| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yong Sze Ong | + 4152 word(s) | 4152 | 2021-11-03 10:34:52 | | | |
| 2 | Peter Tang | Meta information modification | 4152 | 2021-11-11 03:52:16 | | |
Video Upload Options
Alkaloids are natural products that possess numerous pharmacological activities and have been exploited effectively to treat cancer. However, the clinically approved anticancer alkaloids are generally limited by serious side effects due to their lack of specificity to cancer cells, indiscriminate tissue distribution and toxic formulation excipients. Lipid-based nanoparticles represent the most effective drug delivery system concerning clinical translation owing to their unique appealing characteristics for drug delivery.
1. Introduction
2. Nanotechnology
3. Alkaloid
|
Class |
Drugs |
Molecular Formula |
Origin |
Indication/Uses |
|---|---|---|---|---|
|
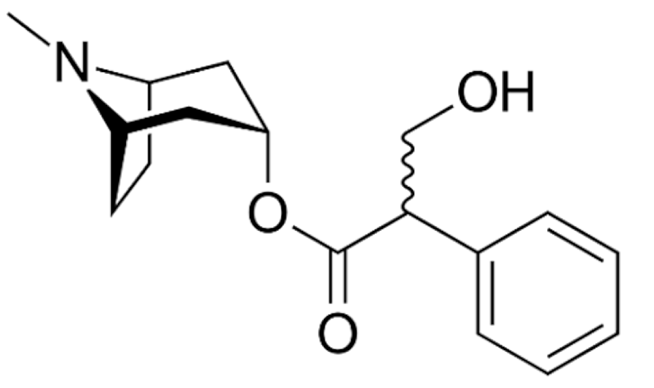
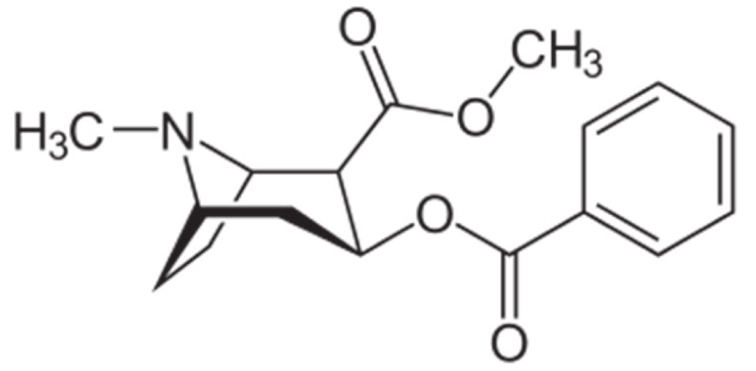
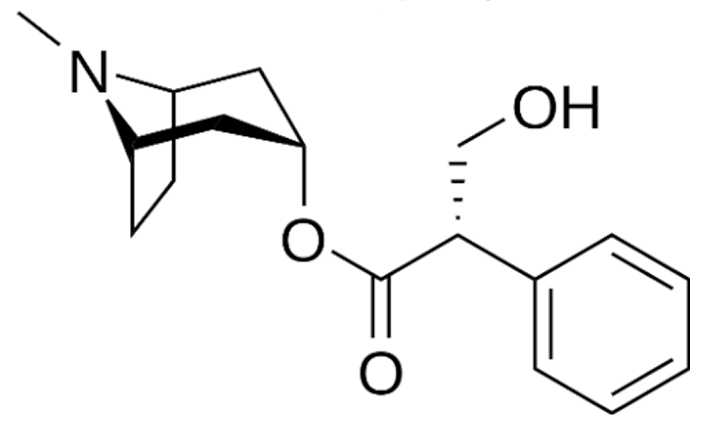
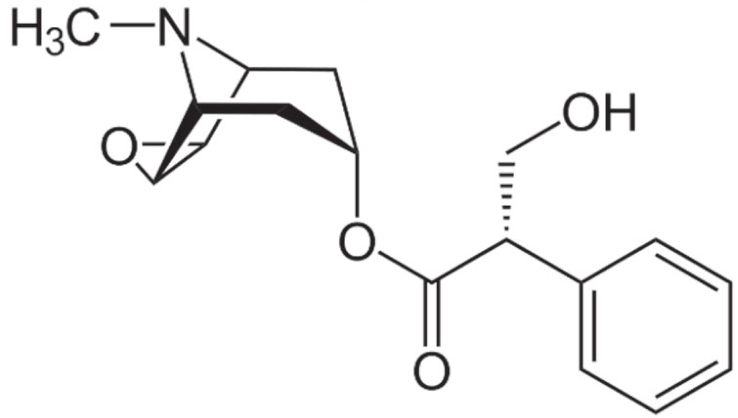
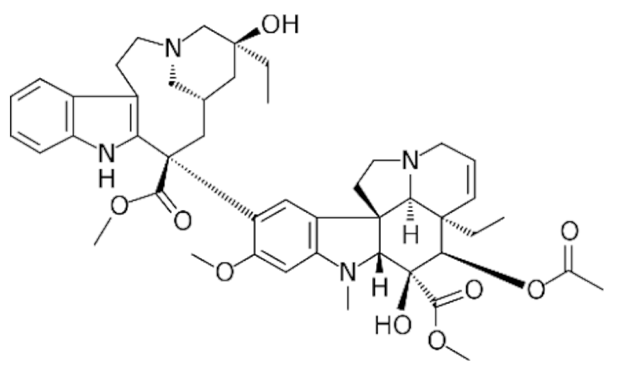
Indole |
Vincristine |
C46H56N4O10 |
Catharanthus roseus |
Anticancer |
|
Vinblastine |
C46H58N4O9 |
|||
|
Vinorelbine |
C45H54N4O8 |
|||
|
Vincamine |
C21H26N2O3 |
Vinca minor |
Primary degenerative and vascular dementia |
|
|
Physostigmine |
C15H21N3O2 |
Physostigma venenosum |
Glaucoma |
|
|
Ajmaline |
C20H26N2O2 |
Rauvolfia serpentina |
Anti-arrhythmic |
|
|
Ajmalicine |
C21H24N2O3 |
Anti-hypertensive |
||
|
Reserpine |
C33H40N2O9 |
Rauvolfia serpentina |
Anti-hypertensive |
|
|
Yohimbine |
C21H26N2O3 |
Erectile dysfunction |
||
|
Strychnine |
C21H22N2O2 |
Strychnos nux-vomica |
Convulsant |
|
|
Mitragynine |
C23H30N2O4 |
Mitragyna speciosa |
Stimulant, analgesic |
|
|
Psilocin |
C12H16N2O |
Psilocybe cubensis |
Hallucinogen |
|
|
Psilocybin |
C12H17N2O4P |
|||
|
Ephedrine |
C6H5CH(OH)CH (CH3)NHCH3 |
Ephedra sinica |
Bronchial asthma |
|
|
Quinoline |
Irinotecan |
C33H38N4O6 |
Catharanthus roseus |
Anticancer |
|
Topotecan |
C23H23N3O5 |
|||
|
Colchicine |
C22H25NO6 |
Colchicum autumnale |
Gout |
|
|
Quinidine |
C20H24N2O2 |
Cinchona officinalis |
Anti-arrhythmic |
|
|
Quinine |
C20H24N2O2 |
Anti-malarial |
||
|
Cinchonine |
C19H22N2O |
|||
|
Cinchonidine |
C19H22N2O |
|||
|
Isoquinolines |
Morphine |
C34H40N2O10S |
Papaver somniferum |
Analgesic |
|
Codeine |
C18H21NO3 |
|||
|
Heroine |
C21H23NO5 |
|||
|
Apomorphine |
C17H17NO2 |
Parkinson’s disease |
||
|
Noscapine |
C22H23NO7 |
Anti-tussive |
||
|
Trabectedin |
C39H43N3O11S |
Ecteinascidia turbinata |
Anticancer |
|
|
Berberine |
C20H18ClNO4 |
Coptis chinensis |
Antimicrobial |
|
|
Tubocurarine |
C37H41ClN2O6 |
Chondrodendron tomentosum |
Skeletal muscle relaxant |
|
|
Atracurium |
C53H72N2O12 |
Leontice leontopetalum |
||
|
Tetrandrine |
C38H42N2O6 |
Stephania tetrandra |
Anti-arrhythmic |
|
|
Galantamine |
C17H21NO3 |
Galanthus nivalis |
Alzheimer’s disease |
|
|
Sanguinarine |
C20H14NO4 |
Sanguinaria canadenis |
Antibacterial, antiplaque |
|
|
Papaverine |
C20H21NO4 |
Papaver somniferum |
Vasodilator |
|
|
Pyrrolidines |
Hygrine |
C8H15NO |
Erythroxylon coca |
Laxative, diuretic |
|
Cuscohygrine |
C13H24N2O |
|||
|
Stachydrine |
C7H14NO2 |
Stachys tuberifera |
Neuroprotectant |
|
|
Pyridines |
Arecoline |
C8H13NO2 |
Areca catechu |
Muscarinic agonist |
|
Ricinine |
C8H8N2O2 |
Ricinus communis |
Insecticide |
|
|
Trigonelline |
C7H7NO2 |
Trigonella foenum-graecum |
Antidiabetic |
|
|
Nicotine |
C10H14N2 |
Nicotiana tabacum |
Smoking cessation |
|
|
Piperidine |
Piperine |
C17H19NO3 |
Piper nigrum |
Anticancer |
|
Piperlongumine |
C17H19NO5 |
Piper longum |
||
|
Pipernonaline |
C21H27NO3 |
Antifungal |
||
|
Tropanes |
Atropine |
C17H23NO3 |
Atropa belladonna |
Anticholinergic |
|
Cocaine |
C17H21NO4 |
Erythroxylum coca |
Local anaesthetic |
|
|
Hyoscyamine |
C17H23NO3 |
Atropa belladonna, Hyoscyamus niger |
Anticholinergic |
|
|
Hyoscine |
C17H21NO4 |
Atropa belladonna |
Motion sickness |
|
|
Indolizidine |
Swainsonine |
C8H15NO3 |
Swainsona canescens |
Anticancer |
|
Castanospermine |
C8H15NO4 |
Castanospermum australe |
Antiviral |
|
|
Securinine |
C13H15NO2 |
Securinega suffruticosa |
Neuroprotection |
|
|
Tylophorine |
C24H27NO4 |
Tylophora indica |
Anticancer |
|
|
Lycorine |
C16H17NO4 |
Clivia miniata |
||
|
Terpenoids |
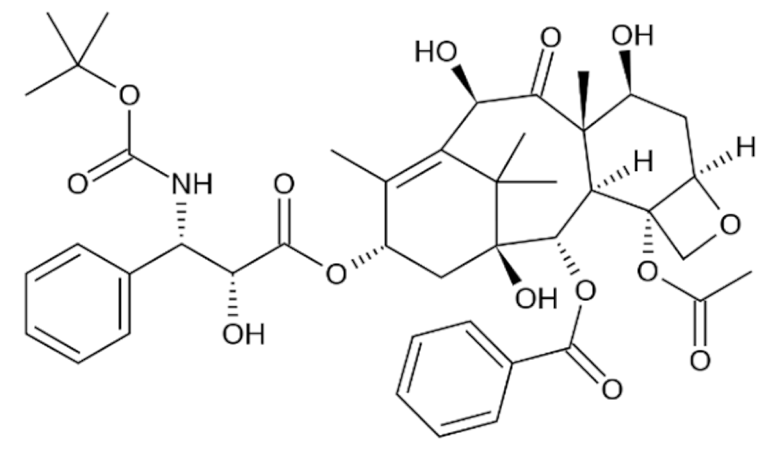
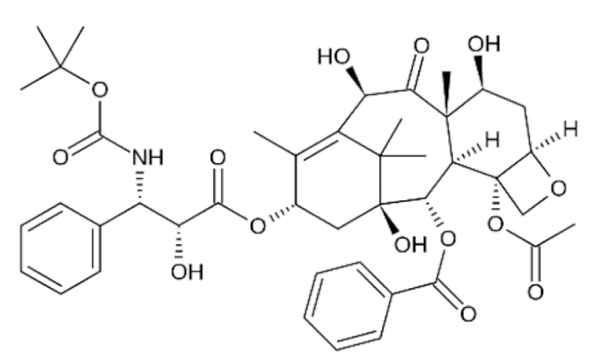
Paclitaxel |
C47H51NO14 |
Taxus brevifolia |
|
|
Docetaxel |
C43H53NO14 |
Taxus baccata |
||
|
Purine |
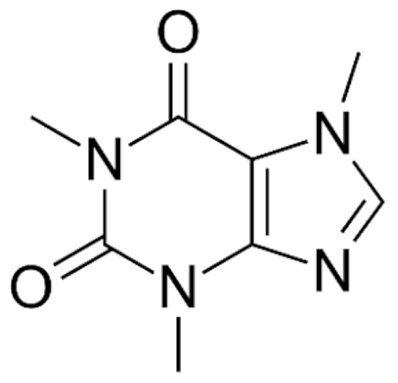
Caffeine |
C8H10N4O2 |
Coffee arabica |
CNS stimulant |
|
Theobromine |
C7H8N4O2 |
Theobroma cacao |
Cardioprotectant |
|
|
Theophylline |
C7H8N4O2 |
COPD and asthma |
||
|
Imidazole |
Pilocarpine |
C11H16N2O2 |
Pilocarpus microphyllus |
Glaucoma |
|
Epiisopiloturine |
C₁₆H₁₈N₂O₃ |
Anthelmintic |
||
|
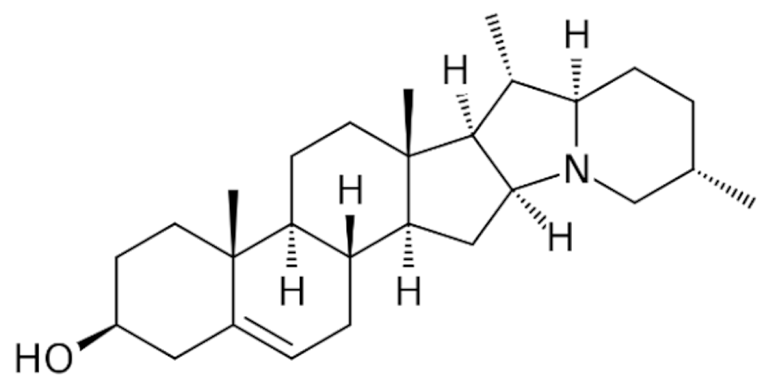
Steroidal |
Pancuronium |
C35H60N2O4 |
Malouetia bequaertiana |
Skeletal muscle relaxant |
|
Conessine |
C24H40N2 |
Holarrhena antidysenterica |
Antidysenteric |
|
|
Solanidine |
C27H43NO |
Solanum tuberosum |
Anticancer |
|
|
Tomatidine |
C27H45NO2 |
Lycopersicon esculentum |
4. Lipid-Based Nanoparticles for Encapsulation of Anticancer Alkaloids
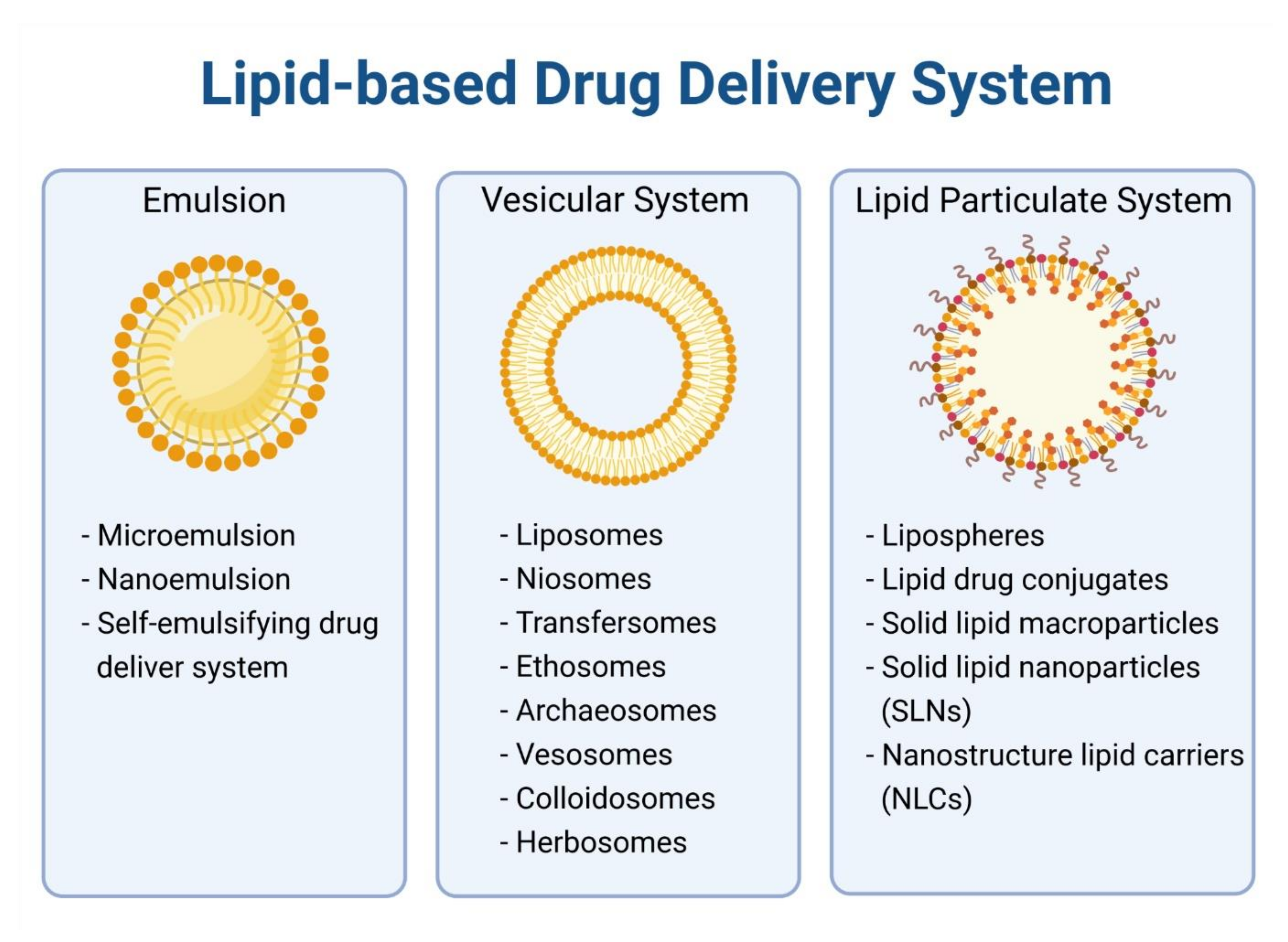
4.1. Liposome
4.2. Micelles
4.3. Solid Lipid Nanoparticles
4.4. Nanostructured Lipid Carriers
|
Class |
Compound |
Type of Lipid Carrier |
Type of Cancer |
In Vitro/In Vivo |
Cell Type/Animal Model |
Ref. |
|---|---|---|---|---|---|---|
|
Terpenoids |
Docetaxel |
Liposome |
Breast |
in vitro; in vivo |
MCF-7/4T1 xenograft mice |
[92] |
|
in vivo |
MDA-MB-435/LCC6 xenograft mice |
[93] |
||||
|
in vitro |
MCF-7 |
[94] |
||||
|
Lung |
in vivo |
A549 xenograft rat |
[95] |
|||
|
in vitro |
A549 |
[96] |
||||
|
in vitro |
A549 |
[94] |
||||
|
Liver |
in vitro |
HepG2 |
[94] |
|||
|
Melanoma |
in vitro; in vivo |
B16F10/B16-F10 xenograft mice |
[97] |
|||
|
Micelle |
Breast |
in vitro |
MCF-7 |
[98] |
||
|
in vitro |
MCF-7 |
[99] |
||||
|
Lung |
in vitro |
A549 |
[99] |
|||
|
Niosome |
Breast |
in vitro; in vivo |
MDA-MB-s31 and MCF-7 |
[100] |
||
|
NLC |
Liver, Ovarian, Lung, Melanoma |
in vitro; in vivo |
HepG2, SKOV3, A549, B16 cells |
[101] |
||
|
Paclitaxel |
Liposome |
Breast |
in vitro; in vivo |
4T1 xenograft mice |
[102] |
|
|
in vivo |
ICR male mice |
[103] |
||||
|
Lung |
in vivo |
A549 xenograft mice |
[104] |
|||
|
in vitro; in vivo |
A549 |
[105] |
||||
|
NLC |
Breast, Ovarian |
in vitro |
MCF-7, SKOV3 |
[106] |
||
|
Ethosome |
Squamous Cell Carcinoma |
in vitro |
DJM-1 |
[107] |
||
|
Micelle |
Glioma |
in vitro; in vivo |
C6/C6 xenograft rat |
[108] |
||
|
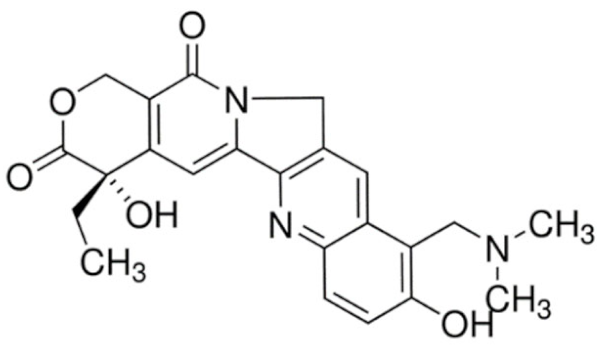
Indole |
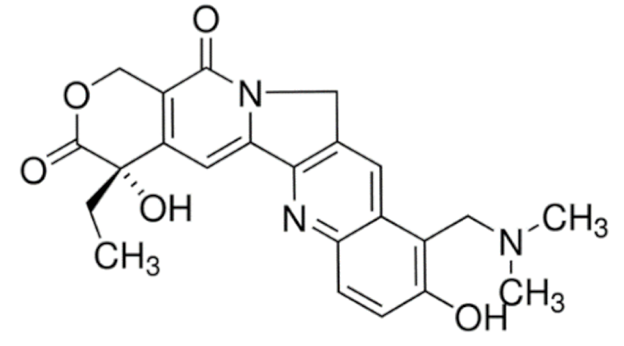
Vincristine |
Liposome |
Acute Lymphoblastic Leukemia |
in vivo |
Namwala xenograft mice |
[49] |
|
Brain Glioma |
in vitro, in vivo |
Glioma bearing mice |
[109] |
|||
|
Nasopharyngeal cancer |
in vitro, in vivo |
KB/KBv200 xenograft mice |
[110] |
|||
|
SLN |
Breast |
in vitro; in vivo |
MDA-MB-231 |
[111] |
||
|
NLC |
Breast |
in vitro |
MCF-7 |
[112] |
||
|
Vinblastine |
Liposome |
Non-Small Cell Lung |
in vitro; in vivo |
LLT/LLT xenograft mice |
[113] |
|
|
Niosome |
Lung |
in vitro; in vivo |
TC-1/TC-1 xenograft mice |
[114] |
||
|
Vinorelbine |
Micelle |
Breast |
in vitro |
MCF-7 |
[115] |
|
|
Quinoline |
Topotecan |
Liposome |
Lung and Adenocarcinoma |
in vitro |
LLC |
[116] |
|
Breast |
in vitro; in vivo |
MCF-7/MCF-7 xenograft mice |
[117] |
|||
|
SLN/NLC |
Leukemia |
in vitro |
K-562 |
[118] |
||
|
Irinotecan |
Liposome |
Colon |
in vivo |
Colo 320DM and Colon 26 xenograft mice |
[119] |
|
|
SLN |
Rectal, Colon |
in vivo |
SCC7 xenograft mice |
[120] |
||
|
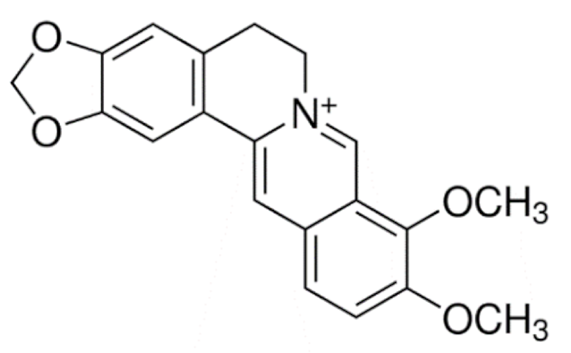
Isoquinoline |
Berberine |
Liposome |
Hepatic |
in vitro; in vivo |
HepG2/HepG2 xenograft mice |
[121] |
|
Breast |
in vitro; in vivo |
MCF-7/MCF-7 CSC xenograft mice |
[122] |
|||
|
SLN |
Breast, Hepatic, Lung |
in vitro |
MCF-7, HepG2, A549 |
[123] |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021.
- Bryant, A.K.; Banegas, M.P.; Martinez, M.E.; Mell, L.K.; Murphy, J.D. Trends in Radiation Therapy among Cancer Survivors in the United States, 2000–2030. Cancer Epidemiol. Biomark. Prev. 2017, 26, 963–970.
- Perera, S.K.; Jacob, S.; Sullivan, R.; Barton, M. Evidence-based benchmarks for use of cancer surgery in high-income countries: A population-based analysis. Lancet Oncol. 2021, 22, 173–181.
- Wilson, B.E.; Jacob, S.; Yap, M.L.; Ferlay, J.; Bray, F.; Barton, M.B. Estimates of global chemotherapy demands and corresponding physician workforce requirements for 2018 and 2040: A population-based study. Lancet Oncol. 2019, 20, 769–780.
- Falzone, L.; Salomone, S.; Libra, M. Evolution of Cancer Pharmacological Treatments at the Turn of the Third Millennium. Front. Pharmacol. 2018, 9, 1300.
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707–723.
- Li, F.S.; Weng, J.K. Demystifying traditional herbal medicine with modern approach. Nat. Plants 2017, 3, 17109.
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; International Natural Product Sciences Taskforce; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216.
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661.
- Davison, E.K.; Brimble, M.A. Natural product derived privileged scaffolds in drug discovery. Curr. Opin Chem Biol. 2019, 52, 1–8.
- Eguchi, R.; Ono, N.; Hirai Morita, A.; Katsuragi, T.; Nakamura, S.; Huang, M.; Altaf-Ul-Amin, M.; Kanaya, S. Classification of alkaloids according to the starting substances of their biosynthetic pathways using graph convolutional neural networks. BMC Bioinform. 2019, 20, 380.
- Ehrenworth, A.M.; Peralta-Yahya, P. Accelerating the semisynthesis of alkaloid-based drugs through metabolic engineering. Nat. Chem. Biol. 2017, 13, 249–258.
- Bharate, S.S.; Mignani, S.; Vishwakarma, R.A. Why Are the Majority of Active Compounds in the CNS Domain Natural Products? A Critical Analysis. J. Med. Chem. 2018, 61, 10345–10374.
- Reuvers, T.G.A.; Kanaar, R.; Nonnekens, J. DNA Damage-Inducing Anticancer Therapies: From Global to Precision Damage. Cancers 2020, 12, 2098.
- Steinmetz, M.O.; Prota, A.E. Microtubule-Targeting Agents: Strategies to Hijack the Cytoskeleton. Trends Cell Biol. 2018, 28, 776–792.
- Douer, D. Efficacy and Safety of Vincristine Sulfate Liposome Injection in the Treatment of Adult Acute Lymphocytic Leukemia. Oncologist 2016, 21, 840–847.
- Innocenti, F.; Schilsky, R.L.; Ramirez, J.; Janisch, L.; Undevia, S.; House, L.K.; Das, S.; Wu, K.; Turcich, M.; Marsh, R.; et al. Dose-finding and pharmacokinetic study to optimize the dosing of irinotecan according to the UGT1A1 genotype of patients with cancer. J. Clin. Oncol. 2014, 32, 2328–2334.
- Rosello, S.; Blasco, I.; Garcia Fabregat, L.; Cervantes, A.; Jordan, K.; Committee, E.G. Management of infusion reactions to systemic anticancer therapy: ESMO Clinical Practice Guidelines. Ann. Oncol. 2017, 28, iv100–iv118.
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951.
- Pelaz, B.; Alexiou, C.; Alvarez-Puebla, R.A.; Alves, F.; Andrews, A.M.; Ashraf, S.; Balogh, L.P.; Ballerini, L.; Bestetti, A.; Brendel, C.; et al. Diverse Applications of Nanomedicine. ACS Nano 2017, 11, 2313–2381.
- Tang, W.; Fan, W.; Lau, J.; Deng, L.; Shen, Z.; Chen, X. Emerging blood-brain-barrier-crossing nanotechnology for brain cancer theranostics. Chem. Soc. Rev. 2019, 48, 2967–3014.
- Lancet, J.E.; Uy, G.L.; Cortes, J.E.; Newell, L.F.; Lin, T.L.; Ritchie, E.K.; Stuart, R.K.; Strickland, S.A.; Hogge, D.; Solomon, S.R.; et al. CPX-351 (cytarabine and daunorubicin) Liposome for Injection Versus Conventional Cytarabine Plus Daunorubicin in Older Patients With Newly Diagnosed Secondary Acute Myeloid Leukemia. J. Clin. Oncol. 2018, 36, 2684–2692.
- Pattni, B.S.; Chupin, V.V.; Torchilin, V.P. New Developments in Liposomal Drug Delivery. Chem. Rev. 2015, 115, 10938–10966.
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51.
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37.
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124.
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The History of Nanoscience and Nanotechnology: From Chemical-Physical Applications to Nanomedicine. Molecules 2019, 25, 112.
- Roco, M.C. The long view of nanotechnology development: The National Nanotechnology Initiative at 10 years. J. Nanopart. Res. 2011, 13, 427–445.
- Porter, A.L.; Youtie, J. How interdisciplinary is nanotechnology? J. Nanopart. Res. 2009, 11, 1023–1041.
- Chen, H.; Gu, Z.; An, H.; Chen, C.; Chen, J.; Cui, R.; Chen, S.; Chen, W.; Chen, X.; Chen, X.; et al. Precise nanomedicine for intelligent therapy of cancer. Sci. China Chem. 2018, 61, 1503–1552.
- Van der Meel, R.; Sulheim, E.; Shi, Y.; Kiessling, F.; Mulder, W.J.M.; Lammers, T. Smart cancer nanomedicine. Nat. Nanotechnol. 2019, 14, 1007–1017.
- Zaytseva, O.; Neumann, G. Carbon nanomaterials: Production, impact on plant development, agricultural and environmental applications. Chem. Biol. Technol. Agric. 2016, 3, 17.
- Liu, Z.; Robinson, J.T.; Tabakman, S.M.; Yang, K.; Dai, H. Carbon materials for drug delivery & cancer therapy. Mater. Today 2011, 14, 316–323.
- Patel, K.D.; Singh, R.K.; Kim, H.-W. Carbon-based nanomaterials as an emerging platform for theranostics. Mater. Horiz. 2019, 6, 434–469.
- Mody, V.V.; Siwale, R.; Singh, A.; Mody, H.R. Introduction to metallic nanoparticles. J. Pharm. Bioallied. Sci. 2010, 2, 282–289.
- Saleh, T.A. Nanomaterials: Classification, properties, and environmental toxicities. Environ. Technol. Innov. 2020, 20, 101067.
- Palazzolo, S.; Bayda, S.; Hadla, M.; Caligiuri, I.; Corona, G.; Toffoli, G.; Rizzolio, F. The Clinical Translation of Organic Nanomaterials for Cancer Therapy: A Focus on Polymeric Nanoparticles, Micelles, Liposomes and Exosomes. Curr. Med. Chem. 2018, 25, 4224–4268.
- Yang, L.; Stockigt, J. Trends for diverse production strategies of plant medicinal alkaloids. Nat. Prod. Rep. 2010, 27, 1469–1479.
- Conroy, T.; Hammel, P.; Hebbar, M.; Ben Abdelghani, M.; Wei, A.C.; Raoul, J.L.; Chone, L.; Francois, E.; Artru, P.; Biagi, J.J.; et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N. Engl. J. Med. 2018, 379, 2395–2406.
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodriguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301.
- Cortinovis, D.; Bidoli, P.; Canova, S.; Colonese, F.; Gemelli, M.; Lavitrano, M.L.; Banna, G.L.; Liu, S.V.; Morabito, A. Novel Cytotoxic Chemotherapies in Small Cell Lung Carcinoma. Cancers 2021, 13, 1152.
- Sweeney, C.J.; Chen, Y.H.; Carducci, M.; Liu, G.; Jarrard, D.F.; Eisenberger, M.; Wong, Y.N.; Hahn, N.; Kohli, M.; Cooney, M.M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2015, 373, 737–746.
- Connors, J.M.; Jurczak, W.; Straus, D.J.; Ansell, S.M.; Kim, W.S.; Gallamini, A.; Younes, A.; Alekseev, S.; Illes, A.; Picardi, M.; et al. Brentuximab Vedotin with Chemotherapy for Stage III or IV Hodgkin’s Lymphoma. N. Engl. J. Med. 2018, 378, 331–344.
- Silva, C.O.; Pinho, J.O.; Lopes, J.M.; Almeida, A.J.; Gaspar, M.M.; Reis, C. Current Trends in Cancer Nanotheranostics: Metallic, Polymeric, and Lipid-Based Systems. Pharmaceutics 2019, 11, 22.
- Lim, S.B.; Banerjee, A.; Önyüksel, H. Improvement of drug safety by the use of lipid-based nanocarriers. J. Control Release 2012, 163, 34–45.
- Rahman, M.A.; Hussain, A.; Hussain, M.S.; Mirza, M.A.; Iqbal, Z. Role of excipients in successful development of self-emulsifying/microemulsifying drug delivery system (SEDDS/SMEDDS). Drug Dev. Ind. Pharm. 2013, 39, 1–19.
- Zhigaltsev, I.V.; Tam, Y.K.; Leung, A.K.K.; Cullis, P.R. Production of limit size nanoliposomal systems with potential utility as ultra-small drug delivery agents. J. Liposome Res. 2016, 26, 96–102.
- Cabral, H.; Matsumoto, Y.; Mizuno, K.; Chen, Q.; Murakami, M.; Kimura, M.; Terada, Y.; Kano, M.R.; Miyazono, K.; Uesaka, M.; et al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat. Nanotechnol. 2011, 6, 815–823.
- Silverman, J.A.; Deitcher, S.R. Marqibo(R) (vincristine sulfate liposome injection) improves the pharmacokinetics and pharmacodynamics of vincristine. Cancer Chemother. Pharm. 2013, 71, 555–564.
- Barenholz, Y. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control Release 2012, 160, 117–134.
- Green, M.R.; Manikhas, G.M.; Orlov, S.; Afanasyev, B.; Makhson, A.M.; Bhar, P.; Hawkins, M.J. Abraxane, a novel Cremophor-free, albumin-bound particle form of paclitaxel for the treatment of advanced non-small-cell lung cancer. Ann. Oncol. 2006, 17, 1263–1268.
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharm. 2015, 6, 286.
- Northfelt, D.W.; Dezube, B.J.; Thommes, J.A.; Miller, B.J.; Fischl, M.A.; Friedman-Kien, A.; Kaplan, L.D.; Du Mond, C.; Mamelok, R.D.; Henry, D.H. Pegylated-liposomal doxorubicin versus doxorubicin, bleomycin, and vincristine in the treatment of AIDS-related Kaposi’s sarcoma: Results of a randomized phase III clinical trial. J. Clin. Oncol. 1998, 16, 2445–2451.
- Yingchoncharoen, P.; Kalinowski, D.S.; Richardson, D.R. Lipid-Based Drug Delivery Systems in Cancer Therapy: What Is Available and What Is Yet to Come. Pharm. Rev. 2016, 68, 701–787.
- Amoabediny, G.; Haghiralsadat, F.; Naderinezhad, S.; Helder, M.N.; Akhoundi Kharanaghi, E.; Mohammadnejad Arough, J.; Zandieh-Doulabi, B. Overview of preparation methods of polymeric and lipid-based (niosome, solid lipid, liposome) nanoparticles: A comprehensive review. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 383–400.
- Gabizon, A.; Shmeeda, H.; Grenader, T. Pharmacological basis of pegylated liposomal doxorubicin: Impact on cancer therapy. Eur. J. Pharm. Sci. 2012, 45, 388–398.
- Steffes, V.M.; Zhang, Z.; MacDonald, S.; Crowe, J.; Ewert, K.K.; Carragher, B.; Potter, C.S.; Safinya, C.R. PEGylation of Paclitaxel-Loaded Cationic Liposomes Drives Steric Stabilization of Bicelles and Vesicles thereby Enhancing Delivery and Cytotoxicity to Human Cancer Cells. ACS Appl. Mater. Interfaces 2020, 12, 151–162.
- Lin, W.; Kampf, N.; Goldberg, R.; Driver, M.J.; Klein, J. Poly-phosphocholinated Liposomes Form Stable Superlubrication Vectors. Langmuir 2019, 35, 6048–6054.
- Cao, Z.; Zhang, L.; Jiang, S. Superhydrophilic Zwitterionic Polymers Stabilize Liposomes. Langmuir 2012, 28, 11625–11632.
- Perez-Herrero, E.; Fernandez-Medarde, A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52–79.
- Lewrick, F.; Süss, R. Remote loading of anthracyclines into liposomes. Methods Mol. Biol. 2010, 605, 139–145.
- Hardiansyah, A.; Huang, L.Y.; Yang, M.C.; Liu, T.Y.; Tsai, S.C.; Yang, C.Y.; Kuo, C.Y.; Chan, T.Y.; Zou, H.M.; Lian, W.N.; et al. Magnetic liposomes for colorectal cancer cells therapy by high-frequency magnetic field treatment. Nanoscale Res. Lett. 2014, 9, 497.
- Park, J.H.; Cho, H.J.; Yoon, H.Y.; Yoon, I.S.; Ko, S.H.; Shim, J.S.; Cho, J.H.; Park, J.H.; Kim, K.; Kwon, I.C.; et al. Hyaluronic acid derivative-coated nanohybrid liposomes for cancer imaging and drug delivery. J. Control Release 2014, 174, 98–108.
- Deshpande, P.; Jhaveri, A.; Pattni, B.; Biswas, S.; Torchilin, V. Transferrin and octaarginine modified dual-functional liposomes with improved cancer cell targeting and enhanced intracellular delivery for the treatment of ovarian cancer. Drug Deliv. 2018, 25, 517–532.
- Ninomiya, K.; Kawabata, S.; Tashita, H.; Shimizu, N. Ultrasound-mediated drug delivery using liposomes modified with a thermosensitive polymer. Ultrason. Sonochem. 2014, 21, 310–316.
- Sesarman, A.; Tefas, L.; Sylvester, B.; Licarete, E.; Rauca, V.; Luput, L.; Patras, L.; Porav, S.; Banciu, M.; Porfire, A. Co-delivery of curcumin and doxorubicin in PEGylated liposomes favored the antineoplastic C26 murine colon carcinoma microenvironment. Drug Deliv. Transl. Res. 2019, 9, 260–272.
- Legut, M.; Lipka, D.; Filipczak, N.; Piwoni, A.; Kozubek, A.; Gubernator, J. Anacardic acid enhances the anticancer activity of liposomal mitoxantrone towards melanoma cell lines - in vitro studies. Int. J. Nanomed. 2014, 9, 653–668.
- Lakkadwala, S.; Singh, J. Dual Functionalized 5-Fluorouracil Liposomes as Highly Efficient Nanomedicine for Glioblastoma Treatment as Assessed in an In Vitro Brain Tumor Model. J. Pharm. Sci. 2018, 107, 2902–2913.
- Ge, X.; Wei, M.; He, S.; Yuan, W.-E. Advances of Non-Ionic Surfactant Vesicles (Niosomes) and Their Application in Drug Delivery. Pharmaceutics 2019, 11, 55.
- Ag Seleci, D.; Seleci, M.; Walter, J.-G.; Stahl, F.; Scheper, T. Niosomes as Nanoparticular Drug Carriers: Fundamentals and Recent Applications. J. Nanomater. 2016, 2016, 7372306.
- Gharbavi, M.; Amani, J.; Kheiri-Manjili, H.; Danafar, H.; Sharafi, A. Niosome: A Promising Nanocarrier for Natural Drug Delivery through Blood-Brain Barrier. Adv. Pharmacol. Sci. 2018, 2018, 6847971.
- You, L.; Liu, X.; Fang, Z.; Xu, Q.; Zhang, Q. Synthesis of multifunctional nano-niosomes as a targeting carrier for treatment of cervical cancer. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 94, 291–302.
- Abdulbaqi, I.M.; Darwis, Y.; Khan, N.A.; Assi, R.A.; Khan, A.A. Ethosomal nanocarriers: The impact of constituents and formulation techniques on ethosomal properties, in vivo studies, and clinical trials. Int. J. Nanomed. 2016, 11, 2279–2304.
- Nainwal, N.; Jawla, S.; Singh, R.; Saharan, V.A. Transdermal applications of ethosomes—A detailed review. J. Liposome Res. 2019, 29, 103–113.
- Chen, M.; Liu, X.; Fahr, A. Skin penetration and deposition of carboxyfluorescein and temoporfin from different lipid vesicular systems: In vitro study with finite and infinite dosage application. Int. J. Pharm. 2011, 408, 223–234.
- Yu, X.; Du, L.; Li, Y.; Fu, G.; Jin, Y. Improved anti-melanoma effect of a transdermal mitoxantrone ethosome gel. Biomed. Pharm. 2015, 73, 6–11.
- Mishra, D.K.; Shandilya, R.; Mishra, P.K. Lipid based nanocarriers: A translational perspective. Nanomedicine 2018, 14, 2023–2050.
- Lu, Y.; Zhang, E.; Yang, J.; Cao, Z. Strategies to improve micelle stability for drug delivery. Nano Res. 2018, 11, 4985–4998.
- Koo, O.M.Y.; Rubinstein, I.; Onyüksel, H. Actively targeted low-dose camptothecin as a safe, long-acting, disease-modifying nanomedicine for rheumatoid arthritis. Pharm. Res. 2011, 28, 776–787.
- Krishnadas, A.; Rubinstein, I.; Önyüksel, H. Sterically Stabilized Phospholipid Mixed Micelles: In Vitro Evaluation as a Novel Carrier for Water-Insoluble Drugs. Pharm. Res. 2003, 20, 297–302.
- Tang, N.; Du, G.; Wang, N.; Liu, C.; Hang, H.; Liang, W. Improving penetration in tumors with nanoassemblies of phospholipids and doxorubicin. J. Natl. Cancer Inst. 2007, 99, 1004–1015.
- Kim, S.; Shi, Y.; Kim, J.Y.; Park, K.; Cheng, J.X. Overcoming the barriers in micellar drug delivery: Loading efficiency, in vivo stability, and micelle-cell interaction. Expert Opin. Drug Deliv. 2010, 7, 49–62.
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Peter, W. The Lipid Bilayer. In Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002.
- Muller, R.H.; Shegokar, R.; Keck, C.M. 20 years of lipid nanoparticles (SLN and NLC): Present state of development and industrial applications. Curr. Drug Discov. Technol. 2011, 8, 207–227.
- Uner, M.; Yener, G. Importance of solid lipid nanoparticles (SLN) in various administration routes and future perspectives. Int. J. Nanomed. 2007, 2, 289–300.
- Wang, W.; Zhang, L.; Chen, T.; Guo, W.; Bao, X.; Wang, D.; Ren, B.; Wang, H.; Li, Y.; Wang, Y.; et al. Anticancer Effects of Resveratrol-Loaded Solid Lipid Nanoparticles on Human Breast Cancer Cells. Molecules 2017, 22, 1814.
- Ghasemiyeh, P.; Mohammadi-Samani, S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: Applications, advantages and disadvantages. Res. Pharm. Sci. 2018, 13, 288–303.
- Muller, R.H.; Radtke, M.; Wissing, S.A. Nanostructured lipid matrices for improved microencapsulation of drugs. Int. J. Pharm. 2002, 242, 121–128.
- Beloqui, A.; Solinis, M.A.; Rodriguez-Gascon, A.; Almeida, A.J.; Preat, V. Nanostructured lipid carriers: Promising drug delivery systems for future clinics. Nanomedicine 2016, 12, 143–161.
- Iqbal, M.A.; Md, S.; Sahni, J.K.; Baboota, S.; Dang, S.; Ali, J. Nanostructured lipid carriers system: Recent advances in drug delivery. J. Drug Target 2012, 20, 813–830.
- Wei, Q.; Yang, Q.; Wang, Q.; Sun, C.; Zhu, Y.; Niu, Y.; Yu, J.; Xu, X. Formulation, Characterization, and Pharmacokinetic Studies of 6-Gingerol-Loaded Nanostructured Lipid Carriers. AAPS PharmSciTech 2018, 19, 3661–3669.
- Vakili-Ghartavol, R.; Rezayat, S.M.; Faridi-Majidi, R.; Sadri, K.; Jaafari, M.R. Optimization of Docetaxel Loading Conditions in Liposomes: Proposing potential products for metastatic breast carcinoma chemotherapy. Sci. Rep. 2020, 10, 5569.
- Zhigaltsev, I.V.; Winters, G.; Srinivasulu, M.; Crawford, J.; Wong, M.; Amankwa, L.; Waterhouse, D.; Masin, D.; Webb, M.; Harasym, N.; et al. Development of a weak-base docetaxel derivative that can be loaded into lipid nanoparticles. J. Control Release 2010, 144, 332–340.
- Chang, M.; Lu, S.; Zhang, F.; Zuo, T.; Guan, Y.; Wei, T.; Shao, W.; Lin, G. RGD-modified pH-sensitive liposomes for docetaxel tumor targeting. Colloids Surf. B Biointerfaces 2015, 129, 175–182.
- Ren, G.; Liu, D.; Guo, W.; Wang, M.; Wu, C.; Guo, M.; Ai, X.; Wang, Y.; He, Z. Docetaxel prodrug liposomes for tumor therapy: Characterization, in vitro and in vivo evaluation. Drug Deliv. 2016, 23, 1272–1281.
- Luo, Q.; Yang, B.; Tao, W.; Li, J.; Kou, L.; Lian, H.; Che, X.; He, Z.; Sun, J. ATB(0,+) transporter-mediated targeting delivery to human lung cancer cells via aspartate-modified docetaxel-loading stealth liposomes. Biomater. Sci. 2017, 5, 295–304.
- Gu, Z.; Wang, Q.; Shi, Y.; Huang, Y.; Zhang, J.; Zhang, X.; Lin, G. Nanotechnology-mediated immunochemotherapy combined with docetaxel and PD-L1 antibody increase therapeutic effects and decrease systemic toxicity. J. Control Release 2018, 286, 369–380.
- Mi, Y.; Liu, Y.; Feng, S.S. Formulation of Docetaxel by folic acid-conjugated d-alpha-tocopheryl polyethylene glycol succinate 2000 (Vitamin E TPGS(2k)) micelles for targeted and synergistic chemotherapy. Biomaterials 2011, 32, 4058–4066.
- Shi, C.; Zhang, Z.; Wang, F.; Ji, X.; Zhao, Z.; Luan, Y. Docetaxel-loaded PEO-PPO-PCL/TPGS mixed micelles for overcoming multidrug resistance and enhancing antitumor efficacy. J. Mater. Chem. B 2015, 3, 4259–4271.
- Liu, H.; Tu, L.; Zhou, Y.; Dang, Z.; Wang, L.; Du, J.; Feng, J.; Hu, K. Improved Bioavailability and Antitumor Effect of Docetaxel by TPGS Modified Proniosomes: In Vitro and In Vivo Evaluations. Sci. Rep. 2017, 7, 43372.
- Liu, D.; Liu, Z.; Wang, L.; Zhang, C.; Zhang, N. Nanostructured lipid carriers as novel carrier for parenteral delivery of docetaxel. Colloids Surf. B Biointerfaces 2011, 85, 262–269.
- Ravar, F.; Saadat, E.; Gholami, M.; Dehghankelishadi, P.; Mahdavi, M.; Azami, S.; Dorkoosh, F.A. Hyaluronic acid-coated liposomes for targeted delivery of paclitaxel, in-vitro characterization and in-vivo evaluation. J. Control Release 2016, 229, 10–22.
- Wang, J.; Jia, J.; Liu, J.; He, H.; Zhang, W.; Li, Z. Tumor targeting effects of a novel modified paclitaxel-loaded discoidal mimic high density lipoproteins. Drug Deliv. 2013, 20, 356–363.
- Miao, Y.Q.; Chen, M.S.; Zhou, X.; Guo, L.M.; Zhu, J.J.; Wang, R.; Zhang, X.X.; Gan, Y. Chitosan oligosaccharide modified liposomes enhance lung cancer delivery of paclitaxel. Acta Pharm. Sin. 2021.
- Jain, S.; Kumar, D.; Swarnakar, N.K.; Thanki, K. Polyelectrolyte stabilized multilayered liposomes for oral delivery of paclitaxel. Biomaterials 2012, 33, 6758–6768.
- Zhang, X.G.; Miao, J.; Dai, Y.Q.; Du, Y.Z.; Yuan, H.; Hu, F.Q. Reversal activity of nanostructured lipid carriers loading cytotoxic drug in multi-drug resistant cancer cells. Int. J. Pharm. 2008, 361, 239–244.
- Paolino, D.; Celia, C.; Trapasso, E.; Cilurzo, F.; Fresta, M. Paclitaxel-loaded ethosomes(R): Potential treatment of squamous cell carcinoma, a malignant transformation of actinic keratoses. Eur. J. Pharm. Biopharm. 2012, 81, 102–112.
- Zhao, B.J.; Ke, X.Y.; Huang, Y.; Chen, X.M.; Zhao, X.; Zhao, B.X.; Lu, W.L.; Lou, J.N.; Zhang, X.; Zhang, Q. The antiangiogenic efficacy of NGR-modified PEG-DSPE micelles containing paclitaxel (NGR-M-PTX) for the treatment of glioma in rats. J. Drug Target. 2011, 19, 382–390.
- Song, X.L.; Liu, S.; Jiang, Y.; Gu, L.Y.; Xiao, Y.; Wang, X.; Cheng, L.; Li, X.T. Targeting vincristine plus tetrandrine liposomes modified with DSPE-PEG2000-transferrin in treatment of brain glioma. Eur. J. Pharm. Sci. 2017, 96, 129–140.
- Wang, C.; Feng, L.; Yang, X.; Wang, F.; Lu, W. Folic acid-conjugated liposomal vincristine for multidrug resistant cancer therapy. Asian J. Pharm. Sci. 2013, 8, 118–127.
- Aboutaleb, E.; Atyabi, F.; Khoshayand, M.R.; Vatanara, A.R.; Ostad, S.N.; Kobarfard, F.; Dinarvand, R. Improved brain delivery of vincristine using dextran sulfate complex solid lipid nanoparticles: Optimization and in vivo evaluation. J. Biomed. Mater. Res. A 2014, 102, 2125–2136.
- Gao, X.; Zhang, J.; Xu, Q.; Huang, Z.; Wang, Y.; Shen, Q. Hyaluronic acid-coated cationic nanostructured lipid carriers for oral vincristine sulfate delivery. Drug Dev. Ind. Pharm. 2017, 43, 661–667.
- Li, X.T.; He, M.L.; Zhou, Z.Y.; Jiang, Y.; Cheng, L. The antitumor activity of PNA modified vinblastine cationic liposomes on Lewis lung tumor cells: In vitro and in vivo evaluation. Int. J. Pharm. 2015, 487, 223–233.
- Amiri, B.; Ahmadvand, H.; Farhadi, A.; Najmafshar, A.; Chiani, M.; Norouzian, D. Delivery of vinblastine-containing niosomes results in potent in vitro/in vivo cytotoxicity on tumor cells. Drug Dev. Ind. Pharm. 2018, 44, 1371–1376.
- Bahadori, F.; Topcu, G.; Eroglu, M.S.; Onyuksel, H. A new lipid-based nano formulation of vinorelbine. AAPS Pharm. Sci. Tech. 2014, 15, 1138–1148.
- Dadashzadeh, S.; Vali, A.M.; Rezaie, M. The effect of PEG coating on in vitro cytotoxicity and in vivo disposition of topotecan loaded liposomes in rats. Int. J. Pharm. 2008, 353, 251–259.
- Yu, Y.; Wang, Z.H.; Zhang, L.; Yao, H.J.; Zhang, Y.; Li, R.J.; Ju, R.J.; Wang, X.X.; Zhou, J.; Li, N.; et al. Mitochondrial targeting topotecan-loaded liposomes for treating drug-resistant breast cancer and inhibiting invasive metastases of melanoma. Biomaterials 2012, 33, 1808–1820.
- Souza, L.G.; Silva, E.J.; Martins, A.L.; Mota, M.F.; Braga, R.C.; Lima, E.M.; Valadares, M.C.; Taveira, S.F.; Marreto, R.N. Development of topotecan loaded lipid nanoparticles for chemical stabilization and prolonged release. Eur. J. Pharm. Biopharm. 2011, 79, 189–196.
- Hattori, Y.; Shi, L.; Ding, W.; Koga, K.; Kawano, K.; Hakoshima, M.; Maitani, Y. Novel irinotecan-loaded liposome using phytic acid with high therapeutic efficacy for colon tumors. J. Control Release 2009, 136, 30–37.
- Din, F.U.; Choi, J.Y.; Kim, D.W.; Mustapha, O.; Kim, D.S.; Thapa, R.K.; Ku, S.K.; Youn, Y.S.; Oh, K.T.; Yong, C.S.; et al. Irinotecan-encapsulated double-reverse thermosensitive nanocarrier system for rectal administration. Drug Deliv. 2017, 24, 502–510.
- Lin, Y.C.; Kuo, J.Y.; Hsu, C.C.; Tsai, W.C.; Li, W.C.; Yu, M.C.; Wen, H.W. Optimizing manufacture of liposomal berberine with evaluation of its antihepatoma effects in a murine xenograft model. Int. J. Pharm. 2013, 441, 381–388.
- Ma, X.; Zhou, J.; Zhang, C.X.; Li, X.Y.; Li, N.; Ju, R.J.; Shi, J.F.; Sun, M.G.; Zhao, W.Y.; Mu, L.M.; et al. Modulation of drug-resistant membrane and apoptosis proteins of breast cancer stem cells by targeting berberine liposomes. Biomaterials 2013, 34, 4452–4465.
- Wang, L.; Li, H.; Wang, S.; Liu, R.; Wu, Z.; Wang, C.; Wang, Y.; Chen, M. Enhancing the antitumor activity of berberine hydrochloride by solid lipid nanoparticle encapsulation. AAPS PharmSciTech 2014, 15, 834–844.