Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | María Alejandra Décima | + 1434 word(s) | 1434 | 2021-11-10 02:58:42 | | | |
| 2 | Camila Xu | Meta information modification | 1434 | 2021-11-11 03:10:39 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Décima, M.A. Carbamazepine in water and wastewater treatment plants. Encyclopedia. Available online: https://encyclopedia.pub/entry/15881 (accessed on 07 February 2026).
Décima MA. Carbamazepine in water and wastewater treatment plants. Encyclopedia. Available at: https://encyclopedia.pub/entry/15881. Accessed February 07, 2026.
Décima, María Alejandra. "Carbamazepine in water and wastewater treatment plants" Encyclopedia, https://encyclopedia.pub/entry/15881 (accessed February 07, 2026).
Décima, M.A. (2021, November 10). Carbamazepine in water and wastewater treatment plants. In Encyclopedia. https://encyclopedia.pub/entry/15881
Décima, María Alejandra. "Carbamazepine in water and wastewater treatment plants." Encyclopedia. Web. 10 November, 2021.
Copy Citation
Carbamazepine (CBZ) is one of the most frequently used drugs for the medical treatment of epilepsy and bipolar disorder, being a mood stabilizer. It is metabolized in the liver and excreted mainly as hydroxylated and conjugated metabolite and only around 5% as an unchanged drug.
carbamazepine
water treatment plants
wastewater treatment plants
1. Carbamazepine Usage and Physicochemical Characteristics
CBZ is one of the most frequently used drugs for the medical treatment of epilepsy and bipolar disorder, being a mood stabilizer [1]. It is metabolized in the liver and excreted mainly as hydroxylated and conjugated metabolite and only around 5% as an unchanged drug [2]. The formula, structure, identifier (i.e., CAS number) and the main physicochemical characteristics of CBZ are summarized in Table 1.
Table 1. Main CBZ physicochemical characteristics.
| CAS n. | Formula | Structure | Molecular Weight | pKa | Log KOW | KH | S |
|---|---|---|---|---|---|---|---|
| 298-46-4 | C15H12N2O | 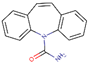 |
236.274 g/mol | 2.3 [3] 13.9 [4] |
2.45 [5] | 2.24 × 1010 atm·m3/mol [6] P | 0.0005 mol/L [5] at 18 mg/L and 25 °C [4] |
CAS n.: Chemical Abstracts Service number; Formula: Chemical formula; pKa: log of acid dissociation constant; Log Kow: log of octanol-water partition coefficient; KH: Henry’s law constant; S: water solubility; P: predicted value.
CBZ is not considered a volatile compound and it is characterized by a value of the octanol-water partitioning coefficient below 3 which entails that it is moderately hydrophobic; it is also considered soluble in water based on the available values of the solubility. Moreover, in the aquatic environment and most of the water and wastewater treatment processes, CBZ is present as a non-ionized form, since its pKa is far from neutral, which is the usual pH condition for these treatments [7]. All these characteristics proved that CBZ is refractory to the traditional water and wastewater treatment processes and particularly to biological processes, as reported by several authors [8][9][10][11]. As a consequence, it is usually found in the effluent of the treatment plants and then in the surface water bodies where it is frequently released. The NORMAN Network database, which includes 20 countries and 25,359 samples, shows that the frequency of detection in this environmental matrix is about 73% [5]. The frequent occurrence of CBZ in the aquatic environment represents a relevant and concerning matter since this substance has adverse toxicological effects [1]. Indeed, CBZ is classified as a toxic compound concerning marine and surface water compartments, being the lowest predicted no-effect concentration (PNEC) below 0.1 µg/L in both compartments (0.005 µg/L and 0.05 µg/L, respectively) according to the criteria proposed by NORMAN Prioritization framework for emerging substances and the REACH regulation [5][12]. Thus, CBZ represents a risk for the environment, but also for human health when surface water is used as a source for drinking water production.
2. Wastewater Treatment Plants (WWTPs)
Frequency of detection (Fd) is the parameter of relevance when evaluating the pollution of water by a contaminant that describes the steadiness of its occurrence. For CBZ, its frequency of detection is often significantly high, defining pollution not randomly but consistently present in wastewater influent and effluent. On this account indeed, Loos et al. (2013) reported a frequency of detection of 90% in WWTPs effluent sampled across the EU [13]; Di Marcantonio et al. (2020) found an Fd of 96% and 91% in the influent and effluent, respectively [8]. According to Thiebault et al. (2017), Suebdi et al. (2015), Tran and Gin (2017) and Rivera-Jaimes et al. (2018), 100% Fd was recorded in both influent and effluent [11][14][15][16]. Table 2 lists the average concentrations at which CBZ was found across the world in the influent and effluent of wastewater treatment plants: even if the values are often in the range of ng/L, there are also exceptions with concentrations reaching the order of magnitude of µg/L.
Table 2. CBZ concentration (ng/L) mean and range in WWTPs worldwide, considering raw wastewater (RWW) and treated wastewater (TWW).
| Location | n° WWTPs | RWW (Mean/Median) | RWW Range | TWW (Mean/Median) | TWW Range | Ref. |
|---|---|---|---|---|---|---|
| China | 5× | 45 | 24–72 | 34 | 24–49 | [17] |
| China | 17 | n.a. | 18 | n.a. | [18] | |
| China | 14 | 10–20 | 16 | 13–21 | [19] | |
| USA | 2× * | 193 | 61–588 | 289 | 91–731 | [15] |
| USA | 5× | 115 | 34–350 | 21 | <LOQ-62 | [20] |
| Canada | 5× | 757 | n.a. | 713 | n.a. | [21] |
| Mexico | * | 214 | 85–380 | 285 | 165–476 | [16] |
| Singapore | * | 323 | 323–339 | 313 | 262–336 | [11] |
| Spain | <LOQ | n.a. | 97 | n.a. | [22] | |
| Spain | (WWTP1) * | 166 | 69–283 | 102 | 29–198 | [23] |
| (WWTP2) * | 172 | 140 | ||||
| Spain | 4× * | 422 | 70–970 | 100 | 60–150 | [24] |
| Turkey | 5× * | 19 | <LOQ-95 | 5 | <LOQ-75 | [25] |
| Italy | 570 | n.a. | 370 | n.a. | [26] | |
| Italy | 76× | 209 | <LOQ-1381 | 193 | <LOQ-890 | [8] |
| Italy | 140 | n.a. | 179 | n.a. | [27] | |
| Switzerland | 256 | n.a. | 251 | n.a. | [27] | |
| Czech Republic | 460 | 210–710 | 510 | 220–730 | [28] | |
| France | 215 | 51–937 | 163 | 5–357 | [14] | |
| Germany | 1536 | 246–815 | 1614 | 1020–2309 | [29] | |
| Germany | 6× | 1900 | 1500–2100 | 2000 | 1800–2200 | [30] |
| Portugal | 2× | 470 | 440–500 | 520 | 500–540 | [30] |
| Portugal | 2× | 95 | 47–226 | 117 | 62.7–245 | [31] |
| African countries | (review) | 117–6145 | 64–1438 | [32] | ||
| India | 1642 | 22–8200 | 393 | 88–900 | [33] |
Data shown are either reported as such in the articles or calculated with the data available (*). The data reported in Italic are medians. When needed, WebPlotDigitizer was used to gain data from graphs.
Regarding time-related patterns for CBZ pollution of wastewaters, seasonality is usually excluded, being CBZ a substance not linked to seasonal illness; moreover, daily patterns have not been detected [34]. However, some studies report that drier seasons exerted an influence on the CBZ concentration detected at WWTPs due to lower dilution, particularly in the case of combined sewers [1].
The behavior of CBZ in WWTPs has been well addressed in the reviews by Couto et al. (2019) [34] and Krzeminski et al. (2019) [35], along with other contaminants of emerging concern (CECs). WWTPs are not (yet) designed to remove CECs, which are hence often discharged in the receiving water body through the treated effluent [34]. Particularly, CBZ seems quite refractory to biological treatments [22][35]; furthermore, it is also not expected to adsorb greatly on solids (medium-low octanol-water partition coefficient) [34] and therefore these can explain the poor removal in WWTPs [10]. The removal reported for CBZ is often even negative, due to recombination processes of precursors, accumulation within the plant, release by solids and sampling strategies that do not consider the plant Hydraulic Retention Time (HRT) [8][11][34][36]. Regarding the influence of the WWTP layout, an interesting study is the one conducted by Di Marcantonio et al. (2020) [8], where 76 WWTPs were analyzed for 2.5 years. According to the results, CBZ showed very low removal efficiencies (lower than 50%), the lowest being recorded for layouts comprising secondary treatments alone, slightly improving where primary or tertiary treatments were also included. Indeed, the review by Yang et al. (2017) and that of Hai et al. (2018) also confirmed the scarce removal by the secondary treatment, regardless of the system used (Conventional Activated Sludge, membrane bioreactor, others) [1][37].
3. Drinking Water Treatment Plants (DWTPs)
Indeed, CBZ is detected at the influent of DWTPs, as shown in Table 3, as in the effluents.
Table 3. CBZ concentration (ng/L) mean and maximum and Fd (%) in DWTPs worldwide.
| Location | n° DWTPs | Water Source | RW | TW | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|
| Fd | Mean/Median | Max | Fd | Mean/Median | Max | ||||
| Canada | 17× | GW/SW | 50 | 3 | 749 | 25 | 0.21 | 601 | [38] |
| Canada | 5× * | SW | 68 | 3.1 | 7.2 | 65.6 | 1.92 | 3.6 | [39] |
| USA | SW | 13 | 1.4 | 1.6 | 13 | 0.3 | 0.4 | [40] | |
| USA | 31× * | GW/SW | 37 | 6.1 | 17.9 | 27 | 3.6 | 6.9 | [41] |
| USA | 29× | GW/SW | 56 | 15.9 | 35.7 | 8 | 17.75 | 26.5 | [42] |
| Japan | 6× * | GW/SW | 39 | 7.3 | 100 | 13 | 1.9 | 25 | [43] |
| Korea | 5× | SW | 100 | 7.7 | 21.1 | 5 | 0.67 | 0.67 | [44] |
| Korea | SW | 92 | 10.3 | 46.4 | 13 | 1.7 | 17.7 | [45] | |
| China | SW | 100 | 1.33–1.82 | 100 | 0.37–1.15 | [46] | |||
| China | SW | 100 | 0.8 | 1.01 | 13 | n.a. | 0.65 | [47] | |
| Sweden | 90× | GW/SW | 41.5 | 0.95 | n.a. | 35 | 0.95 | n.a. | [48] |
| Sweden | 7× * | SW | 100 | 10.48 | 13.44 | 28.5 | 2.91 | 11.32 | [49] |
| Spain | * | SW | 92 | 153 | 245 | 8.3 | 0.02 | 0.09 | [50] |
| Spain | * | GW | 100 | 84.5 | 167 | 89 | 1.1 | 5.7 | [51] |
| Italy | 3× * | SW | 66.6 | 13.8 | 34.57 | 41.7 | 0.2 | 1.20 | [52] |
| Portugal | GW/SW | 96 | 3.3 | 16.8 | 69 | 1.8 | 13.5 | [53] | |
Data shown are either reported as such in the articles or calculated with the data available (*). Fd: frequency of detection (%), GW: groundwater, n.a.: not available, RW: raw water, SW: surface water, TW: treated water. Values in italics are medians; when needed, WebPlotDigitizer was used to gain data from graphs.
The frequency of detection (%) is often quite high and not necessarily decreasing between influent and effluent of the water treatment plant, highlighting the persistence of the compound. The Fd median value, considering the studies reported in Table 3, for raw water is 80%, whereas for treated water it is 25%. The concentration in the effluent is usually lower, confirming at least a partial effect of the water treatment units. The concentrations reported by worldwide example studies in Table 3 shows quite a variability: in raw water, the range spans from almost zero to hundreds of ng/L, whereas in treated water it is reduced to tens of ng/L. However, the presence of CBZ at the effluent of DWTPs is common in different countries and plant layouts. In general, treatments for the removal of colloids and solids are indeed not deemed to be effective against CBZ due to its characteristics, while improved outcomes might be linked to chemical oxidation processes such as ozonation, adsorptive process by granular activated carbon (GAC) and membrane filtration [34].
Simazaki et al. (2015) found that the average removal of CBZ by plants that included ozonation and activated carbon was of 97 ± 2%, whereas the removal was reduced to 62 ± 49% where such processes were not applied [43]. The importance of the oxidation steps in the removal of CBZ is also underlined by Kim et al. (2020) [44]. Pulicharla et al. (2021) found instead the removal of CBZ to be as low as 0–40% even in DWTPs with a layout that included inter-ozonation, activated carbon and sand filtration [39].
High removal efficiencies were also recorded for DWTPs employing Granular Activated Carbon (GAC) and Biological Activated Carbon (BAC) in Italy [52], even though concentration was not always reduced to below the limit of quantification (LOQ). The high removal of CBZ by nanofiltration/reverse osmosis (NF/RO) was instead witnessed by Radjenović et al. (2008) [51] and Al-rifai et al. (2011) [54].
References
- Hai, F.; Yang, S.; Asif, M.; Sencadas, V.; Shawkat, S.; Sanderson-Smith, M.; Gorman, J.; Xu, Z.-Q.; Yamamoto, K. Carbamazepine as a Possible Anthropogenic Marker in Water: Occurrences, Toxicological Effects, Regulations and Removal by Wastewater Treatment Technologies. Water 2018, 10, 107.
- Kim, K.A.; Sae, O.O.; Park, P.W.; Park, J.Y. Effect of probenecid on the pharmacokinetics of carbamazepine in healthy subjects. Eur. J. Clin. Pharmacol. 2005, 61, 275–280.
- Yu, Z.; Peldszus, S.; Huck, P.M. Adsorption characteristics of selected pharmaceuticals and an endocrine disrupting compound-Naproxen, carbamazepine and nonylphenol-on activated carbon. Water Res. 2008, 42, 2873–2882.
- National Center for Biotechnology Information PubChem Compound Summary for CID 2554, Carbamazepine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Carbamazepine (accessed on 6 April 2021).
- Dullio, V.; von der Ohe, P.C. NORMAN Prioritisation Framework for Emerging Substances; NORMAN Association: Verneuil-en-Halatte, France, 2013.
- Williams, A.J.; Grulke, C.M.; Edwards, J.; McEachran, A.D.; Mansouri, K.; Baker, N.C.; Patlewicz, G.; Shah, I.; Wambaugh, J.F.; Judson, R.S.; et al. The CompTox Chemistry Dashboard: A community data resource for environmental chemistry. J. Cheminform. 2017, 9, 61.
- Seeley, I.H. Wastewater Engineering. In Public Works Engineering; Macmillan Education: London, UK, 1992; pp. 160–214. ISBN 9780073401188.
- Di Marcantonio, C.; Chiavola, A.; Dossi, S.; Cecchini, G.; Leoni, S.; Frugis, A.; Spizzirri, M.; Boni, M.R. Occurrence, seasonal variations and removal of Organic Micropollutants in 76 Wastewater Treatment Plants. Process. Saf. Environ. Prot. 2020, 141, 61–72.
- Martínez-Alcalá, I.; Guillén-Navarro, J.M.; Fernández-López, C. Pharmaceutical biological degradation, sorption and mass balance determination in a conventional activated-sludge wastewater treatment plant from Murcia, Spain. Chem. Eng. J. 2017, 316, 332–340.
- Min, X.; Li, W.; Wei, Z.; Spinney, R.; Dionysiou, D.D.; Seo, Y.; Tang, C.J.; Li, Q.; Xiao, R. Sorption and biodegradation of pharmaceuticals in aerobic activated sludge system: A combined experimental and theoretical mechanistic study. Chem. Eng. J. 2018, 342, 211–219.
- Tran, N.H.; Gin, K.Y.H. Occurrence and removal of pharmaceuticals, hormones, personal care products, and endocrine disrupters in a full-scale water reclamation plant. Sci. Total Environ. 2017, 599–600, 1503–1516.
- The European parliament and of the council regulation (EC) No 1907/2006 concerning reach. Off. J. Eur. Union 2006. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2007:136:0003:0280:en:PDF (accessed on 6 April 2021).
- Loos, R.; Carvalho, R.; António, D.C.; Comero, S.; Locoro, G.; Tavazzi, S.; Paracchini, B.; Ghiani, M.; Lettieri, T.; Blaha, L.; et al. EU-wide monitoring survey on emerging polar organic contaminants in wastewater treatment plant effluents. Water Res. 2013, 47, 6475–6487.
- Thiebault, T.; Boussafir, M.; Le Milbeau, C. Occurrence and removal efficiency of pharmaceuticals in an urban wastewater treatment plant: Mass balance, fate and consumption assessment. J. Environ. Chem. Eng. 2017, 5, 2894–2902.
- Subedi, B.; Kannan, K. Occurrence and fate of select psychoactive pharmaceuticals and antihypertensives in two wastewater treatment plants in New York State, USA. Sci. Total Environ. 2015, 514, 273–280.
- Rivera-Jaimes, J.A.; Postigo, C.; Melgoza-Alemán, R.M.; Aceña, J.; Barceló, D.; López de Alda, M. Study of pharmaceuticals in surface and wastewater from Cuernavaca, Morelos, Mexico: Occurrence and environmental risk assessment. Sci. Total Environ. 2018, 613–614, 1263–1274.
- Wu, M.; Xiang, J.; Que, C.; Chen, F.; Xu, G. Occurrence and fate of psychiatric pharmaceuticals in the urban water system of Shanghai, China. Chemosphere 2015, 138, 486–493.
- Wang, D.; Sui, Q.; Lu, S.G.; Zhao, W.T.; Qiu, Z.F.; Miao, Z.W.; Yu, G. Occurrence and removal of six pharmaceuticals and personal care products in a wastewater treatment plant employing anaerobic/anoxic/aerobic and UV processes in Shanghai, China. Environ. Sci. Pollut. Res. 2014, 21, 4276–4285.
- Yan, Q.; Gao, X.; Huang, L.; Gan, X.M.; Zhang, Y.X.; Chen, Y.P.; Peng, X.Y.; Guo, J.S. Occurrence and fate of pharmaceutically active compounds in the largest municipal wastewater treatment plant in Southwest China: Mass balance analysis and consumption back-calculated model. Chemosphere 2014, 99, 160–170.
- Yu, Y.; Wu, L.; Chang, A.C. Seasonal variation of endocrine disrupting compounds, pharmaceuticals and personal care products in wastewater treatment plants. Sci. Total Environ. 2013, 442, 310–316.
- Lajeunesse, A.; Smyth, S.A.; Barclay, K.; Sauvé, S.; Gagnon, C. Distribution of antidepressant residues in wastewater and biosolids following different treatment processes by municipal wastewater treatment plants in Canada. Water Res. 2012, 46, 5600–5612.
- Martínez-Alcalá, I.; Guillén-Navarro, J.M.; Lahora, A. Occurrence and fate of pharmaceuticals in a wastewater treatment plant from southeast of Spain and risk assessment. J. Environ. Manag. 2021, 279, 111565.
- Čelić, M.; Gros, M.; Farré, M.; Barceló, D.; Petrović, M. Pharmaceuticals as chemical markers of wastewater contamination in the vulnerable area of the Ebro Delta (Spain). Sci. Total Environ. 2019, 652, 952–963.
- Martín, J.; Camacho-Muñoz, D.; Santos, J.L.; Aparicio, I.; Alonso, E. Occurrence of pharmaceutical compounds in wastewater and sludge from wastewater treatment plants: Removal and ecotoxicological impact of wastewater discharges and sludge disposal. J. Hazard. Mater. 2012, 239–240, 40–47.
- Komesli, O.T.; Muz, M.; Ak, M.S.; Bakirdere, S.; Gokcay, C.F. Occurrence, fate and removal of endocrine disrupting compounds (EDCs) in Turkish wastewater treatment plants. Chem. Eng. J. 2015, 277, 202–208.
- Verlicchi, P.; Al Aukidy, M.; Jelic, A.; Petrović, M.; Barceló, D. Comparison of measured and predicted concentrations of selected pharmaceuticals in wastewater and surface water: A case study of a catchment area in the Po Valley (Italy). Sci. Total Environ. 2014, 470–471, 844–854.
- Castiglioni, S.; Zuccato, E.; Fattore, E.; Riva, F.; Terzaghi, E.; Koenig, R.; Principi, P.; Di Guardo, A. Micropollutants in Lake Como water in the context of circular economy: A snapshot of water cycle contamination in a changing pollution scenario. J. Hazard. Mater. 2020, 384, 121441.
- Golovko, O.; Kumar, V.; Fedorova, G.; Randak, T.; Grabic, R. Seasonal changes in antibiotics, antidepressants/psychiatric drugs, antihistamines and lipid regulators in a wastewater treatment plant. Chemosphere 2014, 111, 418–426.
- Gurke, R.; Rößler, M.; Marx, C.; Diamond, S.; Schubert, S.; Oertel, R.; Fauler, J. Occurrence and removal of frequently prescribed pharmaceuticals and corresponding metabolites in wastewater of a sewage treatment plant. Sci. Total Environ. 2015, 532, 762–770.
- Bahlmann, A.; Brack, W.; Schneider, R.J.; Krauss, M. Carbamazepine and its metabolites in wastewater: Analytical pitfalls and occurrence in Germany and Portugal. Water Res. 2014, 57, 104–114.
- Paíga, P.; Santos, L.H.M.L.M.; Ramos, S.; Jorge, S.; Silva, J.G.; Delerue-Matos, C. Presence of pharmaceuticals in the Lis river (Portugal): Sources, fate and seasonal variation. Sci. Total Environ. 2016, 573, 164–177.
- K’oreje, K.O.; Okoth, M.; Van Langenhove, H.; Demeestere, K. Occurrence and treatment of contaminants of emerging concern in the African aquatic environment: Literature review and a look ahead. J. Environ. Manag. 2020, 254, 109752.
- Balakrishna, K.; Rath, A.; Praveenkumarreddy, Y.; Guruge, K.S.; Subedi, B. A review of the occurrence of pharmaceuticals and personal care products in Indian water bodies. Ecotoxicol. Environ. Saf. 2017, 137, 113–120.
- Couto, C.F.; Lange, L.C.; Amaral, M.C.S. Occurrence, fate and removal of pharmaceutically active compounds (PhACs) in water and wastewater treatment plants—A review. J. Water Process. Eng. 2019, 32, 100927.
- Krzeminski, P.; Tomei, M.C.; Karaolia, P.; Langenhoff, A.; Almeida, C.M.R.; Felis, E.; Gritten, F.; Andersen, H.R.; Fernandes, T.; Manaia, C.M.; et al. Performance of secondary wastewater treatment methods for the removal of contaminants of emerging concern implicated in crop uptake and antibiotic resistance spread: A review. Sci. Total Environ. 2019, 648, 1052–1081.
- Jelic, A.; Gros, M.; Ginebreda, A.; Cespedes-Sánchez, R.; Ventura, F.; Petrovic, M.; Barcelo, D. Occurrence, partition and removal of pharmaceuticals in sewage water and sludge during wastewater treatment. Water Res. 2011, 45, 1165–1176.
- Yang, Y.; Ok, Y.S.; Kim, K.H.; Kwon, E.E.; Tsang, Y.F. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: A review. Sci. Total Environ. 2017, 596–597, 303–320.
- Kleywegt, S.; Pileggi, V.; Yang, P.; Hao, C.; Zhao, X.; Rocks, C.; Thach, S.; Cheung, P.; Whitehead, B. Pharmaceuticals, hormones and bisphenol A in untreated source and finished drinking water in Ontario, Canada—Occurrence and treatment efficiency. Sci. Total Environ. 2011, 409, 1481–1488.
- Pulicharla, R.; Proulx, F.; Behmel, S.; Sérodes, J.B.; Rodriguez, M.J. Occurrence and seasonality of raw and drinking water contaminants of emerging interest in five water facilities. Sci. Total Environ. 2021, 751, 141748.
- Kim, H.; Homan, M. Evaluation of pharmaceuticals and personal care products (PPCPs) in drinking water originating from Lake Erie. J. Great Lakes Res. 2020, 46, 1321–1330.
- Wang, C.; Shi, H.; Adams, C.D.; Gamagedara, S.; Stayton, I.; Timmons, T.; Ma, Y. Investigation of pharmaceuticals in Missouri natural and drinking water using high performance liquid chromatography-tandem mass spectrometry. Water Res. 2011, 45, 1818–1828.
- Glassmeyer, S.T.; Furlong, E.T.; Kolpin, D.W.; Batt, A.L.; Benson, R.; Boone, J.S.; Conerly, O.; Donohue, M.J.; King, D.N.; Kostich, M.S.; et al. Nationwide reconnaissance of contaminants of emerging concern in source and treated drinking waters of the United States. Sci. Total Environ. 2017, 581–582, 909–922.
- Simazaki, D.; Kubota, R.; Suzuki, T.; Akiba, M.; Nishimura, T.; Kunikane, S. Occurrence of selected pharmaceuticals at drinking water purification plants in Japan and implications for human health. Water Res. 2015, 76, 187–200.
- Kim, K.Y.; Ekpe, O.D.; Lee, H.J.; Oh, J.E. Perfluoroalkyl substances and pharmaceuticals removal in full-scale drinking water treatment plants. J. Hazard. Mater. 2020, 400, 123235.
- Nam, S.W.; Jo, B.-I.; Yoon, Y.; Zoh, K.D. Occurrence and removal of selected micropollutants in a water treatment plant. Chemosphere 2014, 95, 156–165.
- Cai, M.Q.; Wang, R.; Feng, L.; Zhang, L.Q. Determination of selected pharmaceuticals in tap water and drinking water treatment plant by high-performance liquid chromatography-triple quadrupole mass spectrometer in Beijing, China. Environ. Sci. Pollut. Res. 2015, 22, 1854–1867.
- Lin, T.; Yu, S.; Chen, W. Occurrence, removal and risk assessment of pharmaceutical and personal care products (PPCPs) in an advanced drinking water treatment plant (ADWTP) around Taihu Lake in China. Chemosphere 2016, 152, 1–9.
- Karki, A.J.; Cappelli, P.; Dirks, C.; Pekar, H.; Hellenäs, K.E.; Rosén, J.; Westerberg, E. New efficient methodology for screening of selected organic micropollutants in raw- and drinking water from 90 Swedish water treatment plants. Sci. Total Environ. 2020, 724, 138069.
- Tröger, R.; Köhler, S.J.; Franke, V.; Bergstedt, O.; Wiberg, K. A case study of organic micropollutants in a major Swedish water source—Removal efficiency in seven drinking water treatment plants and influence of operational age of granulated active carbon filters. Sci. Total Environ. 2020, 706, 135680.
- Azzouz, A.; Ballesteros, E. Influence of seasonal climate differences on the pharmaceutical, hormone and personal care product removal efficiency of a drinking water treatment plant. Chemosphere 2013, 93, 2046–2054.
- Radjenović, J.; Petrović, M.; Ventura, F.; Barceló, D. Rejection of pharmaceuticals in nanofiltration and reverse osmosis membrane drinking water treatment. Water Res. 2008, 42, 3601–3610.
- Valbonesi, P.; Profita, M.; Vasumini, I.; Fabbri, E. Contaminants of emerging concern in drinking water: Quality assessment by combining chemical and biological analysis. Sci. Total Environ. 2020, 758, 143624.
- de Jesus Gaffney, V.; Almeida, C.M.M.; Rodrigues, A.; Ferreira, E.; Benoliel, M.J.; Cardoso, V.V. Occurrence of pharmaceuticals in a water supply system and related human health risk assessment. Water Res. 2015, 72, 199–208.
- Al-Rifai, J.H.; Khabbaz, H.; Schäfer, A.I. Removal of pharmaceuticals and endocrine disrupting compounds in a water recycling process using reverse osmosis systems. Sep. Purif. Technol. 2011, 77, 60–67.
More
Information
Subjects:
Engineering, Environmental
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.8K
Entry Collection:
Wastewater Treatment
Revisions:
2 times
(View History)
Update Date:
12 Nov 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




