Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Anna Park | + 2045 word(s) | 2045 | 2021-10-27 06:14:06 | | | |
| 2 | Catherine Yang | Meta information modification | 2045 | 2021-11-10 02:02:37 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Park, A. Adipose Tissue Mitochondria. Encyclopedia. Available online: https://encyclopedia.pub/entry/15848 (accessed on 07 February 2026).
Park A. Adipose Tissue Mitochondria. Encyclopedia. Available at: https://encyclopedia.pub/entry/15848. Accessed February 07, 2026.
Park, Anna. "Adipose Tissue Mitochondria" Encyclopedia, https://encyclopedia.pub/entry/15848 (accessed February 07, 2026).
Park, A. (2021, November 10). Adipose Tissue Mitochondria. In Encyclopedia. https://encyclopedia.pub/entry/15848
Park, Anna. "Adipose Tissue Mitochondria." Encyclopedia. Web. 10 November, 2021.
Copy Citation
Mitochondria play a key role in maintaining energy homeostasis in metabolic tissues, including adipose tissues. The two main types of adipose tissues are the white adipose tissue (WAT) and the brown adipose tissue (BAT). WAT primarily stores excess energy, whereas BAT is predominantly responsible for energy expenditure by non-shivering thermogenesis through the mitochondria. WAT in response to appropriate stimuli such as cold exposure and β-adrenergic agonist undergoes browning wherein it acts as BAT, which is characterized by the presence of a higher number of mitochondria.
mitochondria
mitochondrial dysfunction
1. Introduction
Adipose tissue is the major organ that controls the overall energy homeostasis in a living organism. Briefly, in excess energy conditions, the adipose tissue stores the superabundant nutrients in the form of triglycerides, whereas during scarcity of energy, it supplies the nutrients to other tissues through lipolysis [1]. In mammals, there are two different types of adipose tissues, namely, white adipose tissue (WAT) and brown adipose tissue (BAT). Interestingly, WAT and BAT have opposite functions. The WAT stores excess energy as triglycerides, while the BAT is specialized in the dissipation of energy through heat production for maintaining the body temperature and energy consumption. Recent studies have demonstrated that beige adipocytes sporadically reside with white adipocytes and emerge in response to certain environmental cues. Beige adipocytes have the antagonistic functions of WAT and agonistic functions of BAT at the same time. In the basal state, beige adipocytes act as white adipocytes; however, when given signals that require heat production through energy consumption such as cold stimulation, these cells demonstrate BAT-like function and morphology [2].
2. Role of the Mitochondria in Adipocytes
2.1. Adipocyte Differentiation
Mitochondria are key organelles that control the physiological role of adipocytes such as adipocyte differentiation, lipid homeostasis, insulin sensitivity, oxidative capacity, adaptive thermogenesis, and browning of WATs (Figure 1). In particular, adipocyte differentiation is characterized by an induction in mitochondrial metabolism. During adipogenesis, preadipocytes undergo sequential transcriptional regulation by adipogenic regulators such as peroxisome proliferator-activated receptor-γ (PPARγ), CCAAT-enhancer-binding proteins (C/EBPs), and PPARγ coactivator 1-α (PGC1α), thereby leading to the promotion of mitochondrial biogenesis [3]. In addition, mitochondrial remodeling is involved in the quantitative and qualitative changes of the mitochondria during adipocyte differentiation [4]. Within 4 days of adipogenic induction of 3T3-L1, which are immortalized white preadipocytes, the expression of mitochondrial proteins and the number of mitochondria increases dramatically and is accompanied by qualitative changes in the mitochondrial composition, including pyruvate carboxylase and aconitase, which are involved in fatty acid metabolism [5]. Moreover, the increased oxygen consumption rate of the differentiated adipocytes compared to that of the preadipocytes is clear evidence that mitochondria are activated during adipocyte differentiation [6].
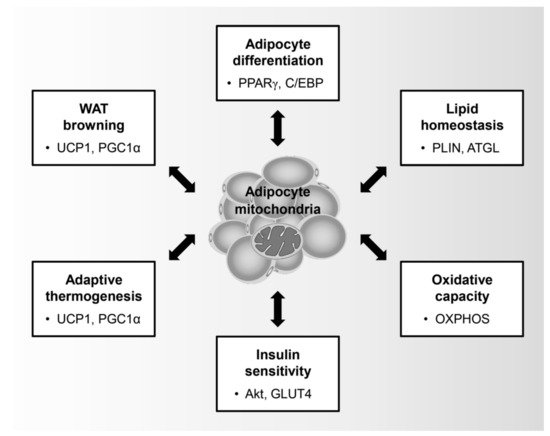
Figure 1. Physiological role of adipocyte mitochondria: Mitochondria in adipocytes regulate adipocyte differentiation, lipid homeostasis, oxidative capacity, insulin sensitivity, adaptive thermogenesis, and browning of white adipose tissues. Peroxisome proliferator-activated receptor-γ (PPARγ); CCAAT-enhancer-binding protein (C/EBP); Perilipin (PLIN); Adipose triglyceride lipase (ATGL); Oxidative phosphorylation (OXPHOS); Glucose transporter type 4 (GLUT4); Uncoupling protein-1 (UCP1); PPARγ coactivator 1-α (PGC1α).
Previous studies have shown that the induction of mitochondria is essential for ATP production, which is necessary for increasing the metabolic activity during the adipogenic program. Despite promoted mitochondrial activity during adipocyte differentiation, the ATP content is decreased by high ATP consumption processes such as lipogenesis [5]. In the adipogenic program, the TCA cycle produces reducing equivalents such as nicotinamide adenine dinucleotide and flavin adenine dinucleotide and achieves oxidation of the acetyl-coenzyme A—an important metabolite produced from the catabolism of glucose and fatty acids [7]. Recently, it was demonstrated that mitochondrial dysfunction adversely affects adipocyte differentiation. Specific inhibitors of mitochondrial di- and tricarboxylate carriers prevent the differentiation of 3T3-L1 adipocytes, suggesting that mitochondria play an essential role in adipogenesis [8].
Mitochondrial reactive oxygen species (ROS) produced by the respiratory chain act as a second messenger and plays a crucial role in numerous cellular signaling pathways inside and outside the mitochondria. Mitochondrial ROS produced by OXPHOS complex III is essential for the initiation of adipocyte differentiation of human mesenchymal stem cells, whereas adipogenesis is inhibited by mitochondria-targeted antioxidants [9]. However, high doses of ROS negatively regulate the proliferation and differentiation of adipocytes [10]. In particular, increased ROS production by rotenone, a complex I inhibitor, or oligomycin, a F0-F1 ATP synthase inhibitor, has been reported to prevent the adipocyte differentiation of human mesenchymal stem cells [11][12]. A number of studies support the importance of mitochondria in adipocyte differentiation. Therefore, it is rational to target mitochondria for the treatment of obesity caused by the abnormal control of adipocyte differentiation.
2.2. Lipid Homeostasis and Oxidative Capacity
Adipose tissues play a key role in maintaining whole-body lipid homeostasis. Adipose tissues store lipids in the form of triglycerides in lipid droplets, thereby preventing ectopic lipid accumulation in other organs. Perilipin (PLIN), a lipid droplet coat protein, is required for the stable storage of lipid metabolites in adipocytes [13]. The triglycerides are broken into fatty acids and glycerol by adipose triglyceride lipase (ATGL)-mediated lipolysis, sequentially used as energy [14]. In obesity, excessive lipogenesis increases the total lipid pool, leading to insulin resistance and hyperglycemia [15]. It has been reported that the mitochondrial dysfunction with an OXPHOS complex III inhibitor causes triglyceride accumulation by inducing excessive lipogenesis in 3T3-L1 preadipocytes [16]. In particular, mitochondria in brown adipocytes are involved in lipid homeostasis by controlling fatty acid oxidation, which is one of the key pathways for lipid clearance [17]. Therefore, ectopic lipid accumulation is promoted by reducing fatty acid oxidation and energy expenditure due to mitochondrial dysfunction in the brown adipocytes of people with obesity and metabolic diseases [18].
A previous study showed that the abundance of mitochondrial populations is halved in adipocytes isolated from epididymal adipose tissues from obese and diabetic ob/ob mice [19]. Moreover, two obese and diabetic mouse models—the genetic model db/db mice and the dietary high-fat diet-fed mice—showed a decreased level of mitochondrial biogenesis regulators, including PGC1α and estrogen-related receptor-α, resulting in the loss of mitochondrial mass and structure [20]. Furthermore, mitochondrial dysfunction caused by tumor necrosis factor-α (TNF-α), which is an inflammatory cytokine that increases in obesity, leads to smaller and condensed mitochondria and inhibits intracellular ATP synthesis in 3T3-L1 adipocytes [21]. In addition, the expression of genes involved in OXPHOS complexes and fatty acid oxidation is decreased by TNF-α in primary adipocytes [22]. Under various pathological conditions, the oxidative capacity, biogenesis, density, and dynamics of mitochondria could be disrupted in the adipocytes, resulting in the development of obesity and metabolic diseases.
The main function of mitochondria in the adipocytes is to produce ATP to support a variety of metabolic pathways, including triglyceride synthesis, gluconeogenesis, and fatty acid re-esterification [7]. Dysregulation of mitochondria destroys the oxidative capacity and eventually fails to generate ATP. The mitochondrial membrane potential and the activity of the respiratory chain complexes are reported to be significantly decreased in the subcutaneous adipose tissues of obese subjects [23]. Moreover, the expression of the genes involved in mitochondrial oxidative pathways, including fatty acid oxidation, TCA cycle, ketogenesis, ketolysis, and branched chain amino acid degradation, is suppressed in obese individuals. One study showed that the protein levels of OXPHOS complexes III, IV, and V are also decreased in the adipose tissues of obese subjects [24]. Inverse correlations have been reported between body mass index and mitochondrial respiratory capacity in human adipose tissues. Furthermore, ADP-stimulated mitochondrial respiration, mtDNA copy number, and OXPHOS complex protein expression are dramatically decreased in the adipose tissues of obese subjects [25]. Thus, previous studies have shown the correlation between mitochondrial oxidative capacity in the adipocytes and systemic energy metabolism.
2.3. Glucose Utilization and Insulin Sensitivity
Insulin is a major anabolic hormone also involved in the regulation of energy and lipid metabolism through the phosphatidylinositol 3-kinase (PI3K)–Akt signaling pathway. The primary function of insulin is to allow glucose uptake into muscle cells and adipocytes to lower the blood glucose level. Binding of insulin to its receptor on adipocytes triggers the phosphorylation and activation of insulin receptor substrate (IRS), and it forms a docking site for PI3K at the cell membrane. When docked, PI3K converts phosphatidylinositol 4,5-bisphosphate (PIP2) to phosphatidylinositol 3,4,5-trisphosphate (PIP3). Subsequently PIP3 activates phosphoinositide-dependent protein kinase 1 (PDK1) and Akt. Akt stimulates translocation of glucose transporter type 4 (GLUT4) translocation to cell membranes, thus allowing glucose to enter the cell [26]. Adipocyte-specific Glut4 knockout mice exhibit systemic glucose intolerance and insulin resistance [27].
Recently, mitochondria in adipocytes have been suggested to play a role in regulating insulin sensitivity. Treatments with respiratory inhibitors or uncoupling reagents that block mitochondrial function were shown to reduce insulin-stimulated glucose uptake in adipocytes [28]. In high-fat-diet-fed rats, mitochondrial biogenesis and the copy number of mitochondrial DNA (mtDNA) were decreased in epididymal adipose tissues, accompanied by hyperglycemia [29]. Further, high levels of glucose and/or free fatty acid attenuated insulin-stimulated glucose uptake by the dysfunctional mitochondria in the adipocytes. This glucose and/or free fatty acid-mediated reduction of glucose uptake was recovered by PGC1α overexpression, which is involved in the normalization of mitochondria [30]. The CR6-interacting factor-1 (Crif1) is a key translational factor responsible for the protein expression of mitochondrial OXPHOS complexes. Crif1 ablation in adipocytes causes loss of OXPHOS complex subunits and respiratory complexes, sequentially resulting in whole-body insulin resistance [31]. These studies suggest that mitochondria in adipocytes are required for regulating glucose use through insulin signaling pathway.
2.4. Thermogenesis and WAT Browning
Recent studies have focused on the role of mitochondria in brown adipocytes that promotes energy consumption via adaptive thermogenesis [32]. Activation of mitochondria in brown adipocytes accelerates heat generation by increasing the inner mitochondrial membrane UCP1 [33]. Activated UCP1 uncouples the electron transport of the respiratory chain, thereby blocking ATP production and dissipating energy in the form of heat [34]. It has been demonstrated that the activity of UCP1 and thermogenesis in mouse BAT is associated with body-weight control and energy homeostasis [35]. Moreover, the adipocyte UCP1 expression is reduced in obese subjects, while metabolic complications are improved with UCP1 activation by various environmental and pharmacological stimuli [36]. Strategies for combating metabolic diseases through the activation of UCP1 have been proposed; for instance, eight subjects with diabetes reported a 43% increase in insulin sensitivity when UCP1 was activated by cold acclimation (14–15 °C) for 10 days [37]. Thus, adaptive thermogenesis-dependent fat burn has emerged as a plausible and safe strategy for relieving the metabolic syndrome.
The most important and well-studied factor that activates thermogenesis is norepinephrine (NE). NE affects cell proliferation and differentiation as well as thermogenesis of brown adipocytes. NE increases cyclic adenosine monophosphate levels via the β3-adrenergic receptor and activates protein kinase A, subsequently leading to lipolysis-mediated production of free fatty acids, one of the acute substrates of thermogenesis [38]. PGC1α has been reported to be essential for thermogenic activation by cold-induced and β3-adrenergic agonists in brown adipocytes [39]. PGC1α was first identified as a cold-induced interacting partner of PPARγ in BAT [40]. Mechanistically, PGC1α promotes mitochondrial biogenesis and oxidative metabolism by inducing UCP1 transcription [38]. Notably, thermogenic activation in brown adipocytes is accompanied by mitochondrial fission and depolarization [41]. These studies suggest that thermogenesis might be regulated by mitochondrial dynamics in brown adipocytes.
A recent study showed that white adipocytes, which have low UCP1 expression and low mitochondrial contents, sometimes act as inducible thermogenic adipocytes that increase UCP1 expression and energy consumption under certain conditions such as cold exposure and β-adrenergic activation. Beige adipocytes (also called “brite”, “brown-like”, or “inducible brown”) are generated by the browning of WAT [42] (Figure 2). Beige adipocytes exhibit UCP1-dependent thermogenesis, and these mitochondria use lipids or carbohydrates as substrates, similar to brown adipocytes [43]. Chronic exercise decreases body weight and fat mass by inducing browning of subcutaneous WAT [44]. In addition, Irisin, a PGC1α-dependent myokine induced by exercise, has been shown to increase energy expenditure and to improve insulin sensitivity by activation of WAT browning [45]. In adults with low compositions of BAT, increased energy expenditure through WAT browning is an attractive strategy for preventing metabolic diseases. Taken together, mitochondria are essential organelles for maintaining the function of adipocytes in metabolic homeostasis.
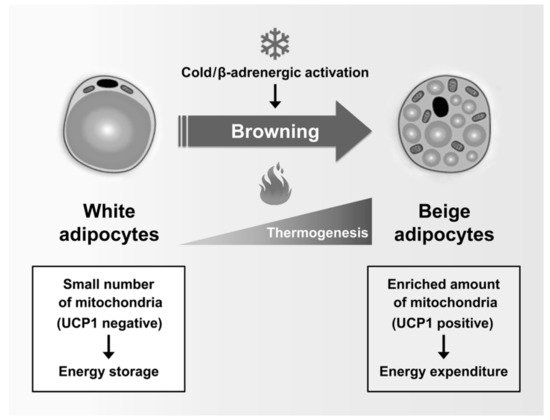
Figure 2. Regulation of adipose tissue browning: Beige adipocytes are generated by the browning of white adipose tissues in response to numerous stimuli, including cold exposure and activation of β-adrenergic receptors. Subsequently, the transcriptional machinery of the browning program activates the expression of characteristic thermogenic genes, leading to the formation of beige adipocytes. Similar to brown adipocytes, beige adipocytes are rich in mitochondria that express UCP1 and can achieve thermogenesis. Notably, beige adipocytes primarily contribute to energy expenditure rather than to energy storage.
References
- Granneman, J.G.; Li, P.; Zhu, Z.; Lu, Y. Metabolic and cellular plasticity in white adipose tissue I: Effects of beta3-adrenergic receptor activation. Am. J. Physiol. Endocrinol. Metab. 2005, 289, E608–E616.
- Park, A.; Kim, W.K.; Bae, K.H. Distinction of white, beige and brown adipocytes derived from mesenchymal stem cells. World J. Stem Cells 2014, 6, 33–42.
- Rosen, E.D.; Spiegelman, B.M. Molecular regulation of adipogenesis. Annu. Rev. Cell Dev. Biol. 2000, 16, 145–171.
- Forni, M.F.; Peloggia, J.; Trudeau, K.; Shirihai, O.; Kowaltowski, A.J. Murine Mesenchymal Stem Cell Commitment to Differentiation Is Regulated by Mitochondrial Dynamics. Stem Cells 2016, 34, 743–755.
- Wilson-Fritch, L.; Burkart, A.; Bell, G.; Mendelson, K.; Leszyk, J.; Nicoloro, S.; Czech, M.; Corvera, S. Mitochondrial biogenesis and remodeling during adipogenesis and in response to the insulin sensitizer rosiglitazone. Mol. Cell Biol. 2003, 23, 1085–1094.
- Si, Y.; Palani, S.; Jayaraman, A.; Lee, K. Effects of forced uncoupling protein 1 expression in 3T3-L1 cells on mitochondrial function and lipid metabolism. J. Lipid Res. 2007, 48, 826–836.
- De Pauw, A.; Tejerina, S.; Raes, M.; Keijer, J.; Arnould, T. Mitochondrial (dys)function in adipocyte (de)differentiation and systemic metabolic alterations. Am. J. Pathol. 2009, 175, 927–939.
- Kajimoto, K.; Terada, H.; Baba, Y.; Shinohara, Y. Essential role of citrate export from mitochondria at early differentiation stage of 3T3-L1 cells for their effective differentiation into fat cells, as revealed by studies using specific inhibitors of mitochondrial di- and tricarboxylate carriers. Mol. Genet. Metab. 2005, 85, 46–53.
- Tormos, K.V.; Anso, E.; Hamanaka, R.B.; Eisenbart, J.; Joseph, J.; Kalyanaraman, B.; Chandel, N.S. Mitochondrial complex III ROS regulate adipocyte differentiation. Cell Metab. 2011, 14, 537–544.
- Boneh, A. Regulation of mitochondrial oxidative phosphorylation by second messenger-mediated signal transduction mechanisms. Cell Mol. Life Sci. 2006, 63, 1236–1248.
- Carriere, A.; Carmona, M.C.; Fernandez, Y.; Rigoulet, M.; Wenger, R.H.; Penicaud, L.; Casteilla, L. Mitochondrial reactive oxygen species control the transcription factor CHOP-10/GADD153 and adipocyte differentiation: A mechanism for hypoxia-dependent effect. J. Biol. Chem. 2004, 279, 40462–40469.
- Zhang, Y.; Marsboom, G.; Toth, P.T.; Rehman, J. Mitochondrial respiration regulates adipogenic differentiation of human mesenchymal stem cells. PLoS ONE 2013, 8, e77077.
- Brasaemle, D.L. Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J. Lipid Res. 2007, 48, 2547–2559.
- Duncan, R.E.; Ahmadian, M.; Jaworski, K.; Sarkadi-Nagy, E.; Sul, H.S. Regulation of lipolysis in adipocytes. Annu. Rev. Nutr. 2007, 27, 79–101.
- Sun, K.; Kusminski, C.M.; Scherer, P.E. Adipose tissue remodeling and obesity. J. Clin. Investig. 2011, 121, 2094–2101.
- Vankoningsloo, S.; Piens, M.; Lecocq, C.; Gilson, A.; De Pauw, A.; Renard, P.; Demazy, C.; Houbion, A.; Raes, M.; Arnould, T. Mitochondrial dysfunction induces triglyceride accumulation in 3T3-L1 cells: role of fatty acid beta-oxidation and glucose. J. Lipid Res. 2005, 46, 1133–1149.
- Vamecq, J.; Dessein, A.F.; Fontaine, M.; Briand, G.; Porchet, N.; Latruffe, N.; Andreolotti, P.; Cherkaoui-Malki, M. Mitochondrial dysfunction and lipid homeostasis. Curr. Drug Metab. 2012, 13, 1388–1400.
- Bournat, J.C.; Brown, C.W. Mitochondrial dysfunction in obesity. Curr. Opin. Endocrinol. Diabetes Obes. 2010, 17, 446–452.
- Wilson-Fritch, L.; Nicoloro, S.; Chouinard, M.; Lazar, M.A.; Chui, P.C.; Leszyk, J.; Straubhaar, J.; Czech, M.P.; Corvera, S. Mitochondrial remodeling in adipose tissue associated with obesity and treatment with rosiglitazone. J. Clin. Investig. 2004, 114, 1281–1289.
- Rong, J.X.; Qiu, Y.; Hansen, M.K.; Zhu, L.; Zhang, V.; Xie, M.; Okamoto, Y.; Mattie, M.D.; Higashiyama, H.; Asano, S.; et al. Adipose mitochondrial biogenesis is suppressed in db/db and high-fat diet-fed mice and improved by rosiglitazone. Diabetes 2007, 56, 1751–1760.
- Chen, X.H.; Zhao, Y.P.; Xue, M.; Ji, C.B.; Gao, C.L.; Zhu, J.G.; Qin, D.N.; Kou, C.Z.; Qin, X.H.; Tong, M.L.; et al. TNF-alpha induces mitochondrial dysfunction in 3T3-L1 adipocytes. Mol. Cell Endocrinol. 2010, 328, 63–69.
- Dahlman, I.; Forsgren, M.; Sjogren, A.; Nordstrom, E.A.; Kaaman, M.; Naslund, E.; Attersand, A.; Arner, P. Downregulation of electron transport chain genes in visceral adipose tissue in type 2 diabetes independent of obesity and possibly involving tumor necrosis factor-alpha. Diabetes 2006, 55, 1792–1799.
- Chattopadhyay, M.; Guhathakurta, I.; Behera, P.; Ranjan, K.R.; Khanna, M.; Mukhopadhyay, S.; Chakrabarti, S. Mitochondrial bioenergetics is not impaired in nonobese subjects with type 2 diabetes mellitus. Metabolism 2011, 60, 1702–1710.
- Heinonen, S.; Buzkova, J.; Muniandy, M.; Kaksonen, R.; Ollikainen, M.; Ismail, K.; Hakkarainen, A.; Lundbom, J.; Lundbom, N.; Vuolteenaho, K.; et al. Impaired Mitochondrial Biogenesis in Adipose Tissue in Acquired Obesity. Diabetes 2015, 64, 3135–3145.
- Fischer, B.; Schottl, T.; Schempp, C.; Fromme, T.; Hauner, H.; Klingenspor, M.; Skurk, T. Inverse relationship between body mass index and mitochondrial oxidative phosphorylation capacity in human subcutaneous adipocytes. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E380–E387.
- Taniguchi, C.M.; Emanuelli, B.; Kahn, C.R. Critical nodes in signalling pathways: insights into insulin action. Nat. Rev. Mol. Cell Biol. 2006, 7, 85–96.
- Abel, E.D.; Peroni, O.; Kim, J.K.; Kim, Y.B.; Boss, O.; Hadro, E.; Minnemann, T.; Shulman, G.I.; Kahn, B.B. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature 2001, 409, 729–733.
- Wang, C.H.; Wang, C.C.; Huang, H.C.; Wei, Y.H. Mitochondrial dysfunction leads to impairment of insulin sensitivity and adiponectin secretion in adipocytes. FEBS J. 2013, 280, 1039–1050.
- Sutherland, L.N.; Capozzi, L.C.; Turchinsky, N.J.; Bell, R.C.; Wright, D.C. Time course of high-fat diet-induced reductions in adipose tissue mitochondrial proteins: potential mechanisms and the relationship to glucose intolerance. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1076–E1083.
- Gao, C.L.; Zhu, C.; Zhao, Y.P.; Chen, X.H.; Ji, C.B.; Zhang, C.M.; Zhu, J.G.; Xia, Z.K.; Tong, M.L.; Guo, X.R. Mitochondrial dysfunction is induced by high levels of glucose and free fatty acids in 3T3-L1 adipocytes. Mol. Cell Endocrinol. 2010, 320, 25–33.
- Ryu, M.J.; Kim, S.J.; Kim, Y.K.; Choi, M.J.; Tadi, S.; Lee, M.H.; Lee, S.E.; Chung, H.K.; Jung, S.B.; Kim, H.J.; et al. Crif1 deficiency reduces adipose OXPHOS capacity and triggers inflammation and insulin resistance in mice. PLoS Genet. 2013, 9, e1003356.
- Wang, W.; Seale, P. Control of brown and beige fat development. Nat. Rev. Mol. Cell Biol. 2016, 17, 691–702.
- Nicholls, D.G.; Locke, R.M. Thermogenic mechanisms in brown fat. Physiol. Rev. 1984, 64, 1–64.
- Zafrir, B. Brown adipose tissue: Research milestones of a potential player in human energy balance and obesity. Horm. Metab. Res. 2013, 45, 774–785.
- Stanford, K.I.; Middelbeek, R.J.; Townsend, K.L.; An, D.; Nygaard, E.B.; Hitchcox, K.M.; Markan, K.R.; Nakano, K.; Hirshman, M.F.; Tseng, Y.H.; et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J. Clin. Investig. 2013, 123, 215–223.
- Cypess, A.M.; Kahn, C.R. Brown fat as a therapy for obesity and diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 2010, 17, 143–149.
- Hanssen, M.J.; Hoeks, J.; Brans, B.; van der Lans, A.A.; Schaart, G.; van den Driessche, J.J.; Jorgensen, J.A.; Boekschoten, M.V.; Hesselink, M.K.; Havekes, B.; et al. Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nat. Med. 2015, 21, 863–865.
- Harms, M.; Seale, P. Brown and beige fat: development, function and therapeutic potential. Nat. Med. 2013, 19, 1252–1263.
- Uldry, M.; Yang, W.; St-Pierre, J.; Lin, J.; Seale, P.; Spiegelman, B.M. Complementary action of the PGC-1 coactivators in mitochondrial biogenesis and brown fat differentiation. Cell Metab. 2006, 3, 333–341.
- Puigserver, P.; Wu, Z.; Park, C.W.; Graves, R.; Wright, M.; Spiegelman, B.M. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 1998, 92, 829–839.
- Wikstrom, J.D.; Mahdaviani, K.; Liesa, M.; Sereda, S.B.; Si, Y.; Las, G.; Twig, G.; Petrovic, N.; Zingaretti, C.; Graham, A.; et al. Hormone-induced mitochondrial fission is utilized by brown adipocytes as an amplification pathway for energy expenditure. EMBO J. 2014, 33, 418–436.
- Giralt, M.; Villarroya, F. White, brown, beige/brite: different adipose cells for different functions? Endocrinology 2013, 154, 2992–3000.
- Shabalina, I.G.; Petrovic, N.; de Jong, J.M.; Kalinovich, A.V.; Cannon, B.; Nedergaard, J. UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell Rep. 2013, 5, 1196–1203.
- De Matteis, R.; Lucertini, F.; Guescini, M.; Polidori, E.; Zeppa, S.; Stocchi, V.; Cinti, S.; Cuppini, R. Exercise as a new physiological stimulus for brown adipose tissue activity. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 582–590.
- Bostrom, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Bostrom, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468.
More
Information
Subjects:
Biology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Entry Collection:
Biopharmaceuticals Technology
Revisions:
2 times
(View History)
Update Date:
10 Nov 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




