
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Franck Biet | + 2283 word(s) | 2283 | 2020-08-07 08:30:27 | | | |
| 2 | Rita Xu | -1329 word(s) | 954 | 2020-08-11 10:34:32 | | |
Video Upload Options
In this study we characterized adhesins heparin-binding hemagglutinin (HBHA) and laminin-binding proteins (LBP) from M. intracellulare subsp chimaera intracellulare complex (MCIC) species isolated from patients with a variety of disease expression, examined the role of these adhesins in binding of M. intracellulare to lung epithelial cells and their degree of conservation within the M. intracellulare subsp chimaera intracellulare complex
1. Introduction
Non-tuberculous mycobacteria (NTM) are an increasing cause of opportunistic diseases in humans [1][2]. Among NTM, the Mycobacterium avium complex (MAC) represents a group with a specific distribution of species according to continent and countries [3]. In addition to severe infection in immune-deficient subjects, such as AIDS patients, the incidence of MAC infections has also recently increased in patients with chronic pulmonary disease and other underlying conditions [4][5]. Due to modifications in mandatory programs of vaccination with bacillus Calmette-Guérin (BCG) in low-incidence countries, an increase in the frequency of adenitis in children was noticed [6] mostly because of MAC infection. MAC is classically divided into Mycobacterium avium and Mycobacterium intracellulare. The M. avium species includes four closely related subspecies, M. avium subsp. paratuberculosis, the etiologic agent of Johne’s disease or paratuberculosis in ruminants [7]. M. avium subsp. avium and M. avium subsp. silvaticum, responsible for avian tuberculosis and infection in wood pigeons, respectively [8] and M. avium subsp. hominissuis, which is usually isolated from pigs but can also be implicated in human infections [9]. Some recently discovered species are very close to M. intracellulare and are termed M. intracellulare subsp. chimaera intracellulare complex (MCIC) [10][11]. M. intracellulare and M. intracellulare subsp. chimaera are associated with infections in humans. M. intracellulare is mainly implicated in pulmonary infections, and M. intracellulare subsp. chimaera [12], was recently associated with fatal infections after cardiac surgery [13]. MAC can be identified by using DNA probes, luminescent systems, DNA sequencing of rpoB, hsp65 and the 16S–23S Intergenic region, or identification of specific insertion sequences [10]. GenoType NTM-DR, a new commercial diagnostic assay, allows differentiation between three MAC species, M. avium, M. intracellulare, M. intracellulare subsp. chimaera, as well as identification of subspecies within the Mycobacterium abscessus complex [14]. Mass spectrometry has also been recently proposed as a useful tool to identify these NTM at the species level [15].
Several studies have shown that pathogenic mycobacteria use the protein or proteoglycan component of the extracellular matrix (ECM) for adherence and invasion of the host [16]. One of the best characterized mycobacterial adhesins is the heparin-binding hemagglutinin (HBHA), initially identified in Mycobacterium tuberculosis and Mycobacterium bovis bacillus Calmette-Guérin (BCG) [17][18]. However, HBHA-like molecules are also present in many other mycobacteria, both pathogenic and nonpathogenic [19][20][21][22]. HBHA is located on the surface of the mycobacteria and mediates binding of the bacilli to epithelial cells and fibroblasts [18] by interacting with sulfated glycoconjugates present on the surfaces of host cells [23]. It also plays a role in the dissemination of M. tuberculosis from the lungs to deeper tissues [24] and has shown promise as a diagnostic target for the detection of latent tuberculosis in humans [25][26][27][28].
Laminin and collagen in the lung also promote adherence to ECM-binding mycobacteria, and mycobacterial laminin-binding proteins (LBP) involved in adherence of mycobacteria to host cells have been identified and characterized. LBP was initially described to play a role in the interaction between Mycobacterium leprae and Schwann cells [29][30][31]. LBP, also referenced to as Lbp/Hlp [32][33], Mdp1, the Mycobacterial DNA-binding protein 1 [30] and hupB the mycobacterial histone-like protein are conserved in mycobacteria, including MAC [32].
2. Adherence of M. intracellulare to Epithelial Cells is Modulated by Heparin and Laminin
Previous reports have shown that adherence of mycobacteria to epithelial cells can be modulated by the addition of an extracellular matrix component [18][32]. Using the recombinant M. intracellulare strain, we tested whether soluble exogenous heparin or laminin can affect the cytoadherence of M. intracellulare to A549 epithelial cells. As observed qualitatively by fluorescence microscopy in Figure 1A, the recombinant M. intracellulare (green) is able to adhere to the A549 epithelial cells, stained with Blue Evans (red). Adherence is inhibited by the addition of heparin and enhanced in the presence of laminin. Quantification of M. intracellulare adherence by luciferase assay indicated that this adhesion to epithelial cells was significantly decreased from 50 to 60% in the presence of exogenous heparin but increased from 35 to 50% in the presence of exogenous laminin (Figure 1B). The diagram in Figure 1C explains how heparin and laminin can modulate the adhesion of bacteria to cells. The addition of heparin decreases the adhesion of bacteria to cells because it represents targets in competition with the heparin present in cell membranes. Conversely, the addition of laminin will indirectly increase the adhesion of bacteria to the cells via the laminin receptor (Figure 1C).
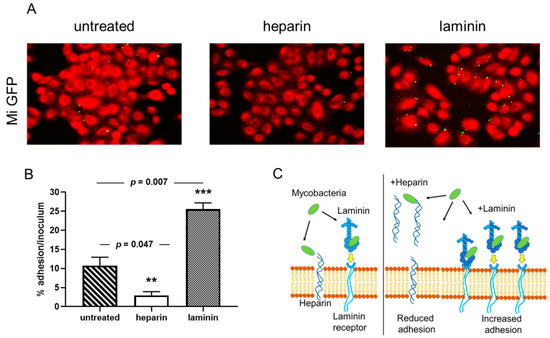
Figure 1. Cytoadherence of M. intracellulare ATCC13950 to A549 epithelial cells inhibited heparin and increased by laminin. A549 cells were infected by Green fluorescent protein (GFP)- and luciferase-producing M. intracellulare ATCC13950 in the presence or absence of heparin or laminin. (A) Fluorescence microscopy analysis of the A549 cells infected by M. intracellulare /GFPlux (green). The samples were fixed with PFA and stained with Evans Blue (red). Images taken with 40× objectives represent the overlay of Evans Blue and GFP signals. (B) Quantification of M. intracellulare /GFPlux adherence by luciferase assays. The percentages of adhesion were calculated by the formula (cell-associated RLU/RLU of the inoculum) × 100. The graph shows the averages of triplicate samples from one representative of three independent experiments. ** p < 0.05; *** p < 0.01. The error bars represent the standard deviation. (C) The diagram gives an illustration of how bacteria bind to cells, either directly on the heparin present on the surface of cells or via laminin which then binds to its cell receptor. In the presence of exogenous heparin or laminin, the adhesion of the bacteria is inhibited or increased.
References
- Dartois, V.; Sizemore, C.; Dick, T. Editorial: NTM-The New Uber-Bugs. Front. Microbiol. 2019, 10, 1299, doi:10.3389/fmicb.2019.01299.
- Johansen, M.D.; Herrmann, J.L.; Kremer, L. Non-tuberculous mycobacteria and the rise of Mycobacterium abscessus. Nat. Rev. Microbiol. 2020, 18, 392–407, doi:10.1038/s41579-020-0331-1.
- Hoefsloot, W.; van Ingen, J.; Andrejak, C.; Angeby, K.; Bauriaud, R.; Bemer, P.; Beylis, N.; Boeree, M.J.; Cacho, J.; Chihota, V., et al. The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: An NTM-NET collaborative study. Eur. Respir. J. 2013, 42, 1604–1613, doi:10.1183/09031936.00149212.
- Kim, S.Y.; Shin, S.H.; Moon, S.M.; Yang, B.; Kim, H.; Kwon, O.J.; Huh, H.J.; Ki, C.S.; Lee, N.Y.; Shin, S.J., et al. Distribution and clinical significance of Mycobacterium avium complex species isolated from respiratory specimens. Diagn. Microbiol. Infect. Dis 2017, 88, 125–137, doi:10.1016/j.diagmicrobio.2017.02.017.
- Falkinham, J.O., 3rd. Epidemiology of infection by nontuberculous mycobacteria. Clin. Microbiol. Rev. 1996, 9, 177–215.
- Lacroix, A.; Piau, C.; Lanotte, P.; Carricajo, A.; Guillouzouic, A.; Peuchant, O.; Cady, A.; Dupin, C.; Fangous, M.S.; Martin, C., et al. Emergence of Nontuberculous Mycobacterial Lymphadenitis in Children After the Discontinuation of Mandatory Bacillus Calmette and GuErin Immunization in France. Pediatr. Infect. Dis. J. 2018, 37, e257–e260, doi:10.1097/INF.0000000000001977.
- Johne, H.A.; Frothingham, L. Ein eigenthuemlicher fall von tuberkulose beim rind. Deutsche Zeitschrift fur tiermedicin und pathologie 1895, 21, 738–454.
- Thorel, M.F.; Krichevsky, M.; Levy-Frebault, V.V. Numerical taxonomy of mycobactin-dependent mycobacteria, emended description of Mycobacterium avium, and description of Mycobacterium avium subsp. avium subsp. nov., Mycobacterium avium subsp. paratuberculosis subsp. nov., and Mycobacterium avium subsp. silvaticum subsp. nov. Int. J. Syst. Bacteriol. 1990, 40, 254–260, doi:10.1099/00207713-40-3-254.
- Mijs, W.; de Haas, P.; Rossau, R.; Van der Laan, T.; Rigouts, L.; Portaels, F.; van Soolingen, D. Molecular evidence to support a proposal to reserve the designation Mycobacterium avium subsp. avium for bird-type isolates and ‘M. avium subsp. hominissuis’ for the human/porcine type of M. avium. Int. J. Syst. Evol. Microbiol. 2002, 52, 1505–1518, doi:10.1099/00207713-52-5-1505.
- van Ingen, J.; Turenne, C.Y.; Tortoli, E.; Wallace, R.J., Jr.; Brown-Elliott, B.A. A definition of the Mycobacterium avium complex for taxonomical and clinical purposes, a review. Int. J. Syst. Evol. Microbiol. 2018, 68, 3666–3677, doi:10.1099/ijsem.0.003026.
- Tortoli, E.; Meehan, C.J.; Grottola, A.; Fregni Serpini, G.; Fabio, A.; Trovato, A.; Pecorari, M.; Cirillo, D.M. Genome-based taxonomic revision detects a number of synonymous taxa in the genus Mycobacterium. Infect. Genet. Evol. 2019, 75, 103983, doi:10.1016/j.meegid.2019.103983.
- Tortoli, E.; Rindi, L.; Garcia, M.J.; Chiaradonna, P.; Dei, R.; Garzelli, C.; Kroppenstedt, R.M.; Lari, N.; Mattei, R.; Mariottini, A., et al. Proposal to elevate the genetic variant MAC-A, included in the Mycobacterium avium complex, to species rank as Mycobacterium chimaera sp. nov. Int. J. Syst. Evol. Microbiol. 2004, 54, 1277–1285, doi:10.1099/ijs.0.02777-0.
- Sax, H.; Bloemberg, G.; Hasse, B.; Sommerstein, R.; Kohler, P.; Achermann, Y.; Rossle, M.; Falk, V.; Kuster, S.P.; Bottger, E.C., et al. Prolonged Outbreak of Mycobacterium chimaera Infection After Open-Chest Heart Surgery. Clin. Infect. Dis. 2015, 61, 67–75, doi:10.1093/cid/civ198.
- Huh, H.J.; Kim, S.Y.; Shim, H.J.; Kim, D.H.; Yoo, I.Y.; Kang, O.K.; Ki, C.S.; Shin, S.Y.; Jhun, B.W.; Shin, S.J., et al. GenoType NTM-DR Performance Evaluation for Identification of Mycobacterium avium Complex and Mycobacterium abscessus and Determination of Clarithromycin and Amikacin Resistance. J. Clin. Microbiol. 2019, 57, doi:10.1128/JCM.00516-19.
- Cao, Y.; Wang, L.; Ma, P.; Fan, W.; Gu, B.; Ju, S. Accuracy of Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry for Identification of Mycobacteria: A systematic review and meta-analysis. Sci. Rep. 2018, 8, 4131, doi:10.1038/s41598-018-22642-w.
- Singh, B.; Fleury, C.; Jalalvand, F.; Riesbeck, K. Human pathogens utilize host extracellular matrix proteins laminin and collagen for adhesion and invasion of the host. FEMS Microbiol. Rev. 2012, 36, 1122–1180, doi:10.1111/j.1574-6976.2012.00340.x.
- Menozzi, F.D.; Bischoff, R.; Fort, E.; Brennan, M.J.; Locht, C. Molecular characterization of the mycobacterial heparin-binding hemagglutinin, a mycobacterial adhesin. Proc. Natl Acad Sci U S A 1998, 95, 12625–12630, doi:10.1073/pnas.95.21.12625.
- Menozzi, F.D.; Rouse, J.H.; Alavi, M.; Laude-Sharp, M.; Muller, J.; Bischoff, R.; Brennan, M.J.; Locht, C. Identification of a heparin-binding hemagglutinin present in mycobacteria. J. Exp. Med. 1996, 184, 993–1001, doi:10.1084/jem.184.3.993.
- Biet, F.; Angela de Melo Marques, M.; Grayon, M.; Xavier da Silveira, E.K.; Brennan, P.J.; Drobecq, H.; Raze, D.; Vidal Pessolani, M.C.; Locht, C.; Menozzi, F.D. Mycobacterium smegmatis produces an HBHA homologue which is not involved in epithelial adherence. Microbes Infect. 2007, 9, 175–182, doi:10.1016/j.micinf.2006.11.007.
- Lefrancois, L.H.; Bodier, C.C.; Cochard, T.; Canepa, S.; Raze, D.; Lanotte, P.; Sevilla, I.A.; Stevenson, K.; Behr, M.A.; Locht, C., et al. Novel feature of Mycobacterium avium subsp. paratuberculosis, highlighted by characterization of the heparin-binding hemagglutinin adhesin. J. Bacteriol. 2013, 195, 4844–4853, doi:10.1128/JB.00671-13.
- Eraghi, V.; Derakhshandeh, A.; Hosseini, A.; Haghkhah, M.; Sechi, L.A.; Motamedi Boroojeni, A. Recombinant fusion protein of Heparin-Binding Hemagglutinin Adhesin and Fibronectin Attachment Protein (rHBHA-FAP) of Mycobacterium avium subsp. paratuberculosis elicits a strong gamma interferon response in peripheral blood mononuclear cell culture. Gut Pathog. 2019, 11, 36, doi:10.1186/s13099-019-0317-6.
- Sechi, L.A.; Ahmed, N.; Felis, G.E.; Dupre, I.; Cannas, S.; Fadda, G.; Bua, A.; Zanetti, S. Immunogenicity and cytoadherence of recombinant heparin binding haemagglutinin (HBHA) of Mycobacterium avium subsp. paratuberculosis: Functional promiscuity or a role in virulence? Vaccine 2006, 24, 236–243, doi:10.1016/j.vaccine.2005.11.030.
- Lebrun, P.; Raze, D.; Fritzinger, B.; Wieruszeski, J.M.; Biet, F.; Dose, A.; Carpentier, M.; Schwarzer, D.; Allain, F.; Lippens, G., et al. Differential contribution of the repeats to heparin binding of HBHA, a major adhesin of Mycobacterium tuberculosis. PLoS ONE 2012, 7, e32421, doi:10.1371/journal.pone.0032421.
- Pethe, K.; Alonso, S.; Biet, F.; Delogu, G.; Brennan, M.J.; Locht, C.; Menozzi, F.D. The heparin-binding haemagglutinin of M. tuberculosis is required for extrapulmonary dissemination. Nature 2001, 412, 190–194, doi:10.1038/35084083.
- Masungi, C.; Temmerman, S.; Van Vooren, J.P.; Drowart, A.; Pethe, K.; Menozzi, F.D.; Locht, C.; Mascart, F. Differential T and B cell responses against Mycobacterium tuberculosis heparin-binding hemagglutinin adhesin in infected healthy individuals and patients with tuberculosis. J. Infect. Dis. 2002, 185, 513–520, doi:10.1086/338833.
- Temmerman, S.; Pethe, K.; Parra, M.; Alonso, S.; Rouanet, C.; Pickett, T.; Drowart, A.; Debrie, A.S.; Delogu, G.; Menozzi, F.D., et al. Methylation-dependent T cell immunity to Mycobacterium tuberculosis heparin-binding hemagglutinin. Nat. Med. 2004, 10, 935–941, doi:10.1038/nm1090.
- Bitti, M.L.; Masala, S.; Capasso, F.; Rapini, N.; Piccinini, S.; Angelini, F.; Pierantozzi, A.; Lidano, R.; Pietrosanti, S.; Paccagnini, D., et al. Mycobacterium avium subsp. paratuberculosis in an Italian cohort of type 1 diabetes pediatric patients. Clin. Dev. Immunol. 2012, 2012, 785262, doi:10.1155/2012/785262.
- Rosu, V.; Ahmed, N.; Paccagnini, D.; Gerlach, G.; Fadda, G.; Hasnain, S.E.; Zanetti, S.; Sechi, L.A. Specific immunoassays confirm association of Mycobacterium avium Subsp. paratuberculosis with type-1 but not type-2 diabetes mellitus. PLoS ONE 2009, 4, e4386, doi:10.1371/journal.pone.0004386.
- de Melo Marques, M.A.; Mahapatra, S.; Nandan, D.; Dick, T.; Sarno, E.N.; Brennan, P.J.; Vidal Pessolani, M.C. Bacterial and host-derived cationic proteins bind alpha2-laminins and enhance Mycobacterium leprae attachment to human Schwann cells. Microbes Infect. 2000, 2, 1407–1417, doi:10.1016/s1286-4579(00)01294-6.
- Shimoji, Y.; Ng, V.; Matsumura, K.; Fischetti, V.A.; Rambukkana, A. A 21-kDa surface protein of Mycobacterium leprae binds peripheral nerve laminin-2 and mediates Schwann cell invasion. Proc. Natl Acad Sci U S A 1999, 96, 9857–9862, doi:10.1073/pnas.96.17.9857.
- Silva, C.A.; Danelishvili, L.; McNamara, M.; Berredo-Pinho, M.; Bildfell, R.; Biet, F.; Rodrigues, L.S.; Oliveira, A.V.; Bermudez, L.E.; Pessolani, M.C. Interaction of Mycobacterium leprae with human airway epithelial cells: Adherence, entry, survival, and identification of potential adhesins by surface proteome analysis. Infect. Immun. 2013, 81, 2645–2659, doi:10.1128/IAI.00147-13.
- Lefrancois, L.H.; Pujol, C.; Bodier, C.C.; Teixeira-Gomez, A.P.; Drobecq, H.; Rosso, M.L.; Raze, D.; Dias, A.A.; Hugot, J.P.; Chacon, O., et al. Characterization of the Mycobacterium avium subsp. paratuberculosis laminin-binding/histone-like protein (Lbp/Hlp) which reacts with sera from patients with Crohn’s disease. Microbes Infect. 2011, 13, 585–594, doi:10.1016/j.micinf.2011.02.002.
- Soares de Lima, C.; Zulianello, L.; Marques, M.A.; Kim, H.; Portugal, M.I.; Antunes, S.L.; Menozzi, F.D.; Ottenhoff, T.H.; Brennan, P.J.; Pessolani, M.C. Mapping the laminin-binding and adhesive domain of the cell surface-associated Hlp/LBP protein from Mycobacterium leprae. Microbes Infect. 2005, 7, 1097–1109, doi:10.1016/j.micinf.2005.02.013.




