In this study we characterized adhesins heparin-binding hemagglutinin (HBHA) and laminin-binding proteins (LBP) from
M. intracellulare
subsp
chimaera
intracellulare
complex (MCIC) species isolated from patients with a variety of disease expression, examined the role of these adhesins in binding of
M. intracellulare
to lung epithelial cells and their degree of conservation within the
M. intracellulare
subsp
chimaera
intracellulare
complex
- Mycobacterium intracellulare
- NTM
- adhesins
1. Introduction
Non-tuberculous mycobacteria (NTM) are an increasing cause of opportunistic diseases in humans [1][2]. Among NTM, the
complex (MAC) represents a group with a specific distribution of species according to continent and countries [3]. In addition to severe infection in immune-deficient subjects, such as AIDS patients, the incidence of MAC infections has also recently increased in patients with chronic pulmonary disease and other underlying conditions [4][5]. Due to modifications in mandatory programs of vaccination with bacillus Calmette-Guérin (BCG) in low-incidence countries, an increase in the frequency of adenitis in children was noticed [6] mostly because of MAC infection. MAC is classically divided into
and
. The
species includes four closely related subspecies,
subsp.
, the etiologic agent of Johne’s disease or paratuberculosis in ruminants [7].
subsp.
and
subsp.
, responsible for avian tuberculosis and infection in wood pigeons, respectively [8] and
subsp.
, which is usually isolated from pigs but can also be implicated in human infections [9]. Some recently discovered species are very close to
and are termed
subsp.
and
subsp.
are associated with infections in humans.
is mainly implicated in pulmonary infections, and
subsp.
[12], was recently associated with fatal infections after cardiac surgery [13]. MAC can be identified by using DNA probes, luminescent systems, DNA sequencing of
,
and the 16S–23S Intergenic region, or identification of specific insertion sequences [10]. GenoType NTM-DR, a new commercial diagnostic assay, allows differentiation between three MAC species,
,
,
subsp.
, as well as identification of subspecies within the
complex [14]. Mass spectrometry has also been recently proposed as a useful tool to identify these NTM at the species level [15].
Several studies have shown that pathogenic mycobacteria use the protein or proteoglycan component of the extracellular matrix (ECM) for adherence and invasion of the host [16]. One of the best characterized mycobacterial adhesins is the heparin-binding hemagglutinin (HBHA), initially identified in Mycobacterium tuberculosis and Mycobacterium bovis bacillus Calmette-Guérin (BCG) [17][18]. However, HBHA-like molecules are also present in many other mycobacteria, both pathogenic and nonpathogenic [19][20][21][22]. HBHA is located on the surface of the mycobacteria and mediates binding of the bacilli to epithelial cells and fibroblasts [18] by interacting with sulfated glycoconjugates present on the surfaces of host cells [23]. It also plays a role in the dissemination of M. tuberculosis from the lungs to deeper tissues [24] and has shown promise as a diagnostic target for the detection of latent tuberculosis in humans [25][26][27][28].
Laminin and collagen in the lung also promote adherence to ECM-binding mycobacteria, and mycobacterial laminin-binding proteins (LBP) involved in adherence of mycobacteria to host cells have been identified and characterized. LBP was initially described to play a role in the interaction between
and Schwann cells [29][30][31]. LBP, also referenced to as Lbp/Hlp [32][33], Mdp1, the Mycobacterial DNA-binding protein 1 [30] and hupB the mycobacterial histone-like protein are conserved in mycobacteria, including MAC [32].
2. Adherence of M. intracellulare to Epithelial Cells is Modulated by Heparin and Laminin
Previous reports have shown that adherence of mycobacteria to epithelial cells can be modulated by the addition of an extracellular matrix component [18,32]. Using the recombinantPrevious reports have shown that adherence of mycobacteria to epithelial cells can be modulated by the addition of an extracellular matrix component [18][32]. Using the recombinant
strain, we tested whether soluble exogenous heparin or laminin can affect the cytoadherence of
to A549 epithelial cells. As observed qualitatively by fluorescence microscopy in Figure 1A, the recombinant
(green) is able to adhere to the A549 epithelial cells, stained with Blue Evans (red). Adherence is inhibited by the addition of heparin and enhanced in the presence of laminin. Quantification of
adherence by luciferase assay indicated that this adhesion to epithelial cells was significantly decreased from 50 to 60% in the presence of exogenous heparin but increased from 35 to 50% in the presence of exogenous laminin (Figure 1B). The diagram in Figure 1C explains how heparin and laminin can modulate the adhesion of bacteria to cells. The addition of heparin decreases the adhesion of bacteria to cells because it represents targets in competition with the heparin present in cell membranes. Conversely, the addition of laminin will indirectly increase the adhesion of bacteria to the cells via the laminin receptor (Figure 1C).
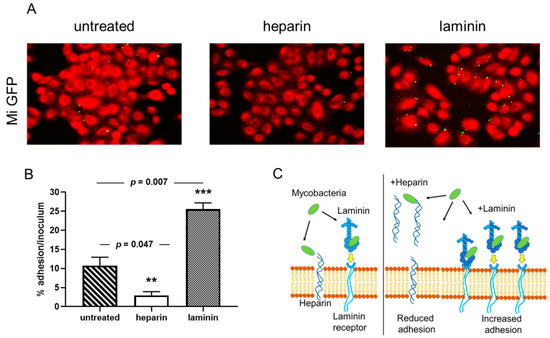
Cytoadherence of
ATCC13950 to A549 epithelial cells inhibited heparin and increased by laminin. A549 cells were infected by Green fluorescent protein (GFP)- and luciferase-producing
ATCC13950 in the presence or absence of heparin or laminin. (
) Fluorescence microscopy analysis of the A549 cells infected by
/GFPlux (green). The samples were fixed with PFA and stained with Evans Blue (red). Images taken with 40× objectives represent the overlay of Evans Blue and GFP signals. (
) Quantification of
/GFPlux adherence by luciferase assays. The percentages of adhesion were calculated by the formula (cell-associated RLU/RLU of the inoculum) × 100. The graph shows the averages of triplicate samples from one representative of three independent experiments. **
< 0.05; ***
< 0.01. The error bars represent the standard deviation. (
References
- Dartois, V.; Sizemore, C.; Dick, T. Editorial: NTM-The New Uber-Bugs. Front. Microbiol. 2019, 10, 1299. [Google Scholar] [CrossRef] [PubMed]
- Johansen, M.D.; Herrmann, J.L.; Kremer, L. Non-tuberculous mycobacteria and the rise of Mycobacterium abscessus. Nat. Rev. Microbiol. 2020, 18, 392–407. [Google Scholar] [CrossRef] [PubMed]
- Hoefsloot, W.; van Ingen, J.; Andrejak, C.; Angeby, K.; Bauriaud, R.; Bemer, P.; Beylis, N.; Boeree, M.J.; Cacho, J.; Chihota, V.; et al. The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: An NTM-NET collaborative study. Eur. Respir. J. 2013, 42, 1604–1613. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Shin, S.H.; Moon, S.M.; Yang, B.; Kim, H.; Kwon, O.J.; Huh, H.J.; Ki, C.S.; Lee, N.Y.; Shin, S.J.; et al. Distribution and clinical significance of Mycobacterium avium complex species isolated from respiratory specimens. Diagn. Microbiol. Infect. Dis 2017, 88, 125–137. [Google Scholar] [CrossRef]
- Falkinham, J.O., 3rd. Epidemiology of infection by nontuberculous mycobacteria. Clin. Microbiol. Rev. 1996, 9, 177–215. [Google Scholar] [CrossRef]
- Lacroix, A.; Piau, C.; Lanotte, P.; Carricajo, A.; Guillouzouic, A.; Peuchant, O.; Cady, A.; Dupin, C.; Fangous, M.S.; Martin, C.; et al. Emergence of Nontuberculous Mycobacterial Lymphadenitis in Children After the Discontinuation of Mandatory Bacillus Calmette and GuErin Immunization in France. Pediatr. Infect. Dis. J. 2018, 37, e257–e260. [Google Scholar] [CrossRef]
- Johne, H.A.; Frothingham, L. Ein eigenthuemlicher fall von tuberkulose beim rind. Deutsche Zeitschrift fur tiermedicin und pathologie 1895, 21, 438–454. [Google Scholar]
- Thorel, M.F.; Krichevsky, M.; Levy-Frebault, V.V. Numerical taxonomy of mycobactin-dependent mycobacteria, emended description of Mycobacterium avium, and description of Mycobacterium avium subsp. avium subsp. nov., Mycobacterium avium subsp. paratuberculosis subsp. nov., and Mycobacterium avium subsp. silvaticum subsp. nov. Int. J. Syst. Bacteriol. 1990, 40, 254–260. [Google Scholar] [CrossRef]
- Mijs, W.; de Haas, P.; Rossau, R.; Van der Laan, T.; Rigouts, L.; Portaels, F.; van Soolingen, D. Molecular evidence to support a proposal to reserve the designation Mycobacterium avium subsp. avium for bird-type isolates and ‘M. avium subsp. hominissuis’ for the human/porcine type of M. avium. Int. J. Syst. Evol. Microbiol. 2002, 52, 1505–1518. [Google Scholar] [CrossRef]
- Van Ingen, J.; Turenne, C.Y.; Tortoli, E.; Wallace, R.J., Jr.; Brown-Elliott, B.A. A definition of the Mycobacterium avium complex for taxonomical and clinical purposes, a review. Int. J. Syst. Evol. Microbiol. 2018, 68, 3666–3677. [Google Scholar] [CrossRef]
- Tortoli, E.; Meehan, C.J.; Grottola, A.; Fregni Serpini, G.; Fabio, A.; Trovato, A.; Pecorari, M.; Cirillo, D.M. Genome-based taxonomic revision detects a number of synonymous taxa in the genus Mycobacterium. Infect. Genet. Evol. 2019, 75, 103983. [Google Scholar] [CrossRef] [PubMed]
- Tortoli, E.; Rindi, L.; Garcia, M.J.; Chiaradonna, P.; Dei, R.; Garzelli, C.; Kroppenstedt, R.M.; Lari, N.; Mattei, R.; Mariottini, A.; et al. Proposal to elevate the genetic variant MAC-A, included in the Mycobacterium avium complex, to species rank as Mycobacterium chimaera sp. nov. Int. J. Syst. Evol. Microbiol. 2004, 54, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Sax, H.; Bloemberg, G.; Hasse, B.; Sommerstein, R.; Kohler, P.; Achermann, Y.; Rossle, M.; Falk, V.; Kuster, S.P.; Bottger, E.C.; et al. Prolonged Outbreak of Mycobacterium chimaera Infection After Open-Chest Heart Surgery. Clin. Infect. Dis. 2015, 61, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Huh, H.J.; Kim, S.Y.; Shim, H.J.; Kim, D.H.; Yoo, I.Y.; Kang, O.K.; Ki, C.S.; Shin, S.Y.; Jhun, B.W.; Shin, S.J.; et al. GenoType NTM-DR Performance Evaluation for Identification of Mycobacterium avium Complex and Mycobacterium abscessus and Determination of Clarithromycin and Amikacin Resistance. J. Clin. Microbiol. 2019, 57. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, L.; Ma, P.; Fan, W.; Gu, B.; Ju, S. Accuracy of Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry for Identification of Mycobacteria: A systematic review and meta-analysis. Sci. Rep. 2018, 8, 4131. [Google Scholar] [CrossRef]
- Singh, B.; Fleury, C.; Jalalvand, F.; Riesbeck, K. Human pathogens utilize host extracellular matrix proteins laminin and collagen for adhesion and invasion of the host. FEMS Microbiol. Rev. 2012, 36, 1122–1180. [Google Scholar] [CrossRef]
- Menozzi, F.D.; Bischoff, R.; Fort, E.; Brennan, M.J.; Locht, C. Molecular characterization of the mycobacterial heparin-binding hemagglutinin, a mycobacterial adhesin. Proc. Natl. Acad. Sci. USA 1998, 95, 12625–12630. [Google Scholar] [CrossRef]
- Menozzi, F.D.; Rouse, J.H.; Alavi, M.; Laude-Sharp, M.; Muller, J.; Bischoff, R.; Brennan, M.J.; Locht, C. Identification of a heparin-binding hemagglutinin present in mycobacteria. J. Exp. Med. 1996, 184, 993–1001. [Google Scholar] [CrossRef]
- Biet, F.; Angela de Melo Marques, M.; Grayon, M.; Xavier da Silveira, E.K.; Brennan, P.J.; Drobecq, H.; Raze, D.; Vidal Pessolani, M.C.; Locht, C.; Menozzi, F.D. Mycobacterium smegmatis produces an HBHA homologue which is not involved in epithelial adherence. Microbes Infect. 2007, 9, 175–182. [Google Scholar] [CrossRef]
- Lefrancois, L.H.; Bodier, C.C.; Cochard, T.; Canepa, S.; Raze, D.; Lanotte, P.; Sevilla, I.A.; Stevenson, K.; Behr, M.A.; Locht, C.; et al. Novel feature of Mycobacterium avium subsp. paratuberculosis, highlighted by characterization of the heparin-binding hemagglutinin adhesin. J. Bacteriol. 2013, 195, 4844–4853. [Google Scholar] [CrossRef]
- Eraghi, V.; Derakhshandeh, A.; Hosseini, A.; Haghkhah, M.; Sechi, L.A.; Motamedi Boroojeni, A. Recombinant fusion protein of Heparin-Binding Hemagglutinin Adhesin and Fibronectin Attachment Protein (rHBHA-FAP) of Mycobacterium avium subsp. paratuberculosis elicits a strong gamma interferon response in peripheral blood mononuclear cell culture. Gut Pathog. 2019, 11, 36. [Google Scholar] [CrossRef] [PubMed]
- Sechi, L.A.; Ahmed, N.; Felis, G.E.; Dupre, I.; Cannas, S.; Fadda, G.; Bua, A.; Zanetti, S. Immunogenicity and cytoadherence of recombinant heparin binding haemagglutinin (HBHA) of Mycobacterium avium subsp. paratuberculosis: Functional promiscuity or a role in virulence? Vaccine 2006, 24, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Lebrun, P.; Raze, D.; Fritzinger, B.; Wieruszeski, J.M.; Biet, F.; Dose, A.; Carpentier, M.; Schwarzer, D.; Allain, F.; Lippens, G.; et al. Differential contribution of the repeats to heparin binding of HBHA, a major adhesin of Mycobacterium tuberculosis. PLoS ONE 2012, 7, e32421. [Google Scholar] [CrossRef] [PubMed]
- Pethe, K.; Alonso, S.; Biet, F.; Delogu, G.; Brennan, M.J.; Locht, C.; Menozzi, F.D. The heparin-binding haemagglutinin of M. tuberculosis is required for extrapulmonary dissemination. Nature 2001, 412, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Masungi, C.; Temmerman, S.; Van Vooren, J.P.; Drowart, A.; Pethe, K.; Menozzi, F.D.; Locht, C.; Mascart, F. Differential T and B cell responses against Mycobacterium tuberculosis heparin-binding hemagglutinin adhesin in infected healthy individuals and patients with tuberculosis. J. Infect. Dis. 2002, 185, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Temmerman, S.; Pethe, K.; Parra, M.; Alonso, S.; Rouanet, C.; Pickett, T.; Drowart, A.; Debrie, A.S.; Delogu, G.; Menozzi, F.D.; et al. Methylation-dependent T cell immunity to Mycobacterium tuberculosis heparin-binding hemagglutinin. Nat. Med. 2004, 10, 935–941. [Google Scholar] [CrossRef]
- Bitti, M.L.; Masala, S.; Capasso, F.; Rapini, N.; Piccinini, S.; Angelini, F.; Pierantozzi, A.; Lidano, R.; Pietrosanti, S.; Paccagnini, D.; et al. Mycobacterium avium subsp. paratuberculosis in an Italian cohort of type 1 diabetes pediatric patients. Clin. Dev. Immunol. 2012, 2012, 785262. [Google Scholar] [CrossRef]
- Rosu, V.; Ahmed, N.; Paccagnini, D.; Gerlach, G.; Fadda, G.; Hasnain, S.E.; Zanetti, S.; Sechi, L.A. Specific immunoassays confirm association of Mycobacterium avium Subsp. paratuberculosis with type-1 but not type-2 diabetes mellitus. PLoS ONE 2009, 4, e4386. [Google Scholar] [CrossRef] [PubMed]
- De Melo Marques, M.A.; Mahapatra, S.; Nandan, D.; Dick, T.; Sarno, E.N.; Brennan, P.J.; Vidal Pessolani, M.C. Bacterial and host-derived cationic proteins bind alpha2-laminins and enhance Mycobacterium leprae attachment to human Schwann cells. Microbes Infect. 2000, 2, 1407–1417. [Google Scholar] [CrossRef]
- Shimoji, Y.; Ng, V.; Matsumura, K.; Fischetti, V.A.; Rambukkana, A. A 21-kDa surface protein of Mycobacterium leprae binds peripheral nerve laminin-2 and mediates Schwann cell invasion. Proc. Natl. Acad. Sci. USA 1999, 96, 9857–9862. [Google Scholar] [CrossRef]
- Silva, C.A.; Danelishvili, L.; McNamara, M.; Berredo-Pinho, M.; Bildfell, R.; Biet, F.; Rodrigues, L.S.; Oliveira, A.V.; Bermudez, L.E.; Pessolani, M.C. Interaction of Mycobacterium leprae with human airway epithelial cells: Adherence, entry, survival, and identification of potential adhesins by surface proteome analysis. Infect. Immun. 2013, 81, 2645–2659. [Google Scholar] [CrossRef] [PubMed]
- Lefrancois, L.H.; Pujol, C.; Bodier, C.C.; Teixeira-Gomez, A.P.; Drobecq, H.; Rosso, M.L.; Raze, D.; Dias, A.A.; Hugot, J.P.; Chacon, O.; et al. Characterization of the Mycobacterium avium subsp. paratuberculosis laminin-binding/histone-like protein (Lbp/Hlp) which reacts with sera from patients with Crohn’s disease. Microbes Infect. 2011, 13, 585–594. [Google Scholar] [CrossRef] [PubMed]
) The diagram gives an illustration of how bacteria bind to cells, either directly on the heparin present on the surface of cells or via laminin which then binds to its cell receptor. In the presence of exogenous heparin or laminin, the adhesion of the bacteria is inhibited or increased.
References
- Dartois, V.; Sizemore, C.; Dick, T. Editorial: NTM-The New Uber-Bugs. Front. Microbiol. 2019, 10, 1299, doi:10.3389/fmicb.2019.01299.
- Johansen, M.D.; Herrmann, J.L.; Kremer, L. Non-tuberculous mycobacteria and the rise of Mycobacterium abscessus. Nat. Rev. Microbiol. 2020, 18, 392–407, doi:10.1038/s41579-020-0331-1.
- Hoefsloot, W.; van Ingen, J.; Andrejak, C.; Angeby, K.; Bauriaud, R.; Bemer, P.; Beylis, N.; Boeree, M.J.; Cacho, J.; Chihota, V., et al. The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: An NTM-NET collaborative study. Eur. Respir. J. 2013, 42, 1604–1613, doi:10.1183/09031936.00149212.
- Kim, S.Y.; Shin, S.H.; Moon, S.M.; Yang, B.; Kim, H.; Kwon, O.J.; Huh, H.J.; Ki, C.S.; Lee, N.Y.; Shin, S.J., et al. Distribution and clinical significance of Mycobacterium avium complex species isolated from respiratory specimens. Diagn. Microbiol. Infect. Dis 2017, 88, 125–137, doi:10.1016/j.diagmicrobio.2017.02.017.
- Falkinham, J.O., 3rd. Epidemiology of infection by nontuberculous mycobacteria. Clin. Microbiol. Rev. 1996, 9, 177–215.
- Lacroix, A.; Piau, C.; Lanotte, P.; Carricajo, A.; Guillouzouic, A.; Peuchant, O.; Cady, A.; Dupin, C.; Fangous, M.S.; Martin, C., et al. Emergence of Nontuberculous Mycobacterial Lymphadenitis in Children After the Discontinuation of Mandatory Bacillus Calmette and GuErin Immunization in France. Pediatr. Infect. Dis. J. 2018, 37, e257–e260, doi:10.1097/INF.0000000000001977.
- Johne, H.A.; Frothingham, L. Ein eigenthuemlicher fall von tuberkulose beim rind. Deutsche Zeitschrift fur tiermedicin und pathologie 1895, 21, 738–454.
- Thorel, M.F.; Krichevsky, M.; Levy-Frebault, V.V. Numerical taxonomy of mycobactin-dependent mycobacteria, emended description of Mycobacterium avium, and description of Mycobacterium avium subsp. avium subsp. nov., Mycobacterium avium subsp. paratuberculosis subsp. nov., and Mycobacterium avium subsp. silvaticum subsp. nov. Int. J. Syst. Bacteriol. 1990, 40, 254–260, doi:10.1099/00207713-40-3-254.
- Mijs, W.; de Haas, P.; Rossau, R.; Van der Laan, T.; Rigouts, L.; Portaels, F.; van Soolingen, D. Molecular evidence to support a proposal to reserve the designation Mycobacterium avium subsp. avium for bird-type isolates and ‘M. avium subsp. hominissuis’ for the human/porcine type of M. avium. Int. J. Syst. Evol. Microbiol. 2002, 52, 1505–1518, doi:10.1099/00207713-52-5-1505.
- van Ingen, J.; Turenne, C.Y.; Tortoli, E.; Wallace, R.J., Jr.; Brown-Elliott, B.A. A definition of the Mycobacterium avium complex for taxonomical and clinical purposes, a review. Int. J. Syst. Evol. Microbiol. 2018, 68, 3666–3677, doi:10.1099/ijsem.0.003026.
- Tortoli, E.; Meehan, C.J.; Grottola, A.; Fregni Serpini, G.; Fabio, A.; Trovato, A.; Pecorari, M.; Cirillo, D.M. Genome-based taxonomic revision detects a number of synonymous taxa in the genus Mycobacterium. Infect. Genet. Evol. 2019, 75, 103983, doi:10.1016/j.meegid.2019.103983.
- Tortoli, E.; Rindi, L.; Garcia, M.J.; Chiaradonna, P.; Dei, R.; Garzelli, C.; Kroppenstedt, R.M.; Lari, N.; Mattei, R.; Mariottini, A., et al. Proposal to elevate the genetic variant MAC-A, included in the Mycobacterium avium complex, to species rank as Mycobacterium chimaera sp. nov. Int. J. Syst. Evol. Microbiol. 2004, 54, 1277–1285, doi:10.1099/ijs.0.02777-0.
- Sax, H.; Bloemberg, G.; Hasse, B.; Sommerstein, R.; Kohler, P.; Achermann, Y.; Rossle, M.; Falk, V.; Kuster, S.P.; Bottger, E.C., et al. Prolonged Outbreak of Mycobacterium chimaera Infection After Open-Chest Heart Surgery. Clin. Infect. Dis. 2015, 61, 67–75, doi:10.1093/cid/civ198.
- Huh, H.J.; Kim, S.Y.; Shim, H.J.; Kim, D.H.; Yoo, I.Y.; Kang, O.K.; Ki, C.S.; Shin, S.Y.; Jhun, B.W.; Shin, S.J., et al. GenoType NTM-DR Performance Evaluation for Identification of Mycobacterium avium Complex and Mycobacterium abscessus and Determination of Clarithromycin and Amikacin Resistance. J. Clin. Microbiol. 2019, 57, doi:10.1128/JCM.00516-19.
- Cao, Y.; Wang, L.; Ma, P.; Fan, W.; Gu, B.; Ju, S. Accuracy of Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry for Identification of Mycobacteria: A systematic review and meta-analysis. Sci. Rep. 2018, 8, 4131, doi:10.1038/s41598-018-22642-w.
- Singh, B.; Fleury, C.; Jalalvand, F.; Riesbeck, K. Human pathogens utilize host extracellular matrix proteins laminin and collagen for adhesion and invasion of the host. FEMS Microbiol. Rev. 2012, 36, 1122–1180, doi:10.1111/j.1574-6976.2012.00340.x.
- Menozzi, F.D.; Bischoff, R.; Fort, E.; Brennan, M.J.; Locht, C. Molecular characterization of the mycobacterial heparin-binding hemagglutinin, a mycobacterial adhesin. Proc. Natl Acad Sci U S A 1998, 95, 12625–12630, doi:10.1073/pnas.95.21.12625.
- Menozzi, F.D.; Rouse, J.H.; Alavi, M.; Laude-Sharp, M.; Muller, J.; Bischoff, R.; Brennan, M.J.; Locht, C. Identification of a heparin-binding hemagglutinin present in mycobacteria. J. Exp. Med. 1996, 184, 993–1001, doi:10.1084/jem.184.3.993.
- Biet, F.; Angela de Melo Marques, M.; Grayon, M.; Xavier da Silveira, E.K.; Brennan, P.J.; Drobecq, H.; Raze, D.; Vidal Pessolani, M.C.; Locht, C.; Menozzi, F.D. Mycobacterium smegmatis produces an HBHA homologue which is not involved in epithelial adherence. Microbes Infect. 2007, 9, 175–182, doi:10.1016/j.micinf.2006.11.007.
- Lefrancois, L.H.; Bodier, C.C.; Cochard, T.; Canepa, S.; Raze, D.; Lanotte, P.; Sevilla, I.A.; Stevenson, K.; Behr, M.A.; Locht, C., et al. Novel feature of Mycobacterium avium subsp. paratuberculosis, highlighted by characterization of the heparin-binding hemagglutinin adhesin. J. Bacteriol. 2013, 195, 4844–4853, doi:10.1128/JB.00671-13.
- Eraghi, V.; Derakhshandeh, A.; Hosseini, A.; Haghkhah, M.; Sechi, L.A.; Motamedi Boroojeni, A. Recombinant fusion protein of Heparin-Binding Hemagglutinin Adhesin and Fibronectin Attachment Protein (rHBHA-FAP) of Mycobacterium avium subsp. paratuberculosis elicits a strong gamma interferon response in peripheral blood mononuclear cell culture. Gut Pathog. 2019, 11, 36, doi:10.1186/s13099-019-0317-6.
- Sechi, L.A.; Ahmed, N.; Felis, G.E.; Dupre, I.; Cannas, S.; Fadda, G.; Bua, A.; Zanetti, S. Immunogenicity and cytoadherence of recombinant heparin binding haemagglutinin (HBHA) of Mycobacterium avium subsp. paratuberculosis: Functional promiscuity or a role in virulence? Vaccine 2006, 24, 236–243, doi:10.1016/j.vaccine.2005.11.030.
- Lebrun, P.; Raze, D.; Fritzinger, B.; Wieruszeski, J.M.; Biet, F.; Dose, A.; Carpentier, M.; Schwarzer, D.; Allain, F.; Lippens, G., et al. Differential contribution of the repeats to heparin binding of HBHA, a major adhesin of Mycobacterium tuberculosis. PLoS ONE 2012, 7, e32421, doi:10.1371/journal.pone.0032421.
- Pethe, K.; Alonso, S.; Biet, F.; Delogu, G.; Brennan, M.J.; Locht, C.; Menozzi, F.D. The heparin-binding haemagglutinin of M. tuberculosis is required for extrapulmonary dissemination. Nature 2001, 412, 190–194, doi:10.1038/35084083.
- Masungi, C.; Temmerman, S.; Van Vooren, J.P.; Drowart, A.; Pethe, K.; Menozzi, F.D.; Locht, C.; Mascart, F. Differential T and B cell responses against Mycobacterium tuberculosis heparin-binding hemagglutinin adhesin in infected healthy individuals and patients with tuberculosis. J. Infect. Dis. 2002, 185, 513–520, doi:10.1086/338833.
- Temmerman, S.; Pethe, K.; Parra, M.; Alonso, S.; Rouanet, C.; Pickett, T.; Drowart, A.; Debrie, A.S.; Delogu, G.; Menozzi, F.D., et al. Methylation-dependent T cell immunity to Mycobacterium tuberculosis heparin-binding hemagglutinin. Nat. Med. 2004, 10, 935–941, doi:10.1038/nm1090.
- Bitti, M.L.; Masala, S.; Capasso, F.; Rapini, N.; Piccinini, S.; Angelini, F.; Pierantozzi, A.; Lidano, R.; Pietrosanti, S.; Paccagnini, D., et al. Mycobacterium avium subsp. paratuberculosis in an Italian cohort of type 1 diabetes pediatric patients. Clin. Dev. Immunol. 2012, 2012, 785262, doi:10.1155/2012/785262.
- Rosu, V.; Ahmed, N.; Paccagnini, D.; Gerlach, G.; Fadda, G.; Hasnain, S.E.; Zanetti, S.; Sechi, L.A. Specific immunoassays confirm association of Mycobacterium avium Subsp. paratuberculosis with type-1 but not type-2 diabetes mellitus. PLoS ONE 2009, 4, e4386, doi:10.1371/journal.pone.0004386.
- de Melo Marques, M.A.; Mahapatra, S.; Nandan, D.; Dick, T.; Sarno, E.N.; Brennan, P.J.; Vidal Pessolani, M.C. Bacterial and host-derived cationic proteins bind alpha2-laminins and enhance Mycobacterium leprae attachment to human Schwann cells. Microbes Infect. 2000, 2, 1407–1417, doi:10.1016/s1286-4579(00)01294-6.
- Shimoji, Y.; Ng, V.; Matsumura, K.; Fischetti, V.A.; Rambukkana, A. A 21-kDa surface protein of Mycobacterium leprae binds peripheral nerve laminin-2 and mediates Schwann cell invasion. Proc. Natl Acad Sci U S A 1999, 96, 9857–9862, doi:10.1073/pnas.96.17.9857.
- Silva, C.A.; Danelishvili, L.; McNamara, M.; Berredo-Pinho, M.; Bildfell, R.; Biet, F.; Rodrigues, L.S.; Oliveira, A.V.; Bermudez, L.E.; Pessolani, M.C. Interaction of Mycobacterium leprae with human airway epithelial cells: Adherence, entry, survival, and identification of potential adhesins by surface proteome analysis. Infect. Immun. 2013, 81, 2645–2659, doi:10.1128/IAI.00147-13.
- Lefrancois, L.H.; Pujol, C.; Bodier, C.C.; Teixeira-Gomez, A.P.; Drobecq, H.; Rosso, M.L.; Raze, D.; Dias, A.A.; Hugot, J.P.; Chacon, O., et al. Characterization of the Mycobacterium avium subsp. paratuberculosis laminin-binding/histone-like protein (Lbp/Hlp) which reacts with sera from patients with Crohn’s disease. Microbes Infect. 2011, 13, 585–594, doi:10.1016/j.micinf.2011.02.002.
- Soares de Lima, C.; Zulianello, L.; Marques, M.A.; Kim, H.; Portugal, M.I.; Antunes, S.L.; Menozzi, F.D.; Ottenhoff, T.H.; Brennan, P.J.; Pessolani, M.C. Mapping the laminin-binding and adhesive domain of the cell surface-associated Hlp/LBP protein from Mycobacterium leprae. Microbes Infect. 2005, 7, 1097–1109, doi:10.1016/j.micinf.2005.02.013.
