Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hyo-Jin An | + 1572 word(s) | 1572 | 2021-11-11 05:09:04 | | | |
| 2 | Rita Xu | Meta information modification | 1572 | 2021-11-11 07:09:31 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
An, H. Oleanolic Acid. Encyclopedia. Available online: https://encyclopedia.pub/entry/15895 (accessed on 07 February 2026).
An H. Oleanolic Acid. Encyclopedia. Available at: https://encyclopedia.pub/entry/15895. Accessed February 07, 2026.
An, Hyo-Jin. "Oleanolic Acid" Encyclopedia, https://encyclopedia.pub/entry/15895 (accessed February 07, 2026).
An, H. (2021, November 11). Oleanolic Acid. In Encyclopedia. https://encyclopedia.pub/entry/15895
An, Hyo-Jin. "Oleanolic Acid." Encyclopedia. Web. 11 November, 2021.
Copy Citation
Oleanolic acid (OA) is a pentacyclic triterpenoid, abundantly found in plants of the Oleaceae family, and is well known for its beneficial pharmacological activities.
oleanolic acid
atopic dermatitis
1. Introduction
Allergic inflammation is characterized by pathophysiological or hypersensitivity disorders, including allergic asthma, allergic rhinitis, anaphylaxis, and atopic dermatitis (AD), after exposure to allergens [1]. AD is a chronic inflammatory skin disease that arises from the complicated interaction of innate and adaptive immune responses based on genetics, environmental factors, immune abnormalities, and skin barrier functions [2]. The characteristic features of AD include itchy, swollen, red, and cracked skin with inflammatory cell accumulation in AD skin lesions. Although the pathogenesis of AD is not clear, it is known that several cells and factors are associated with its development. The pathological processes of AD are thought to be mediated by Th1/ Th2 balance, which is skewed toward Th2 in AD. Th2 cells are mainly activated in the acute phase of AD, while Th1 cells mediate the alteration of expression in chronic AD [3]. The standard treatment for AD involves the application of topical corticosteroids or the administration of immunosuppressive agents; however, protracted use of these agents can cause various side effects, such as skin atrophy, bleeding, vasodilation, and organ toxicity. For this reason, medicines originating from herbal sources may be preferred to steroids, and may be used in combination with other methods, such as enhancing immunity, reducing house mite dust, and dietary restrictions [4][5].
Numerous intracellular signal transduction triggered by ligand–cell surface receptor binding is mediated by transcription factors. Nuclear factor (NF)-κB and the signal transducer and activator of transcription (STAT)−1 are pivotal transcription factors associated with the allergic inflammatory response [6]. Upon stimulation, the inhibitor κB (IκB)-α protein is phosphorylated, leading to the ubiquitination and proteasomal degradation of IκB. Sequentially activated NF-κB and interferon (IFN)-γ-activated STAT 1 in the cytoplasm translocate into the nucleus, where they engage in the expression of numerous pro-inflammatory mediators. Thus, these transcription factors are important pharmacological targets for the discovery of novel therapeutics to treat allergic disorders [7][8].
Oleanolic acid (OA) is a pentacyclic triterpenoid that is abundant in plants of the Oleaceae family, such as Olea europaea. OA is ubiquitously found in food and plants, where it exists as a free acid or as an aglycone of triterpenoid saponins, such as ursolic acid, moronic acid, and betulinic acid [9]. To date, various reports have described the pharmacological activities of OA, including its antioxidant [10], anti-inflammatory [11][12], anti-asthmatic [13], anti-diabetic [14][15], anti-tumor [16], hepatoprotective [17], immunomodulatory [18], anti-parasitic [19], and anti-hypertensive [20] properties. Despite the fact that OA is a well-known active component contained in various plants, studies on its effect on AD are insufficient. As it is important to study natural materials that are effective against allergic diseases, we focused on OA that exhibits a wide range of biological activities, such as anti-inflammatory and anti-asthmatic effects, as a feasible active compound for allergic diseases. Previously, we reported the anti-allergic effect of OA, demonstrating that OA exerted an inhibitory effect on mast cell-mediated allergic inflammation in vivo and in vitro [21]. Allergic response and inflammation can trigger AD and worsen the condition, thus, controlling allergic and inflammatory reaction could be important strategy in the manage of AD. These results prompted us to investigate its potential effect on other allergic diseases, such as AD. As AD is mainly the beginning of a series of allergic disorders, we hypothesized that OA would attenuate AD-like symptoms.
2. OA Attenuated AD Lesions in DNCB-Induced AD Mice
The repeated topical application of DNCB on the dorsal skin of mice induces AD skin symptoms. DNCB is a “contact sensitizer” that induces contact hypersensitivity of the skin in mice, which is considered to be a cell-mediated response [22]. To investigate the remedial effects of OA on AD mice, we administered OA following the induction of AD mouse skin. The experimental procedure is summarized in Figure 1A. On the day of sacrifice, severe AD-like lesions, such as erythema, edema, hemorrhage, scarring, dryness, excoriation, and erosion were observed on the dorsal skin of DNCB-induced AD mice. However, topical application of dexamethasone (10 μM), a well-known therapeutic agent for AD, and OA (10 and 50 μM) for 3 weeks significantly alleviated these AD skin symptoms compared to the DNCB group (p < 0.001) (Figure 1B,C).
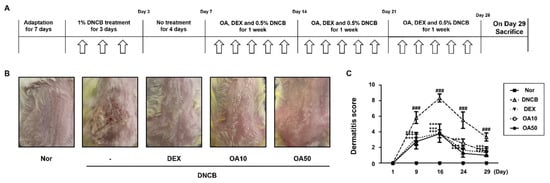
Figure 1. Effects of OA on DNCB-induced AD skin lesions in ICR mice. (A) Experimental schedule for the induction of AD. (B) Effect of OA on clinical features of DNCB-induced AD skin lesions. White arrows indicated DNCB treatment. (C) Effects of OA on dermatitis score. Densitometric analysis was performed using Bio-Rad Quantity One® Software. The data shown represent mean ± S.D. (n = 6) of three independent experiments. ### p < 0.001 vs. the control group; *** p < 0.001 vs. DNCB-treated group.
3. OA Improved the Histological Observations and Histamine Release in DNCB-Induced AD Mice
Histological alterations, such as epidermal hyperplasia and infiltration of lymphocytes and mast cells in the skin, are the main hallmarks of AD [23]. Improvements in clinical skin conditions following OA treatment were confirmed by histological examination. Histological analysis was performed on atopic skin tissues. The excised skin from each group was stained with hematoxylin and eosin (H&E) or toluidine blue, and histological alterations were observed microscopically. H&E-stained tissue sections revealed that the thickness of epidermal and dermal tissues was greater in the DNCB-treated group (91.84 ± 7.60, 474.66 ± 43.65 μm, respectively, p < 0.001) than the control group due to edema, hyperkeratosis, and hyperplasia (Figure 2A). However, treatment with 10 and 50 μM OA markedly attenuated the epidermal (47.16 ± 5.98 and 52.65 ± 9.56 μm, p < 0.001) and dermal thickening (258.65 ± 17.56 and 292.65 ± 25.61 μm, p < 0.001) (Figure 2B,C). In the toluidine blue-stained tissue sections, mast cell infiltration, an indicator of inflammation, was noticeably increased in the DNCB-treated group compared to the control group (73.67 ± 12.06 cells, p < 0.001). Treatment with 10 and 50 μM OA attenuated the infiltration of inflammatory cells, particularly mast cells, as evidenced by toluidine blue staining (28.5 ± 6.98, 25.5 ± 3.73 cells, respectively, p < 0.001) (Figure 3A,B). As mast cells are sources of histamine, which is the most potent mediator involved in AD symptoms [24], histamine levels in the serum were also examined. The results showed that treatment with 50 μM OA remarkably inhibited histamine release (271.91 ± 35.75 ng/mL, p < 0.001) compared to the DNCB-treated group (411.81 ± 60.12 ng/mL, p < 0.001) (Figure 3C).
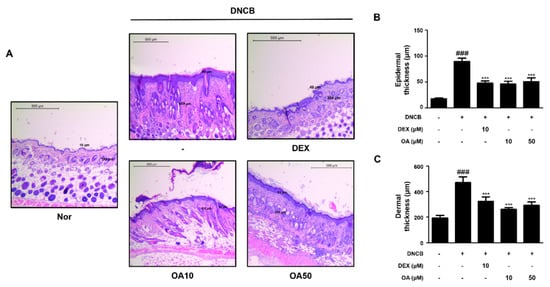
Figure 2. Effect of OA on epidermal and dermal thickness in DNCB-induced AD skin lesions. (A) H&E stained AD mouse skin lesions (scale bar = 500 μm). (B) Determination of epidermal thickness and (C) dermal thickness. Epidermal and dermal thickness in H&E stained sections were measured under a microscope. The data shown represent mean ± S.D. (n = 6) of three independent experiments. ### p < 0.001 vs. the control group; *** p < 0.001 vs. DNCB-treated group.
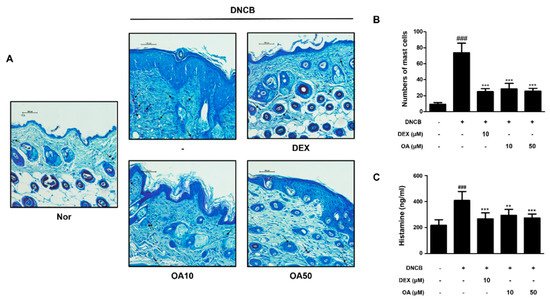
Figure 3. Effect of OA on mast cell infiltration and serum histamine level in DNCB-induced AD skin lesions. (A) Toluidine blue stained AD mouse skin lesions (scale bar = 100 μm). Black arrows indicated stained mast cells. (B) Number of mast cells per mm section. Mast cell infiltration in toluidine blue stained sections is expressed as the average total count in five fields. (C) Histamine release in mouse serum was measured using an ELISA kit. The data shown represent mean ± S.D. (n = 6) of three independent experiments. ### p < 0.001 vs. the control group; ** p < 0.01 and *** p < 0.001 vs. DNCB-treated group.
4. OA Suppressed the mRNA Expression of AD-Related Cytokines and Activation of IκB and STAT1 in DNCB-Induced AD Mice
Next, we investigated whether OA inhibited the signature cytokines of AD in the dorsal tissues of DNCB-induced AD mice. Above all, thymus and activation-regulated chemokine (TARC)/CCL17, are members of the CC chemokine subfamily and are involved in the recruitment of Th2 lymphocytes and the continuation of Th2 immune responses [25]. In addition, thymic stromal lymphopoietin (TSLP) is known to provoke dendritic cell-mediated Th2 responses and is highly expressed in activated mast cells and skin of AD, which triggers allergic inflammation. Therefore, these cytokines are considered mediators of inflammatory skin diseases, such as AD . As shown in Figure 4A, the mRNA expression levels of TSLP and TARC were markedly (p < 0.001) increased by repetitive treatment with DNCB, while OA reduced the expression levels of TSLP and TARC by approximately basal levels (p < 0.001). In line with these results, Th2-type cytokines, including IL-4, IL-5, and IL-13, were downregulated by OA treatment compared to DNCB-induced AD mice (p < 0.001) (Figure 4B).
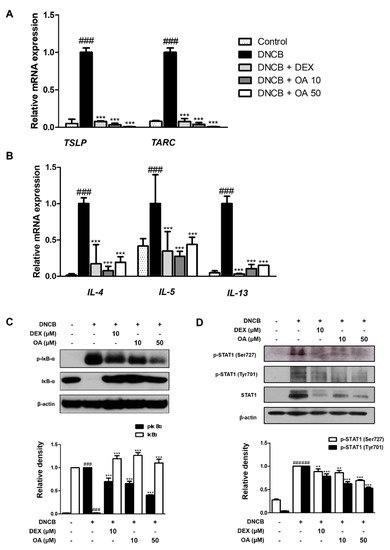
Figure 4. Effect of OA on AD cytokines and IκB, STAT1 activation in DNCB-induced AD skin lesions. Total RNA prepared from the dorsal tissue, and the level of (A) TARC, TSLP, (B) IL-4, IL-5, and IL-13 were determined by quantitative qRT-PCR. Expression of IκB (C) and STAT1 (D) was determined by Western blot analysis using specific antibodies. Densitometric analysis was performed using Bio-Rad Quantity One® Software. The data shown represent mean ± S.D. (n = 6) of three independent experiments. ### p < 0.001 vs. the control group; ** p < 0.01 and *** p < 0.001 vs. DNCB-treated group.
To investigate the signaling pathways involved in the inhibitory effect of OA on cytokine production, we examined the phosphorylation and degradation of IκB and activation of STAT1 in DNCB-induced AD mice. The results demonstrated that the phosphorylation and degradation of IκB induced by DNCB were significantly (p < 0.001) inhibited by treatment with OA (Figure 4C). In addition, OA inhibited the DNCB-induced phosphorylation of STAT1 at residues Ser727 and Tyr701 with significance (Figure 4D). Considering our results, it can be presumed that the NF-κB and STAT1 signaling pathways are involved in the inhibitory effect of OA on the cytokine profiles of DNCB-induced AD-like skin.
References
- Jeong, H.J.; Kim, H.Y.; Kim, H.M. Molecular mechanisms of anti-inflammatory effect of chrysophanol, an active component of AST2017-01 on atopic dermatitis in vitro models. Int. Immunopharmacol. 2018, 54, 238–244.
- Hou, D.D.; Di, Z.H.; Qi, R.Q.; Wang, H.X.; Zheng, S.; Hong, Y.X.; Guo, H.; Chen, H.D.; Gao, X.H. Sea Buckthorn (Hippophae rhamnoides L.) Oil Improves Atopic Dermatitis-Like Skin Lesions via Inhibition of NF-kappaB and STAT1 Activation. Skin Pharmacol. Physiol. 2017, 30, 268–276.
- Yang, G.; Cheon, S.-Y.; Chung, K.-S.; Lee, S.-J.; Hong, C.-H.; Lee, K.-T.; Jang, D.-S.; Jeong, J.-C.; Kwon, O.-K.; Nam, J.-H.; et al. Solanum tuberosum L. cv. Jayoung Epidermis Extract Inhibits Mite Antigen-Induced Atopic Dermatitis in NC/Nga Mice by Regulating the Th1/Th2 Balance and Expression of Filaggrin. J. Med. Food 2015, 18, 1013–1021.
- Jung, M.; Lee, T.H.; Oh, H.J.; Kim, H.; Son, Y.; Lee, E.H.; Kim, J. Inhibitory effect of 5,6-dihydroergosteol-glucoside on atopic dermatitis-like skin lesions via suppression of NF-kappaB and STAT activation. J. Dermatol. Sci. 2015, 79, 252–261.
- Lee, H.; Ha, H.; Lee, J.K.; Park, S.J.; Jeong, S.I.; Shin, H.K. The Leaves of Broussonetia kazinoki Siebold Inhibit Atopic Dermatitis-Like Response on Mite Allergen-Treated Nc/Nga Mice. Biomol. Ther. 2014, 22, 438–444.
- Choi, J.K.; Jang, Y.H.; Lee, S.; Lee, S.R.; Choi, Y.A.; Jin, M.; Choi, J.H.; Park, J.H.; Park, P.H.; Choi, H.; et al. Chrysin attenuates atopic dermatitis by suppressing inflammation of keratinocytes. Food Chem. Toxicol. 2017, 110, 142–150.
- Jung, M.R.; Lee, T.H.; Bang, M.H.; Kim, H.; Son, Y.; Chung, D.K.; Kim, J. Suppression of thymus- and activation-regulated chemokine (TARC/CCL17) production by 3-O-beta-D-glucopyanosylspinasterol via blocking NF-kappaB and STAT1 signaling pathways in TNF-alpha and IFN-gamma-induced HaCaT keratinocytes. Biochem. Biophys. Res. Commun. 2012, 427, 236–241.
- Han, E.H.; Hwang, Y.P.; Choi, J.H.; Yang, J.H.; Seo, J.K.; Chung, Y.C.; Jeong, H.G. Psidium guajava extract inhibits thymus and activation-regulated chemokine (TARC/CCL17) production in human keratinocytes by inducing heme oxygenase-1 and blocking NF-kappaB and STAT1 activation. Environ. Toxicol. Pharmacol. 2011, 32, 136–145.
- Ayeleso, T.B.; Matumba, M.G.; Mukwevho, E. Oleanolic Acid and Its Derivatives: Biological Activities and Therapeutic Potential in Chronic Diseases. Molecules 2017, 22, 1915.
- Sultana, N.; Ata, A. Oleanolic acid and related derivatives as medicinally important compounds. J. Enzyme Inhib. Med. Chem. 2008, 23, 739–756.
- Giner-Larza, E.M.; Manez, S.; Recio, M.C.; Giner, R.M.; Prieto, J.M.; Cerda-Nicolas, M.; Rios, J.L. Oleanonic acid, a 3-oxotriterpene from Pistacia, inhibits leukotriene synthesis and has anti-inflammatory activity. Eur. J. Pharmacol. 2001, 428, 137–143.
- Dzubak, P.; Hajduch, M.; Vydra, D.; Hustova, A.; Kvasnica, M.; Biedermann, D.; Markova, L.; Urban, M.; Sarek, J. Pharmacological activities of natural triterpenoids and their therapeutic implications. Nat. Prod. Rep. 2006, 23, 394–411.
- Lee, J.Y.; Moon, H.; Kim, C.J. Effects of hydroxy pentacyclic triterpene acids from Forsythia viridissima on asthmatic responses to ovalbumin challenge in conscious guinea pigs. Biol. Pharm. Bull. 2010, 33, 230–237.
- Li, M.; Han, Z.; Bei, W.; Rong, X.; Guo, J.; Hu, X. Oleanolic Acid Attenuates Insulin Resistance via NF-kappaB to Regulate the IRS1-GLUT4 Pathway in HepG2 Cells. Evid. Based Complement. Altern. Med. 2015, 2015, 643102.
- Li, Y.; Wang, J.; Gu, T.; Yamahara, J.; Li, Y. Oleanolic acid supplement attenuates liquid fructose-induced adipose tissue insulin resistance through the insulin receptor substrate-1/phosphatidylinositol 3-kinase/Akt signaling pathway in rats. Toxicol. Appl. Pharmacol. 2014, 277, 155–163.
- Petronelli, A.; Pannitteri, G.; Testa, U. Triterpenoids as new promising anticancer drugs. Anticancer Drugs 2009, 20, 880–892.
- Wang, X.; Ye, X.-L.; Liu, R.; Chen, H.-L.; Bai, H.; Liang, X.; Zhang, X.-D.; Wang, Z.; Li, W.-L.; Hai, C.-X. Antioxidant activities of oleanolic acid in vitro: Possible role of Nrf2 and MAP kinases. Chem. Biol. Interact. 2010, 184, 328–337.
- Martin, R.; Hernandez, M.; Cordova, C.; Nieto, M.L. Natural triterpenes modulate immune-inflammatory markers of experimental autoimmune encephalomyelitis: Therapeutic implications for multiple sclerosis. Br. Pharmacol. J. 2012, 166, 1708–1723.
- Sifaoui, I.; Lopez-Arencibia, A.; Martin-Navarro, C.M.; Reyes-Batlle, M.; Mejri, M.; Valladares, B.; Lorenzo-Morales, J.; Abderabba, M.; Pinero, J.E. Selective activity of Oleanolic and Maslinic Acids on the Amastigote form of Leishmania spp. Iran J. Pharm. Res. 2017, 16, 1190–1193.
- Somova, L.I.; Shode, F.O.; Ramnanan, P.; Nadar, A. Antihypertensive, antiatherosclerotic and antioxidant activity of triterpenoids isolated from Olea europaea, subspecies africana leaves. J. Ethnopharmacol. 2003, 84, 299–305.
- Kang, Y.M.; Lee, M.; An, H.J. Oleanolic acid protects against mast cell-mediated allergic responses by suppressing Akt/NF-kappaB and STAT1 activation. Phytomedicine 2021, 80, 153340.
- Zhang, E.Y.; Chen, A.Y.; Zhu, B.T. Mechanism of dinitrochlorobenzene-induced dermatitis in mice: Role of specific antibodies in pathogenesis. PLoS ONE 2009, 4, e7703.
- Yang, I.J.; Lee, D.U.; Shin, H.M. Inhibitory Effect of Valencene on the Development of Atopic Dermatitis-Like Skin Lesions in NC/Nga Mice. Evid. Based Complement. Altern. Med. 2016, 2016, 9370893.
- Cho, M.S.; Park, W.S.; Jung, W.K.; Qian, Z.J.; Lee, D.S.; Choi, J.S.; Lee, D.Y.; Park, S.G.; Seo, S.K.; Kim, H.J.; et al. Caffeic acid phenethyl ester promotes anti-inflammatory effects by inhibiting MAPK and NF-kappaB signaling in activated HMC-1 human mast cells. Pharm. Biol. 2014, 52, 926–932.
- Park, E.J.; Park, K.C.; Eo, H.; Seo, J.; Son, M.; Kim, K.H.; Chang, Y.-S.; Cho, S.-H.; Min, K.-U.; Jin, M.; et al. Suppression of spontaneous dermatitis in NC/Nga murine model by PG102 isolated from Actinidia arguta. J. Investig. Dermatol. 2007, 127, 1154–1160.
More
Information
Subjects:
Allergy
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Revisions:
2 times
(View History)
Update Date:
11 Nov 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




