples include solid–lipid, hybrid polymer–lipid, and hybrid organic–inorganic NPs [32][33].
2.2. Natural/Synthetic Nanocarriers
Natural products are often attractive due to their abundance and higher biocompatibility, as well as the capacity adapt through biochemical mechanisms [34]. Naturally occurring substances offer many benefits over their synthetic counterparts. Natural bioactive compounds encapsulated in nanocarriers can result in increased in vivo stability and water solubility, a longer circulation time of the natural product in the blood, improved biodistribution and targeting of BC cells, and controlled and sustained drug release. They are considered potent antioxidants with closer proximity and positive effects on cancer-specific pathways, with reduced side effects [35]. Natural therapeutic drugs accumulate more appropriately for longer at the tumor site through active or passive targeting of the breast tumor tissue [36]. Several in vitro and in vivo BC model studies were established and validated the antitumor functions of nano-encapsulated phytochemicals (Table 1). Furthermore, experimental studies were established using liposomes, polymers, magnetic NPs, lipid-based NPs, and protein-based NPs, confirming the potential effects of plant-based natural products for BC therapy. The association of NPs with recognized plant-based antitumor compounds has been considered a promising method to reduce tumor growth and their adverse effects [37].
Natural materials can be rapidly metabolized and removed by the body system through hydrolytic or enzymatic degradation [36]. The most common issue with natural products is that of the immune response, which can readily occur upon administration into the body. This issue is due to the protein-derived materials; however, the response is often less severe with the administration of polysaccharide (chitosan)-derived NPs [38]. This immunogenic effect can be minimized by either chemical alteration or purification to eliminate the immunogenic constituents [39].
Presently, drug delivery takes place using NP-based-synthetic substances, as they allow an appropriate control over the physicochemical nature of the nanoproducts. NP-based synthetic substances are stable, safe, biologically inert, and are in circulation for a longer time, improving the distribution of therapeutic BC drugs to the tumor sites. The NPs can stimulate the generation of a corona of plasma proteins near the surface. Thus, extremely charged NPs are engulfed more rapidly by the mononuclear phagocyte system than neutrally charged NPs [40][41]. Hence, using synthetic nanocarriers, the hydrophobicity and surface charges of NPs can be suitably adjusted, improving their half-life in the circulation. Furthermore, their surface functions can be quickly engineered to improve their conjugation to the targeted receptors.
Table 1. Anti-BC effects of natural product-based nano-formulations.
| Drug | Nanocarriers | Natural Compound |
Size | The Outcome of the Study | BC Types | References |
|---|---|---|---|---|---|---|
| Doxorubicin | Poly-glycerol-malic acid-dodecanedioic acid | Curcumin | ~110–218 nm | Significantly increased cytotoxicity, apoptotic cell death, and cellular intake compared to free drug in MCF-7 and MDA-MB-231 | Luminal BC and TNBC | [42] |
| Doxorubicin | Silver NPs | Andrographolide | ~450 nm | Significantly increased cytotoxicity, apoptotic cell death, and cellular intake compared to free drug in MDA-MB-453 | TNBC | [36] |
| Adriamycin | Silver NPs | Camellia sinensis | ~220 nm | Significantly increased cytotoxicity, apoptotic cell death, and cellular intake compared to free drug in MCF-7 | Luminal BC | [43] |
| Doxorubicin | Folate and chitosan | Ursolic acid | ~420 nm | Anticancer effects in an MCF-7 xenograft mouse model | Luminal BC | [38] |
| Doxorubicin | Lipid carriers (precirol® ATO 5, vitamin E, poloxamer 188, Tween 80) | Sulforaphane/Isothiocyanate | 145 nm | Anticancer effects in an MCF-7 xenograft mouse model | Luminal BC | [37] |
| Doxorubicin | Hydrophobically modified glycol chitosan with 5 beta-cholanic acid | Camptothecin | 280–330 nm | Anticancer effects in an MDA-MB-231 xenograft mousemodel | TNBC | [44] |
| Doxorubicin | Phytosome | Quercetin | ~85 nm | Anticancer effects in an MCF-7 xenograft mouse model | Luminal BC | [39] |
| Doxorubicin | PEGylated liposome |
Gambogic acid | ~107 nm | Anticancer effects in an MDA-MB-231 orthotopic xenograft mouse model |
TNBC | [45] |
2.3. Geometric Morphometry
Nanomedicine, as a discipline that involves the fields of chemistry, engineering, and material science, utilizes the unique features of NPs to design improved therapeutic BC interventions. Size, shape, density, and consistency are the key factors to be considered for not only DDSs but also for the engineering of NPs, as these factors regulate in vivo drug loading, stability, circulation, targeting capability, drug release, biodegradability, and toxicity [46]. For instance, particles that are smaller in size have a higher likelihood of accumulation during incubation and storage in vitro, and characteristically have a longer half-life in circulation in vivo [47]. Several investigations have confirmed that NPs display more benefits over micrometer-sized fragments in the range of 0.1–100 μm for DDSs [48]. The biodegradation of polymer NPs can be potentially affected by their size due to rapid degradation products.
The shape of NPs is also equally significant in the use of DDSs. Spherical NPs are generally a worthy candidate for DDSs. In addition, the morphology of anisotropy can also offer greater productivity due to their higher ratios between surface area and volume. They permit the nanocarrier to assume an encouraging shape for attachment to the cell through sharp ends and corners. Through these mechanisms NPs can cross cell membranes, and have been subjected to a wide range of investigations [49][50].
2.4. Surface Properties
The surface of the NPs represents another key factor determining the drug-loading efficacy, release profile, half-life in circulation, tumor targeting, and drug clearance. In fact, NPs generally have a hydrophilic surface to prevent protein adsorption and hence avoid uptake by the immune system [51]. This mechanism is generally achieved by coating the surface of NPs with a hydrophilic polymer (PEG), impacting toxicity, immunogenicity, and biodistribution [52].
The surface charge of NPs is often employed based on the zeta potential. It is determined according to the electrostatic potential of NPs, composition, and the medium used. Charged NPs with a zeta potential greater than 30 mV are indicated to be stable in suspensions, and these surface charges can generally avert the particles from aggregation. Furthermore, the surfaces of cells and blood vessels include several negatively charged ions, which resist negatively charged NPs. When the surface charge of NPs is higher, they can be hunted by the immune system, resulting in a higher clearance of NPs. Thus, the surface charge has a crucial role in minimizing the generic interactions between NPs and the immune system, averting NP loss in undesired settings. Surface hydrophilic PEG chains capsulated with NPs are frequently used to minimize generic interactions. PEGylation is considered to shield NPs such as liposomes, polymer NPs, and micelles from premature clearance during circulation. Various studies have suggested that PEGylated liposomal doxorubicin shows a prolonged half-life in the circulation and elevated stability, which is suggested to be linked to improved BC treatment efficacy [53][54][55][56][57].
Polysaccharides represent another natural surface polymer and are frequently used with NPs. They have been extensively employed in several DDSs and in tissue engineering due to their improved biocompatibility and accessibility, as well as their simple changeability. Dextran, chitosan, hyaluronic acid, fucoidan, and heparin have been employed as standard stealth-coating materials in NPs for BC therapy [58][59]. NPs coated with polysaccharides have more competent cellular uptake than other NPs due to their specific attachment with various receptors on the surface of the BC cells [60]. Hence, polysaccharides have received much attention in the field of nanomedicine.
2.5. Ligands
Several methods and tools are presently accessible to shield NPs for the active targeting of BC cells. Previously, monoclonal antibodies were employed to target epitopes on the cell surface; however, the widespread screening of peptide and aptamer archives has significantly extended the number of ligands available for targeted BC therapy [61]. Various ligands are presently employed, viz., antibodies, peptides, aptamers, oligosaccharides, and small molecules, and can precisely identify and attach to an overexpressed target on the surface of the cell [62][59][60][63]. This novel targeting mode involves a type of molecular recognition that initiates the binding of the ligand receptor. This conjugation allows the NPs to fix to the surface of the tumor cell selectively. Earlier studies have also confirmed this potential binding and validated these NPs as being effective in vitro and in vivo [64][65][66][67][68]. For instance, when attaching to a targeting ligand, the NPs generally demonstrate advanced internalization and are subjected to receptor-mediated endocytosis [16][69]. Based on strong conjugation with ligand, the binding affinity increases and thus promotes more effective receptor-mediated endocytosis.
Monoclonal antibodies are extensively employed as targeting ligands due to their high attaching affinity and specificity for targeting BC cells, as well as their easy accessibility. Numerous investigations have been performed using monoclonal antibodies that conjugate with all families of NPs, such as superparamagnetic iron oxide nanoparticles [70], quantum dots [71], liposomes [3], and silver nanocages [36], to contribute BC-specific targeting capacity. Using bioengineering, the monoclonal antibodies edit the redundant parts of the single-chain variable fragments, reducing the size with respect to the original antibody as well as the immunogenicity [72]. This chimeric antigen receptor-engineered T-cell is a promising tool and has extended to the treatment of other cancers, including B-cell leukemia and lymphoma [72].
Other noteworthy ligands are aptamers and peptides, which are characterized by feasible targeting methods with numerous advantages. The use of peptides shows numerous advantages, including lower molecular weight, tissue diffusion potential, loss of immunogenicity, ease of construction, and relative flexibility in chemical conjugation methods [73]. Similarly, aptamers are synthetic nucleic acid oligomers that can provide multifaceted three-dimensional structures that firmly bind to surface markers with high specificity [74]. Recently, a double aptamer–NP conjugate-based complex and adenosine triphosphate aptamer-conjugated CdTe quantum dots showed high potency for the efficient detection, monitoring, and treatment of BC [74].
Ligands such as folic acid, epidermal growth factor, and transferrin are presently more attractive for BC targeting due to their better attachment to their respective receptors with greater affinity and less immunogenicity [75][76][77]. Targeting ligands are nowadays receiving great attention due to their accessibility, assortment, high affinity, ease of attachment, and cost-effectiveness. Several ligands have been reported to conjugate with various receptors and NP families, as described in Table 2.
Table 2. Targeting ligands employed for BC therapy.
| Type of Nps | Therapeutic BC Drug |
Size of the Nps |
Ligands Used for Engineering | The Outcome of the Study | BC Types | Reference |
|---|---|---|---|---|---|---|
| Albumin-bound NPs | 2-methoxy-estradiol | ~240 nm | Bovine serum albumin | Significantly enhanced cytotoxicity and cellular uptake when compared with the free drug examined in the SK-BR-3 and MCF-7 cell lines and tumor-bearing mice | HER2 + BC | [73] |
| Chitosan | Doxorubicin | ~50 nm | Anti-HER2 peptide (5–10%) and O-succinyl chitosan graft Pluronic® F127 | Significantly enhanced cytotoxicity and cellular uptake when compared with the free drug in the MCF-7 cell line | HER2 + BC | [78] |
| Iron oxide | siRNA | 130 nm | Caffeic acid, calcium phosphate, iron oxide, PEG-polyanion block copolymer | Significantly enhanced cytotoxicity and cellular uptake when compared with free drug on HCC1954. mRNA expression was decreased by 38% when compared with naked siRNA | HER2 + BC | [79] |
| Iron oxide | Baicalein | 100 nm | PEG-coated iron oxide magnetic NPs | Significantly increased anti-apoptotic activity | TNBC | [80] |
| Liposome | Doxorubicin | ~80 nm | 1,2-Distearoyl-sn-glycero-3-phosphorylethanolamine, Distearoylphosphatidylcholine, HER2pep-K3-palmitic acid conjugate, mPEG2000 | Significantly enhanced cytotoxicity and cellular uptake and reduced systemic toxicity when compared with the free drug in BT-474, SK-BR-3, and MCF-7 cell lines. | HER2 + BC | [63] |
| Liposome | Anti-IL6R antibody, doxorubicin |
~100 nm | 1,2-dioleoyl-sn-glycero-3-phosphocholine, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine, cholesterol | Significantly increased tumor-targeting efficacy with anti-tumor metastasis effects in BALB/c mice bearing 4T1 cells | Luminal BC | [81] |
| Liposome | Doxorubicin | 194 nm | 1,2-distearoyl-sn-glycero-3-phosphoryl ethanolamine, estrone conjugated dipalmitoyl phosphatidylcholine- PEG2000-NH2 liposomes | Significantly increased uptake in MCF-7 BC cell lines and decreased uptake in MDA-MB-231 BC cell lines | Luminal BC | [82] |
| PolymericNPs | Curcumin | ~10 nm | Chitosan NPs with an apoptosis-inducing ligand (TRAIL) | Significantly reduced tumor volume when compared to control when tested in BALB/c mice | TNBC | [83] |
| Polymeric NPs | Trastuzumab | ~125 nm | Antigen-binding fragments cut from trastuzumab)-modified NPs (Fab’-NPs) with curcumin | Significantly increased cytotoxicity and cellular uptake when compared with the free drug in the MDA-MB-453 cell lines and a xenograft mice model. | HER2 + BC | [84] |
| Polymeric NPs | Paclitaxel | ~225 nm | Poly(lactic-co-glycolic acid) NP coated with hyaluronic acid | Significantly increased cytotoxicity and cellular uptake when compared with the free drug in MDA-MB-231. | TNBC | [85] |
| Polymeric NPs | Paclitaxel | 131.7 nm | Hyaluronic acid-coated polyethylenimine-poly(d,l-lactide-co-glycolide) NPs with miR-542-3p | Significantly increased cytotoxicity and cellular uptake when compared with the free drug in MDA-MB-231. | TNBC | [86] |
| Polymeric NPs | Gambogic acid | 121.5 nm | Hyaluronic acid-coated polyethylenimine-poly(d,l-lactide-co-glycolide) NPs with RAIL plasmid (pTRAIL) and gambogic acid | Significantly increased cytotoxicity, apoptotic cell death, and cellular uptake when compared with the free drug in MDA-MB-231. | TNBC | [87] |
| Polymeric NPs | Thymoquinone | ~22 nm | Pluronic® F127 NPs, hyaluronic acid-conjugated Pluronic® P123. | Significantly reduced cell growth and migration of MDA-MB-231 cell lines and xenograft Balb/c mice | TNBC | [88] |
| Solid–lipid NPs | Di-allyl-disulfide | ~116 nm | Pluronic F-68, solid–lipid NPs engineered with palmitic acid and soya lecithin and surface-modified with glycation end product antibodies | Significantly enhanced cytotoxicity and cellular uptake, augmented activity at the tumor site, and reduced systemic toxicity when compared with the free drug in MDA-MB231 | TNBC | [89] |
2.6. Polymeric Nanocarriers
Earlier, NPs carriers were developed and examined using a variety of materials, including monosaccharides, polysaccharides, proteins, synthetic polymers, metals, lipids, and organic/inorganic compounds. As the main prerequisite for designing NP carriers, the size, shape, composition, surface properties, and biodegradability are characteristics which must be accurately engineered and improved to achieve site-specific drug discharge with therapeutically dose-dependent optimum effects [90]. Engineered NPs activated with precise ligands can target BC cells using an appropriate method and can transport encapsulated payloads efficiently. Furthermore, advanced drug loading, enhanced half-life in the circulation, organized release, and selective delivery of NPs can also achieved by adapting the size, structure, composition, and surface properties. In the design of the engineered NPs, polymers (proteins, lipids, liposomes, dendrimers, hydrogels, organic/inorganic materials) and ligands (nucleic acids, peptides, oligosaccharides, small molecules, and antibody fragments) have been included on the surface of NPs to improve their targeting efficacy (Figure 2).
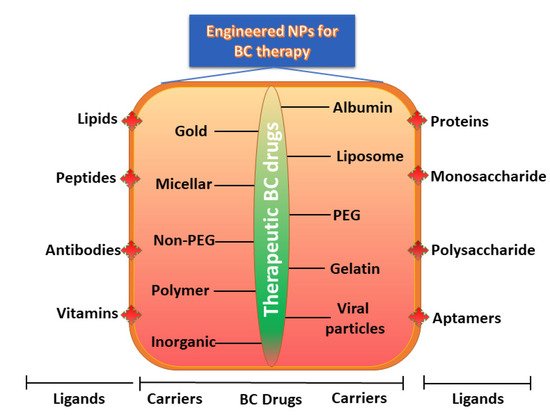
Figure 2. Engineered NPs for BC therapy.
2.6.1. Conjugation with Polymeric Protein
Successful DDSs are based on the attachment of the therapeutic BC drug to proteins for targeted drug delivery. These nanocarriers are directed to the BC through conjugation with the so-called antibody-conjugated NPs. These systems can protect the chemical structure of therapeutic drugs and deliver them to the BC site using a well-controlled method. Upon stimulation, the attachment of antibody to the drug is readily degradable, reducing toxicity [91]. Therapeutic choices may be limited for certain BC subtypes, and hence nanomedicine offers hope for patients with difficult-to-treat BC [92].
A major benefit arises from the smaller size (<10 nm) of such conjugates, which leads to a comparatively longer half-life in the circulation, and makes their extravasation into the BC region more successful when compared to NPs of greater sizes [93][94]. Studies have indicated that protease-cleavable conjugates are more stable than disulfides, although all of them can be engineered.
Standard chemotherapy shows low response rates and short progression-free survival among patients with pretreated metastatic TNBC. However, recent clinical studies showed that sacituzumab govitecan (an antibody–drug conjugate) is well engineered. This conjugate was heavily pre-administered to patients with metastatic TNBC. The outcomes showed improved primary endpoints with fewer complications. The secondary endpoints were progression-free and overall survival, which were found to be improved [95][96].
2.6.2. Liposomes
Liposomes are spherical vesicles (ranging from 50 to 500 nm in size) with a lipid bilayer, and are generated while the lipid connects with the aqueous solution. The most commonly employed lipids are phosphatidylcholine-enriched phospholipids, which produce liposomes. They can be potentially stabilized by strengthening the bilayer with an amphiphilic, long-chain polymer holding PEG at one end, which can simultaneously decrease opsonization and lengthen the circulation time in the blood [82]. Polymeric compounds with appropriate end groups for attachments with antibodies or ligands can also be implanted into the liposome bilayer; therefore, construction-targeted delivery is conceivable. As liposomes bilayers likely mimic those of cells, they can rapidly merge with the plasma membrane [81]. When they are internalized by cells through endocytosis or passive diffusion, the lipid bilayer undergoes rapid degradation due to the acidic environment generated by the endosomes and lysosomes [63].
As shown in the illustration in Figure 2, several engineered polymeric liposomes loaded with therapeutic drugs have used for BC [97][54][98]. Passive tissue targeting is achieved by the extravasation of NPs due to the increased vascular permeability of the BC [99]. Active cellular targeting can also be achieved using the engineered liposomes of NPs with ligands that promote cell-specific recognition and binding. Based on cellular penetration, NPs can release their contents close to the BC cells [53]. Cyclic octapeptide LXY (Cys-Asp-Gly-Phe (3,5-DiF)-Gly-Hyp-Asn-Cys)-attached liposomes carrying the therapeutic drugs doxorubicin and rapamycin targeted over-expressing integrin-α3 in a TNBC-bearing mouse model [100]. These outcomes strongly indicate that targeted combinational therapy can provide a rational approach to improve the therapeutic outcomes of TNBC. Similarly, increased antitumor activity in a TNBC xenograft mouse model has also been revealed with doxorubicin and sorafenib-loaded liposomes [101]. Taken together, research on engineered polymeric liposomes suggests the potential efficacy of the drug-loaded polymer-link liposomes platform in BC therapy.
2.6.3. Lipid–Hybrid Polymer
Polymer NPs are perhaps the most widely studied carrier systems targeting drug delivery. Various synthetic polymers have been employed and investigated based on the potential effects of their hydrophobic and biodegradable nature. Furthermore, many natural polymers (such as chitosan, poly (lactic acid), poly (glycolic acid), poly (lactic-co-glycolic acid), gelatin, poly (alkyl cyanoacrylates), and poly (ε-caprolactone)) have also been employed for drug delivery in BC treatment [102][103]. When liposomes and polymeric NPs were developed, a new class of lipid-hybrid NPs providing the characteristics of both systems was also established. These lipid-hybrid NP incorporate high drug-encapsulation materials and show precise drug release, with outstanding targeting capabilities. Non-targeted drug delivery of platinum–mitaplatin using poly-D, L-lactic-co-glycolic-acid–block-PEG NPs resulted a higher degree of tumor inhibition in the TNBC xenograft mouse model [104].
Recently, anastrozole-loaded PEGylated polymer–lipid hybrid NPs showed high entrapment efficacy (80%), size consistency, and relatively low zeta-potential values (−0.50 to 6.01), and were found to induce apoptosis in ER-positive BC cells [105]. Similarly, another study showed that nanocarriers of a polymer–lipid hybrid encapsulating psoralen optimized its hydrophilic nature and bioavailability, which improved systemic delivery [106]. Li et al. [107] developed salinomycin-loaded polymer–lipid hybrid anti-HER2 NPs and investigated the anti-tumor activity. The outcome showed that the polymer–lipid hybrid was a promising candidate targeting both HER2 + breast CSCs and BC cells.
2.6.4. Dendrimers
Dendrimers are another important type of synthetic nanocarrier (with size ranges from 10 nm to 100 nm) generated by branched monomers of divergent or convergent synthesis. They appear as liposomes, showing a cavity-enriched spherical shape with a hydrophobic core and hydrophilic periphery, and are a distinctive carrier for the delivery of siRNA [108]. Wang et al. [109] established an antisense oligo attached to poly (amidoamine) dendrimers with links to the receptors of vascular endothelial growth factor, and showed a significant decrease in tumor vascularization in the TNBC xenograft mouse model. Another study reported the novel dendrimer G4PAMAM conjugated with GdDOTA and DL680, administered in TNBC xenograft mice as a model for tumor imaging and drug delivery. The outcome of MRI scan and infra-red fluorescence imaging showed the emission of NPs and a significant fluorescence signal in the tumor, demonstrating the selective delivery of a small-sized (GdDOTA)42-G4-DL680 dendrimeric agent to TNBC tumors that circumvented other adjacent primary organs [110]. Hence, the dendrimer is a potential nanocarrier and targeted diagnostic and therapeutic agent in the TNBC tumor mouse model.
3. Engineered NPs Increases the Circulation Half-Life
In principle, an NP-based delivery system should integrate high drug loading capability, a long circulation half-life, effective targeting capacity, discharge programmability, stimuli receptiveness, and diagnostic features. Negatively or neutrally charged NPs generally have a longer blood half-life than positively charged NPs. Using synthetic materials, the surface charges and hydrophobicity of NPs can be suitably tuned to elevate their blood half-life. Based on a longer circulation half-life, NPs can pass the lesion multiple times, with a greater chance of accruing on the lesion site [111][112]. NPs larger than 200 nm are favorably excreted by the spleen. Hence, by selecting a suitable size, surface modifications in the form of PEGylation or the use of rigid NPs will result in the longest blood half-life (2–100 folds), allowing accrual in the spleen with a high percentage and thus the improvement of overall pharmacokinetic parameters [113]. For instance, PEGylated liposomal doxorubicin exhibited a prolonged circulation half-life, which is alleged to be associated with enhanced therapeutic efficacy in BC. Studies revealed that about 50% of polystyrene NPs with the size of 250 nm and coated with poloxamine 908 accrued in the spleen almost 24 h after injection [114]. To avoid clearance by the spleen or MPS, the surface of NPs should to be cautiously engineered to avoid or at least alleviate opsonization [115].
References
- Ganesan, K.; Xu, B. Deep frying cooking oils promote the high risk of metastases in the breast—A critical review. Food Chem. Toxicol. 2020, 144, 111648.
- Fisusi, F.A.; Akala, E.O. Drug Combinations in Breast Cancer Therapy. Pharm. Nanotechnol. 2019, 7, 3–23.
- Tampaki, E.C.; Tampakis, A.; Alifieris, C.E.; Krikelis, D.; Pazaiti, A.; Kontos, M.; Trafalis, D.T. Efficacy and Safety of Neoadjuvant Treatment with Bevacizumab, Liposomal Doxorubicin, Cyclophosphamide and Paclitaxel Combination in Locally/Regionally Advanced, HER2-Negative, Grade III at Premenopausal Status Breast Cancer: A Phase II Study. Clin. Drug Investig. 2018, 38, 639–648.
- Taylor, C.W.; Kirby, A.M. Cardiac Side-effects From Breast Cancer Radiotherapy. Clin. Oncol. 2015, 27, 621–629.
- Zhu, H.; You, J.; Wen, Y.; Jia, L.; Gao, F.; Ganesan, K.; Chen, J. Tumorigenic risk of Angelica sinensis on ER-positive breast cancer growth through ER-induced stemness in vitro and in vivo. J. Ethnopharmacol. 2021, 280, 114415.
- Ganesan, K.; Xu, B. Molecular targets of vitexin and isovitexin in cancer therapy: A critical review. Ann. N. Y. Acad. Sci. 2017, 1401, 102–113.
- Wu, D.; Si, M.; Xue, H.-Y.; Wong, H.-L. Nanomedicine applications in the treatment of breast cancer: Current state of the art. Int. J. Nanomed. 2017, 12, 5879–5892.
- Sinn, H.P.; Kreipe, H. A Brief Overview of the WHO Classification of Breast Tumors, 4th Edition, Focusing on Issues and Updates from the 3rd Edition. Breast Care 2013, 8, 149–154.
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760.
- Ganesan, K.; Sukalingam, K.; Xu, B. Impact of consumption of repeatedly heated cooking oils on the incidence of various cancers—A critical review. Crit. Rev. Food Sci. Nutr. 2019, 59, 488–505.
- Khoobchandani, M.; Katti, K.K.; Karikachery, A.R.; Thipe, V.C.; Srisrimal, D.; Dhurvas Mohandoss, D.K.; Darshakumar, R.D.; Joshi, C.M.; Katti, K.V. New Approaches in Breast Cancer Therapy Through Green Nanotechnology and Nano-Ayurvedic Medicine—Pre-Clinical and Pilot Human Clinical Investigations. Int. J. Nanomed. 2020, 15, 181–197.
- Xiao, K.; Liu, Q.; Suby, N.; Xiao, W.; Agrawal, R.; Vu, M.; Zhang, H.; Luo, Y.; Li, Y.; Lam, K.S. LHRH-Targeted Redox-Responsive Crosslinked Micelles Impart Selective Drug Delivery and Effective Chemotherapy in Triple-Negative Breast Cancer. Adv. Healthc. Mater. 2021, 10, e2001196.
- Mickymaray, S. One-step Synthesis of Silver Nanoparticles Using Saudi Arabian Desert Seasonal Plant Sisymbrium irio and Antibacterial Activity Against Multidrug-Resistant Bacterial Strains. Biomolecules 2019, 9, 662.
- Ke, Y.; Al Aboody, M.S.; Alturaiki, W.; Alsagaby, S.A.; Alfaiz, F.A.; Veeraraghavan, V.P.; Mickymaray, S. Photosynthesized gold nanoparticles from Catharanthus roseus induces caspase-mediated apoptosis in cervical cancer cells (HeLa). Artif. Cells Nanomed. Biotechnol. 2019, 47, 1938–1946.
- Alsagaby, S.A.; Vijayakumar, R.; Premanathan, M.; Mickymaray, S.; Alturaiki, W.; Al-Baradie, R.S.; AlGhamdi, S.; Aziz, M.A.; Alhumaydhi, F.A.; Alzahrani, F.A.; et al. Transcriptomics-Based Characterization of the Toxicity of ZnO Nanoparticles Against Chronic Myeloid Leukemia Cells. Int. J. Nanomed. 2020, 15, 7901–7921.
- Mamnoon, B.; Loganathan, J.; Confeld, M.I.; De Fonseka, N.; Feng, L.; Froberg, J.; Choi, Y.; Tuvin, D.M.; Sathish, V.; Mallik, S. Targeted polymeric nanoparticles for drug delivery to hypoxic, triple-negative breast tumors. ACS Appl. Bio. Mater. 2021, 4, 1450–1460.
- Chen, H.; Feng, X.; Gao, L.; Mickymaray, S.; Paramasivam, A.; Abdulaziz Alfaiz, F.; Almasmoum, H.A.; Ghaith, M.M.; Almaimani, R.A.; Aziz Ibrahim, I.A. Inhibiting the PI3K/AKT/mTOR signalling pathway with copper oxide nanoparticles from Houttuynia cordata plant: Attenuating the proliferation of cervical cancer cells. Artif. Cells Nanomed. Biotechnol. 2021, 49, 240–249.
- O’Brien, M.E.; Wigler, N.; Inbar, M.; Rosso, R.; Grischke, E.; Santoro, A.; Catane, R.; Kieback, D.G.; Tomczak, P.; Ackland, S.P.; et al. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann. Oncol. 2004, 15, 440–449.
- Montero, A.J.; Adams, B.; Diaz-Montero, C.M.; Glück, S. Nab-paclitaxel in the treatment of metastatic breast cancer: A comprehensive review. Expert Rev. Clin. Pharmacol. 2011, 4, 329–334.
- Vishnu, P.; Roy, V. Nab-Paclitaxel: A Novel Formulation of Taxane for Treatment of Breast Cancer. Women’s Health 2010, 6, 495–506.
- Zhao, P.; Astruc, D. Docetaxel nanotechnology in anticancer therapy. ChemMedChem 2012, 7, 952–972.
- Ghafari, M.; Haghiralsadat, F.; Khanamani Falahati-pour, S.; Zavar Reza, J. Development of a novel liposomal nanoparticle formulation of cisplatin to breast cancer therapy. J. Cell. Biochem. 2020, 121, 3584–3592.
- Kumari, P.; Ghosh, B.; Biswas, S. Nanocarriers for cancer-targeted drug delivery. J. Drug Target. 2016, 24, 179–191.
- Navya, P.N.; Kaphle, A.; Srinivas, S.P.; Bhargava, S.K.; Rotello, V.M.; Daima, H.K. Current trends and challenges in cancer management and therapy using designer nanomaterials. Nano Converg. 2019, 6, 23.
- Sabourian, P.; Yazdani, G.; Ashraf, S.S.; Frounchi, M.; Mashayekhan, S.; Kiani, S.; Kakkar, A. Effect of Physico-Chemical Properties of Nanoparticles on Their Intracellular Uptake. Int. J. Mol. Sci. 2020, 21, 8019.
- Jokerst, J.V.; Lobovkina, T.; Zare, R.N.; Gambhir, S.S. Nanoparticle PEGylation for imaging and therapy. Nanomedicine 2011, 6, 715–728.
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48.
- Bayda, S.; Hadla, M.; Palazzolo, S.; Riello, P.; Corona, G.; Toffoli, G.; Rizzolio, F. Inorganic Nanoparticles for Cancer Therapy: A Transition from Lab to Clinic. Curr. Med. Chem. 2018, 25, 4269–4303.
- Yang, Y.; Yu, C. Advances in silica based nanoparticles for targeted cancer therapy. Nanomedicine 2016, 12, 317–332.
- Tang, W.L.; Tang, W.H.; Li, S.D. Cancer theranostic applications of lipid-based nanoparticles. Drug Discov. Today 2018, 23, 1159–1166.
- García-Pinel, B.; Porras-Alcalá, C.; Ortega-Rodríguez, A.; Sarabia, F.; Prados, J.; Melguizo, C.; López-Romero, J.M. Lipid-Based Nanoparticles: Application and Recent Advances in Cancer Treatment. Nanomaterials 2019, 9, 638.
- Date, T.; Nimbalkar, V.; Kamat, J.; Mittal, A.; Mahato, R.I.; Chitkara, D. Lipid-polymer hybrid nanocarriers for delivering cancer therapeutics. J. Control. Release 2018, 271, 60–73.
- Guo, J.; Huang, L. Membrane-core nanoparticles for cancer nanomedicine. Adv. Drug Deliv. Rev. 2020, 156, 23–39.
- Ombredane, A.S.; Silva, V.R.P.; Andrade, L.R.; Pinheiro, W.O.; Simonelly, M.; Oliveira, J.V.; Pinheiro, A.C.; Gonçalves, G.F.; Felice, G.J.; Garcia, M.P.; et al. In Vivo Efficacy and Toxicity of Curcumin Nanoparticles in Breast Cancer Treatment: A Systematic Review. Front. Oncol. 2021, 11, 612903.
- Kashyap, D.; Tuli, H.S.; Yerer, M.B.; Sharma, A.; Sak, K.; Srivastava, S.; Pandey, A.; Garg, V.K.; Sethi, G.; Bishayee, A. Natural product-based nanoformulations for cancer therapy: Opportunities and challenges. Semin. Cancer Biol. 2021, 69, 5–23.
- Karuppiah, A.; Rajan, R.; Ramanathan, M.; Nagarajan, A. Cytotoxicity and Synergistic Effect of Biogenically Synthesized Ternary Therapeutic Nano Conjugates Comprising Plant Active Principle, Silver and Anticancer Drug on MDA-MB-453 Breast Cancer Cell Line. Asian Pac. J. Cancer Prev. 2020, 21, 195–204.
- Soni, K.; Rizwanullah, M.; Kohli, K. Development and optimization of sulforaphane-loaded nanostructured lipid carriers by the Box-Behnken design for improved oral efficacy against cancer: In vitro, ex vivo and in vivo assessments. Artif. Cells Nanomed. Biotechnol. 2018, 46, 15–31.
- Jin, H.; Pi, J.; Yang, F.; Jiang, J.; Wang, X.; Bai, H.; Shao, M.; Huang, L.; Zhu, H.; Yang, P.; et al. Folate-Chitosan Nanoparticles Loaded with Ursolic Acid Confer Anti-Breast Cancer Activities in vitro and in vivo. Sci. Rep. 2016, 6, 30782.
- Minaei, A.; Sabzichi, M.; Ramezani, F.; Hamishehkar, H.; Samadi, N. Co-delivery with nano-quercetin enhances doxorubicin-mediated cytotoxicity against MCF-7 cells. Mol. Biol. Rep. 2016, 43, 99–105.
- Cedervall, T.; Lynch, I.; Lindman, S.; Berggård, T.; Thulin, E.; Nilsson, H.; Dawson, K.A.; Linse, S. Understanding the nanoparticle-protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc. Natl. Acad. Sci. USA 2007, 104, 2050–2055.
- Dobrovolskaia, M.A.; McNeil, S.E. Immunological properties of engineered nanomaterials. Nat. Nanotechnol. 2007, 2, 469–478.
- Kumari, M.; Sharma, N.; Manchanda, R.; Gupta, N.; Syed, A.; Bahkali, A.H.; Nimesh, S. PGMD/curcumin nanoparticles for the treatment of breast cancer. Sci. Rep. 2021, 11, 3824.
- Yadav, A.; Mendhulkar, V.D. Antiproliferative activity of Camellia sinensis mediated silver nanoparticles on three different human cancer cell lines. J. Cancer Res. Ther. 2018, 14, 1316–1324.
- Min, K.H.; Park, K.; Kim, Y.S.; Bae, S.M.; Lee, S.; Jo, H.G.; Park, R.W.; Kim, I.S.; Jeong, S.Y.; Kim, K.; et al. Hydrophobically modified glycol chitosan nanoparticles-encapsulated camptothecin enhance the drug stability and tumor targeting in cancer therapy. J. Control. Release 2008, 127, 208–218.
- Doddapaneni, R.; Patel, K.; Owaid, I.H.; Singh, M. Tumor neovasculature-targeted cationic PEGylated liposomes of gambogic acid for the treatment of triple-negative breast cancer. Drug Deliv. 2016, 23, 1232–1241.
- Toy, R.; Hayden, E.; Shoup, C.; Baskaran, H.; Karathanasis, E. The effects of particle size, density and shape on margination of nanoparticles in microcirculation. Nanotechnology 2011, 22, 115101.
- Toy, R.; Peiris, P.M.; Ghaghada, K.B.; Karathanasis, E. Shaping cancer nanomedicine: The effect of particle shape on the in vivo journey of nanoparticles. Nanomedicine 2014, 9, 121–134.
- White, B.E.; White, M.K.; Adhvaryu, H.; Makhoul, I.; Nima, Z.A.; Biris, A.S.; Ali, N. Nanotechnology approaches to addressing HER2-positive breast cancer. Cancer Nanotechnol. 2020, 11, 12.
- Shen, Z.; Ye, H.; Yi, X.; Li, Y. Membrane Wrapping Efficiency of Elastic Nanoparticles during Endocytosis: Size and Shape Matter. ACS Nano 2019, 13, 215–228.
- Tang, H.; Zhang, H.; Ye, H.; Zheng, Y. Receptor-Mediated Endocytosis of Nanoparticles: Roles of Shapes, Orientations, and Rotations of Nanoparticles. J. Phys. Chem. B 2018, 122, 171–180.
- Kim, S.; Seo, J.; Park, H.H.; Kim, N.; Oh, J.W.; Nam, J.M. Plasmonic Nanoparticle-Interfaced Lipid Bilayer Membranes. Acc. Chem. Res. 2019, 52, 2793–2805.
- Khor, S.Y.; Vu, M.N.; Pilkington, E.H.; Johnston, A.P.R.; Whittaker, M.R.; Quinn, J.F.; Truong, N.P.; Davis, T.P. Elucidating the Influences of Size, Surface Chemistry, and Dynamic Flow on Cellular Association of Nanoparticles Made by Polymerization-Induced Self-Assembly. Small 2018, 14, e1801702.
- Basho, R.K.; Gilcrease, M.; Murthy, R.K.; Helgason, T.; Karp, D.D.; Meric-Bernstam, F.; Hess, K.R.; Herbrich, S.M.; Valero, V.; Albarracin, C.; et al. Targeting the PI3K/AKT/mTOR Pathway for the Treatment of Mesenchymal Triple-Negative Breast Cancer: Evidence From a Phase 1 Trial of mTOR Inhibition in Combination With Liposomal Doxorubicin and Bevacizumab. JAMA Oncol. 2017, 3, 509–515.
- Khallaf, S.M.; Roshdy, J.; Ibrahim, A. Pegylated liposomal doxorubicin in patients with metastatic triple-negative breast cancer: 8-year experience of a single center. J. Egypt. Natl. Cancer Inst. 2020, 32, 20.
- Jehn, C.F.; Hemmati, P.; Lehenbauer-Dehm, S.; Kümmel, S.; Flath, B.; Schmid, P. Biweekly Pegylated Liposomal Doxorubicin (Caelyx) in Heavily Pretreated Metastatic Breast Cancer: A Phase 2 Study. Clin. Breast Cancer 2016, 16, 514–519.
- Martin-Romano, P.; Baraibar, I.; Espinós, J.; Legaspi, J.; López-Picazo, J.M.; Aramendía, J.M.; Fernández, O.A.; Santisteban, M. Combination of pegylated liposomal doxorubicin plus gemcitabine in heavily pretreated metastatic breast cancer patients: Long-term results from a single institution experience. Breast J. 2018, 24, 473–479.
- Smorenburg, C.H.; de Groot, S.M.; van Leeuwen-Stok, A.E.; Hamaker, M.E.; Wymenga, A.N.; de Graaf, H.; de Jongh, F.E.; Braun, J.J.; Los, M.; Maartense, E.; et al. A randomized phase III study comparing pegylated liposomal doxorubicin with capecitabine as first-line chemotherapy in elderly patients with metastatic breast cancer: Results of the OMEGA study of the Dutch Breast Cancer Research Group BOOG. Ann. Oncol. 2014, 25, 599–605.
- Chen, H.H.; Lu, I.L.; Liu, T.I.; Tsai, Y.C.; Chiang, W.H.; Lin, S.C.; Chiu, H.C. Indocyanine green/doxorubicin-encapsulated functionalized nanoparticles for effective combination therapy against human MDR breast cancer. Colloids Surf. B Biointerfaces 2019, 177, 294–305.
- Jafari, M.; Sriram, V.; Xu, Z.; Harris, G.M.; Lee, J.Y. Fucoidan-Doxorubicin Nanoparticles Targeting P-Selectin for Effective Breast Cancer Therapy. Carbohydr. Polym. 2020, 249, 116837.
- Sui, J.; He, M.; Yang, Y.; Ma, M.; Guo, Z.; Zhao, M.; Liang, J.; Sun, Y.; Fan, Y.; Zhang, X. Reversing P-Glycoprotein-Associated Multidrug Resistance of Breast Cancer by Targeted Acid-Cleavable Polysaccharide Nanoparticles with Lapatinib Sensitization. ACS Appl. Mater. Interfaces 2020, 12, 51198–51211.
- Kostryukova, L.V.; Tereshkina, Y.A.; Korotkevich, E.I.; Prozorovsky, V.N.; Torkhovskaya, T.I.; Morozevich, G.E.; Toropygin, I.Y.; Konstantinov, M.A.; Tikhonova, E.G. Targeted drug delivery system for doxorubicin based on a specific peptide and phospholipid nanoparticles. Biomed. Khim. 2020, 66, 464–468.
- Chowdhury, N.; Chaudhry, S.; Hall, N.; Olverson, G.; Zhang, Q.-J.; Mandal, T.; Dash, S.; Kundu, A. Targeted Delivery of Doxorubicin Liposomes for Her-2+ Breast Cancer Treatment. AAPS PharmSciTech 2020, 21, 202.
- Kim, B.; Shin, J.; Wu, J.; Omstead, D.T.; Kiziltepe, T.; Littlepage, L.E.; Bilgicer, B. Engineering peptide-targeted liposomal nanoparticles optimized for improved selectivity for HER2-positive breast cancer cells to achieve enhanced in vivo efficacy. J. Control. Release 2020, 322, 530–541.
- Shieh, M.J.; Hsu, C.Y.; Huang, L.Y.; Chen, H.Y.; Huang, F.H.; Lai, P.S. Reversal of doxorubicin-resistance by multifunctional nanoparticles in MCF-7/ADR cells. J. Control. Release 2011, 152, 418–425.
- Liu, Z.; Balasubramanian, V.; Bhat, C.; Vahermo, M.; Mäkilä, E.; Kemell, M.; Fontana, F.; Janoniene, A.; Petrikaite, V.; Salonen, J.; et al. Quercetin-Based Modified Porous Silicon Nanoparticles for Enhanced Inhibition of Doxorubicin-Resistant Cancer Cells. Adv. Healthc. Mater. 2017, 6, 1601009.
- Yalcin, S.; Unsoy, G.; Mutlu, P.; Khodadust, R.; Gunduz, U. Polyhydroxybutyrate-coated magnetic nanoparticles for doxorubicin delivery: Cytotoxic effect against doxorubicin-resistant breast cancer cell line. Am. J. Ther. 2014, 21, 453–461.
- Ota, K.; Ito, K.; Akahira, J.; Sato, N.; Onogawa, T.; Moriya, T.; Unno, M.; Abe, T.; Niikura, H.; Takano, T.; et al. Expression of organic cation transporter SLC22A16 in human epithelial ovarian cancer: A possible role of the adriamycin importer. Int. J. Gynecol. Pathol. 2007, 26, 334–340.
- Bazylińska, U.; Zieliński, W.; Kulbacka, J.; Samoć, M.; Wilk, K.A. New diamidequat-type surfactants in fabrication of long-sustained theranostic nanocapsules: Colloidal stability, drug delivery and bioimaging. Colloids Surf. B Biointerfaces 2016, 137, 121–132.
- Liao, W.S.; Ho, Y.; Lin, Y.W.; Naveen Raj, E.; Liu, K.K.; Chen, C.; Zhou, X.Z.; Lu, K.P.; Chao, J.I. Targeting EGFR of triple-negative breast cancer enhances the therapeutic efficacy of paclitaxel- and cetuximab-conjugated nanodiamond nanocomposite. Acta Biomater. 2019, 86, 395–405.
- Bano, K.; Bajwa, S.Z.; Ihsan, A.; Hussain, I.; Jameel, N.; Rehman, A.; Taj, A.; Younus, S.; Zubair Iqbal, M.; Butt, F.K.; et al. Synthesis of SPIONs-CNT Based Novel Nanocomposite for Effective Amperometric Sensing of First-Line Antituberculosis Drug Rifampicin. J. Nanosci. Nanotechnol. 2020, 20, 2130–2137.
- Tade, R.S.; Patil, P.O. Theranostic Prospects of Graphene Quantum Dots in Breast Cancer. ACS Biomater. Sci. Eng. 2020, 6, 5987–6008.
- Nakajima, M.; Sakoda, Y.; Adachi, K.; Nagano, H.; Tamada, K. Improved survival of chimeric antigen receptor-engineered T (CAR-T) and tumor-specific T cells caused by anti-programmed cell death protein 1 single-chain variable fragment-producing CAR-T cells. Cancer Sci. 2019, 110, 3079–3088.
- Zhang, N.; Zhang, J.; Wang, P.; Liu, X.; Huo, P.; Xu, Y.; Chen, W.; Xu, H.; Tian, Q. Investigation of an antitumor drug-delivery system based on anti-HER2 antibody-conjugated BSA nanoparticles. Anticancer Drugs 2018, 29, 307–322.
- Mohammadinejad, A.; Taghdisi, S.M.; Es’haghi, Z.; Abnous, K.; Mohajeri, S.A. Targeted imaging of breast cancer cells using two different kinds of aptamers-functionalized nanoparticles. Eur. J. Pharm. Sci. 2019, 134, 60–68.
- Narmani, A.; Rezvani, M.; Farhood, B.; Darkhor, P.; Mohammadnejad, J.; Amini, B.; Refahi, S.; Abdi Goushbolagh, N. Folic acid functionalized nanoparticles as pharmaceutical carriers in drug delivery systems. Drug Dev. Res. 2019, 80, 404–424.
- Du, C.; Qi, Y.; Zhang, Y.; Wang, Y.; Zhao, X.; Min, H.; Han, X.; Lang, J.; Qin, H.; Shi, Q.; et al. Epidermal Growth Factor Receptor-Targeting Peptide Nanoparticles Simultaneously Deliver Gemcitabine and Olaparib To Treat Pancreatic Cancer with Breast Cancer 2 ( BRCA2) Mutation. ACS Nano 2018, 12, 10785–10796.
- Bhagwat, G.S.; Athawale, R.B.; Gude, R.P.; Md, S.; Alhakamy, N.A.; Fahmy, U.A.; Kesharwani, P. Formulation and Development of Transferrin Targeted Solid Lipid Nanoparticles for Breast Cancer Therapy. Front. Pharmacol. 2020, 11, 614290.
- Naruphontjirakul, P.; Viravaidya-Pasuwat, K. Development of anti-HER2-targeted doxorubicin-core-shell chitosan nanoparticles for the treatment of human breast cancer. Int. J. Nanomed. 2019, 14, 4105–4121.
- Cristofolini, T.; Dalmina, M.; Sierra, J.A.; Silva, A.H.; Pasa, A.A.; Pittella, F.; Creczynski-Pasa, T.B. Multifunctional hybrid nanoparticles as magnetic delivery systems for siRNA targeting the HER2 gene in breast cancer cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 109, 110555.
- Kavithaa, K.; Paulpandi, M.; Padma, P.R.; Sumathi, S. Induction of intrinsic apoptotic pathway and cell cycle arrest via baicalein loaded iron oxide nanoparticles as a competent nano-mediated system for triple negative breast cancer therapy. RSC Adv. 2016, 6, 64531–64543.
- Guo, C.; Chen, Y.; Gao, W.; Chang, A.; Ye, Y.; Shen, W.; Luo, Y.; Yang, S.; Sun, P.; Xiang, R.; et al. Liposomal Nanoparticles Carrying anti-IL6R Antibody to the Tumour Microenvironment Inhibit Metastasis in Two Molecular Subtypes of Breast Cancer Mouse Models. Theranostics 2017, 7, 775–788.
- Salkho, N.M.; Paul, V.; Kawak, P.; Vitor, R.F.; Martins, A.M.; Al Sayah, M.; Husseini, G.A. Ultrasonically controlled estrone-modified liposomes for estrogen-positive breast cancer therapy. Artif. Cells Nanomed. Biotechnol. 2018, 46, 462–472.
- Kamalabadi-Farahani, M.; Vasei, M.; Ahmadbeigi, N.; Ebrahimi-Barough, S.; Soleimani, M.; Roozafzoon, R. Anti-tumour effects of TRAIL-expressing human placental derived mesenchymal stem cells with curcumin-loaded chitosan nanoparticles in a mice model of triple negative breast cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, S1011–S1021.
- Duan, D.; Wang, A.; Ni, L.; Zhang, L.; Yan, X.; Jiang, Y.; Mu, H.; Wu, Z.; Sun, K.; Li, Y. Trastuzumab-and Fab’ fragment-modified curcumin PEG-PLGA nanoparticles: Preparation and evaluation in vitro and in vivo. Int. J. Nanomed. 2018, 13, 1831–1840.
- Cerqueira, B.B.S.; Lasham, A.; Shelling, A.N.; Al-Kassas, R. Development of biodegradable PLGA nanoparticles surface engineered with hyaluronic acid for targeted delivery of paclitaxel to triple negative breast cancer cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 593–600.
- Wang, S.; Zhang, J.; Wang, Y.; Chen, M. Hyaluronic acid-coated PEI-PLGA nanoparticles mediated co-delivery of doxorubicin and miR-542-3p for triple negative breast cancer therapy. Nanomedicine 2016, 12, 411–420.
- Wang, S.; Shao, M.; Zhong, Z.; Wang, A.; Cao, J.; Lu, Y.; Wang, Y.; Zhang, J. Co-delivery of gambogic acid and TRAIL plasmid by hyaluronic acid grafted PEI-PLGA nanoparticles for the treatment of triple negative breast cancer. Drug Deliv. 2017, 24, 1791–1800.
- Bhattacharya, S.; Ghosh, A.; Maiti, S.; Ahir, M.; Debnath, G.H.; Gupta, P.; Bhattacharjee, M.; Ghosh, S.; Chattopadhyay, S.; Mukherjee, P.; et al. Delivery of thymoquinone through hyaluronic acid-decorated mixed Pluronic® nanoparticles to attenuate angiogenesis and metastasis of triple-negative breast cancer. J. Control. Release 2020, 322, 357–374.
- Siddhartha, V.T.; Pindiprolu, S.; Chintamaneni, P.K.; Tummala, S.; Nandha Kumar, S. RAGE receptor targeted bioconjuguate lipid nanoparticles of diallyl disulfide for improved apoptotic activity in triple negative breast cancer: In vitro studies. Artif. Cells Nanomed. Biotechnol. 2018, 46, 387–397.
- Dusinska, M.; Magdolenova, Z.; Fjellsbø, L.M. Toxicological aspects for nanomaterial in humans. Methods Mol. Biol. 2013, 948, 1–12.
- Juan, A.; Cimas, F.J.; Bravo, I.; Pandiella, A.; Ocaña, A.; Alonso-Moreno, C. Antibody Conjugation of Nanoparticles as Therapeutics for Breast Cancer Treatment. Int. J. Mol. Sci. 2020, 21, 6018.
- Okines, A.F.C.; Ulrich, L. Investigational antibody-drug conjugates in clinical trials for the treatment of breast cancer. Expert Opin. Investig. Drugs 2021, 30, 789–795.
- Dillman, R.O. Cancer immunotherapy. Cancer Biother. Radiopharm. 2011, 26, 1–64.
- Cersosimo, R.J. Monoclonal antibodies in the treatment of cancer, Part 1. Am. J. Health Syst. Pharm. 2003, 60, 1531–1548.
- Bardia, A.; Mayer, I.A.; Diamond, J.R.; Moroose, R.L.; Isakoff, S.J.; Starodub, A.N.; Shah, N.C.; O’Shaughnessy, J.; Kalinsky, K.; Guarino, M.; et al. Efficacy and Safety of Anti-Trop-2 Antibody Drug Conjugate Sacituzumab Govitecan (IMMU-132) in Heavily Pretreated Patients With Metastatic Triple-Negative Breast Cancer. J. Clin. Oncol. 2017, 35, 2141–2148.
- Bardia, A.; Mayer, I.A.; Vahdat, L.T.; Tolaney, S.M.; Isakoff, S.J.; Diamond, J.R.; O’Shaughnessy, J.; Moroose, R.L.; Santin, A.D.; Abramson, V.G.; et al. Sacituzumab Govitecan-hziy in Refractory Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2019, 380, 741–751.
- Ardavanis, A.; Mavroudis, D.; Kalbakis, K.; Malamos, N.; Syrigos, K.; Vamvakas, L.; Kotsakis, A.; Kentepozidis, N.; Kouroussis, C.; Agelaki, S.; et al. Pegylated liposomal doxorubicin in combination with vinorelbine as salvage treatment in pretreated patients with advanced breast cancer: A multicentre phase II study. Cancer Chemother. Pharmacol. 2006, 58, 742–748.
- Yang, F.O.; Hsu, N.C.; Moi, S.H.; Lu, Y.C.; Hsieh, C.M.; Chang, K.J.; Chen, D.R.; Tu, C.W.; Wang, H.C.; Hou, M.F. Efficacy and toxicity of pegylated liposomal doxorubicin-based chemotherapy in early-stage breast cancer: A multicenter retrospective case-control study. Asia Pac. J. Clin. Oncol. 2018, 14, 198–203.
- Chan, S.; Davidson, N.; Juozaityte, E.; Erdkamp, F.; Pluzanska, A.; Azarnia, N.; Lee, L.W. Phase III trial of liposomal doxorubicin and cyclophosphamide compared with epirubicin and cyclophosphamide as first-line therapy for metastatic breast cancer. Ann. Oncol. 2004, 15, 1527–1534.
- Dai, W.; Yang, F.; Ma, L.; Fan, Y.; He, B.; He, Q.; Wang, X.; Zhang, H.; Zhang, Q. Combined mTOR inhibitor rapamycin and doxorubicin-loaded cyclic octapeptide modified liposomes for targeting integrin α3 in triple-negative breast cancer. Biomaterials 2014, 35, 5347–5358.
- Lee, S.M.; Ahn, R.W.; Chen, F.; Fought, A.J.; O’Halloran, T.V.; Cryns, V.L.; Nguyen, S.T. Biological evaluation of pH-responsive polymer-caged nanobins for breast cancer therapy. ACS Nano 2010, 4, 4971–4978.
- Naskar, S.; Koutsu, K.; Sharma, S. Chitosan-based nanoparticles as drug delivery systems: A review on two decades of research. J. Drug Target. 2019, 27, 379–393.
- Elzoghby, A.O. Gelatin-based nanoparticles as drug and gene delivery systems: Reviewing three decades of research. J. Control. Release 2013, 172, 1075–1091.
- Johnstone, T.C.; Kulak, N.; Pridgen, E.M.; Farokhzad, O.C.; Langer, R.; Lippard, S.J. Nanoparticle encapsulation of mitaplatin and the effect thereof on in vivo properties. ACS Nano 2013, 7, 5675–5683.
- Massadeh, S.; Omer, M.E.; Alterawi, A.; Ali, R.; Alanazi, F.H.; Almutairi, F.; Almotairi, W.; Alobaidi, F.F.; Alhelal, K.; Almutairi, M.S.; et al. Optimized Polyethylene Glycolylated Polymer-Lipid Hybrid Nanoparticles as a Potential Breast Cancer Treatment. Pharmaceutics 2020, 12, 666.
- Du, M.; Ouyang, Y.; Meng, F.; Zhang, X.; Ma, Q.; Zhuang, Y.; Liu, H.; Pang, M.; Cai, T.; Cai, Y. Polymer-lipid hybrid nanoparticles: A novel drug delivery system for enhancing the activity of Psoralen against breast cancer. Int. J. Pharm. 2019, 561, 274–282.
- Li, J.; Xu, W.; Yuan, X.; Chen, H.; Song, H.; Wang, B.; Han, J. Polymer-lipid hybrid anti-HER2 nanoparticles for targeted salinomycin delivery to HER2-positive breast cancer stem cells and cancer cells. Int. J. Nanomed. 2017, 12, 6909–6921.
- Tambe, V.; Thakkar, S.; Raval, N.; Sharma, D.; Kalia, K.; Tekade, R.K. Surface Engineered Dendrimers in siRNA Delivery and Gene Silencing. Curr. Pharm. Des. 2017, 23, 2952–2975.
- Wang, P.; Zhao, X.H.; Wang, Z.Y.; Meng, M.; Li, X.; Ning, Q. Generation 4 polyamidoamine dendrimers is a novel candidate of nano-carrier for gene delivery agents in breast cancer treatment. Cancer Lett. 2010, 298, 34–49.
- Zhang, L.; Varma, N.R.; Gang, Z.Z.; Ewing, J.R.; Arbab, A.S.; Ali, M.M. Targeting Triple Negative Breast Cancer with a Small-sized Paramagnetic Nanoparticle. J. Nanomed. Nanotechnol. 2016, 7, 404.
- Jin-Wook, Y.; Elizabeth, C.; Samir, M. Factors that Control the Circulation Time of Nanoparticles in Blood: Challenges, Solutions and Future Prospects. Curr. Pharm. Des. 2010, 16, 2298–2307.
- Zu, M.; Ma, Y.; Cannup, B.; Xie, D.; Jung, Y.; Zhang, J.; Yang, C.; Gao, F.; Merlin, D.; Xiao, B. Oral delivery of natural active small molecules by polymeric nanoparticles for the treatment of inflammatory bowel diseases. Adv. Drug Deliv. Rev. 2021, 176, 113887.
- Sun, T.; Zhang, Y.S.; Pang, B.; Hyun, D.C.; Yang, M.; Xia, Y. Engineered nanoparticles for drug delivery in cancer therapy. Angew. Chem. Int. Ed. Engl. 2014, 53, 12320–12364.
- Zaman, R.; Islam, R.A.; Ibnat, N.; Othman, I.; Zaini, A.; Lee, C.Y.; Chowdhury, E.H. Current strategies in extending half-lives of therapeutic proteins. J. Control. Release 2019, 301, 176–189.
- Baharifar, H.; Khoobi, M.; Arbabi Bidgoli, S.; Amani, A. Preparation of PEG-grafted chitosan/streptokinase nanoparticles to improve biological half-life and reduce immunogenicity of the enzyme. Int. J. Biol Macromol. 2020, 143, 181–189.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
958
Entry Collection:
Biopharmaceuticals Technology
Revisions:
2 times
(View History)
Update Date:
12 Nov 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




