Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maria-del-Carmen Cardenas-Aguayo | + 2199 word(s) | 2199 | 2021-11-01 09:26:41 | | | |
| 2 | Camila Xu | Meta information modification | 2199 | 2021-11-05 01:42:53 | | | | |
| 3 | Lindsay Dong | Meta information modification | 2199 | 2021-11-12 02:32:38 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Cardenas-Aguayo, M. α-Synuclein. Encyclopedia. Available online: https://encyclopedia.pub/entry/15704 (accessed on 06 March 2026).
Cardenas-Aguayo M. α-Synuclein. Encyclopedia. Available at: https://encyclopedia.pub/entry/15704. Accessed March 06, 2026.
Cardenas-Aguayo, Maria-Del-Carmen. "α-Synuclein" Encyclopedia, https://encyclopedia.pub/entry/15704 (accessed March 06, 2026).
Cardenas-Aguayo, M. (2021, November 04). α-Synuclein. In Encyclopedia. https://encyclopedia.pub/entry/15704
Cardenas-Aguayo, Maria-Del-Carmen. "α-Synuclein." Encyclopedia. Web. 04 November, 2021.
Copy Citation
The α-syn, encoded by the SNCA1/PARK1 gene, is a ubiquitous protein that is abundantly expressed in kidneys and blood cells, but highly enriched in the brain, particularly in the presynaptic terminals of the neocortex, hippocampus, substantia nigra (SN), thalamus, and cerebellum. Interestingly, it has been found expressed in the cytoplasm of astrocytes and oligodendrocytes in healthy individuals.
α-synuclein
neuroinflammation
neurotrophic factors
1. Structure and Physiology of α-Synuclein
The α-syn, encoded by the SNCA1/PARK1 gene, is a ubiquitous protein that is abundantly expressed in kidneys and blood cells [1], but highly enriched in the brain, particularly in the presynaptic terminals of the neocortex, hippocampus, substantia nigra (SN), thalamus, and cerebellum. Interestingly, it has been found expressed in the cytoplasm of astrocytes and oligodendrocytes in healthy individuals [2][3]. Different scientific approaches have converged in the description of α-syn as an intrinsically disordered protein (IDP), with unusual structural properties [4]. Three distinct domains (Figure 1) confer dynamic structural flexibility and remarkable conformational plasticity [4][5][6][7]; (1) The N-terminal region (amino acids 1–65) confers an α-helical structure involved in lipid membranes binding and possibly promotes α-syn oligomerization; (2) The central region (amino acids 61–95) includes the NACore phosphate-binding loop, which has been implicated in the formation of amyloid fibrils and the stabilization of the pathogenic conformation of α-syn; (3) The C-terminal region (amino-acids 96–140) is associated with the major sites of metal binding and posttranslational modification, involved in modulating the protein structure, physiological functions, and toxicity.
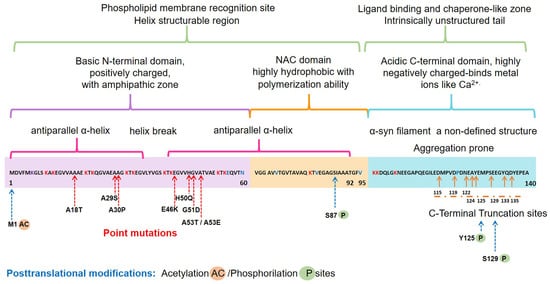
Figure 1. Schematic representation of the native α-syn monomeric structure, highlighting features linked to its biochemical function and dysfunction. Abbreviation: NAC, non-amyloid β-component.
Concerning the physiological roles of this protein, several studies have considered the subcellular localization of α-syn, which ranges from the nucleus to mitochondria and nerve terminals, to propose the following functions [8][9][10][11][12]: (i) neuronal health maintenance; (ii) synaptic plasticity; (iii) membrane biogenesis; iv) mitigation of oxidative stress; (v) regulation of synaptic vesicle trafficking; (vi) neurotransmitter release.
2. Pathological α-Syn Aggregates and Prion-like Properties
Several lines of evidence have proposed that native α-syn exists predominantly as an IDP monomer, which is typically found in an unfolded state and soluble in the cytosol, minimally phosphorylated in the healthy human brain. However, this dynamic protein can convert to various conformations such as helically folded tetramers resisting aggregation, pathologic oligomers, small aggregates, protofibrils, or irreversible insoluble amyloid fibrils with a stabilizing β-sheet structure [6][13][14]. Most α-syn forms exist in a dynamic equilibrium with each other, but perturbation of neuronal homeostasis is a starting point for pathological α-syn insolubility, self-assembly, β-sheet stacking, and misfolding. Cellular environmental cues combined with genetic factors contribute to the posttranslational modifications of the unfolded monomeric α-syn that lead to dysfunctional, neurotoxic, and pathological aggregates with a high degree of β-sheet structure [15].
Interestingly, all the known mutations associated with familial forms of PD are clustered within the N-terminal region, causing misfolding and/or aggregation of the mutant α-syn [6]. Furthermore, N-terminal acetylation could be critical for both aggregation and function [16]. In the C-terminal region, posttranslational modifications have been described that promote a tendency to protein aggregation. Examples are the C-terminal truncation, which results in increased filament assembly, and the phosphorylation at S129 (pS129), which regulates membrane-binding and enhances interactions with metal ions and other proteins (Figure 1). Highlighting that pS129 α-syn modulates key events in the pathogenesis of synucleinopathy such as: (i) variations in the fibrillar structure; (ii) different propagation properties; (iii) increase in cytotoxicity [17].
Indeed, a diversity of pathogenic properties of the misfolded conformations and accumulating aggregates of α-syn have been associated with: (i) mitochondrial dysfunction; (ii) endoplasmic reticulum stress; (iii) proteostasis dysregulation; (iv) synaptic impairment; (v) cell apoptosis; (vi) neuroinflammation; and (vii) neurodegeneration [18][19][20][21][22][23].
Notably, the pathological α-syn aggregates may spread from one neuron to another, causing Lewy pathology in the whole brain [24]. However, α-synuclein-positive inclusions have also been found in the cytoplasm of oligodendrocytes, an event that occurs in the α-synucleinopathy called multiple system atrophy. Specifically, Braak et. al. (2002) suggested that pathological forms of the α-syn act in a prion-like manner, trafficking between cells in a non-random way/form [24]. They hypothesized that PD pathology initiates in the peripheral nervous system, gaining access to the central nervous system (CNS) through retrograde transport via the olfactory tract and the vagal nerve [24][25]. It has been argued that the release and propagation mechanisms of α-syn between neuroanatomically connected regions can be through exosomes, classical exocytosis, trans-synaptic junctions, tunneling nanotubes, and direct penetration [26]. Last but not least, recent studies have suggested that α-syn misfolding and aggregation trigger microglial activation, leading to neuroinflammation and cellular metabolic stress, enhancing the aggregation and spreading of α-syn and affecting its prion-like transmissibility and pathogenicity (Braak’s hypothesis) [27][28][29][30].
3. The Misfolding of α-Synuclein and Its Association with Neuroinflammation in Parkinson’s Disease
A hypothesis claims that chronic neuroinflammation can lead to neuronal damage, neuronal circuitry disturbances, and ultimately, neurodegeneration in PD [31][32]. Hence, chronic neuroinflammation is relevant when considering the pathophysiological mechanisms involved in PD progression and proposing appropriate therapeutic approaches. The brain is an organ susceptible to external stimuli. However, internal stimuli can also alter the delicate homeostasis of the neuronal microenvironment maintained by microglia and astrocytes, considered the brain’s absorptive, excretory, and defense systems [33]. These cells display a Janus-like face because they help eliminate neurotoxins and pathogens, and conversely, they can also cause neuroinflammation, neurotoxicity, and neurodegeneration. Thus, neuroinflammation is a complex pathological condition where different cells and humoral factors converge to resolve the damage as a first intention and later they aggravate the disease in the long term. The cellular actors are activated microglia, reactive astrocytes, and infiltrated lymphocytes, whereas the humoral factors are a great variety of pro-inflammatory molecules. A resulting critical event from the flare-up between cells and humoral factors activities is the loss of BBB permeability that allows molecules to cross from one side to another of the brain [32].
In PD, microglia activation can arise from several factors or causes. The preference of activated microglia for brain areas enriched with pathological α-syn aggregates supports its close association with the neurodegeneration process in PD [34][35]. Multiple studies have shown that extracellular α-syn stimulates microglial cells to produce pro-inflammatory molecules such as interleukin (IL)-1β, IL-6, tumoral necrosis factor-alpha (TNF-α), cyclooxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS), and reactive oxygen species (ROS) [36][37][38][39][40]. The combined neuroinflammation and oxidative stress can promote the neurodegenerative process and further aggravate it [41][42].
Furthermore, neuron-derived α-syn can stimulate astrocytes to produce and release pro-inflammatory cytokines and chemokines, which in turn can recruit activated microglial cells [43] and differentiate them to an M1 (pro-inflammatory) or M2 (anti-inflammatory) phenotype [44]. Therefore, pathological α-syn also behaves as a chemokine to concentrate activated microglia in the affected anatomical areas in PD [45][46].
The relevance of activated microglia in neuroinflammation and neurodegeneration relies on its ability to convert physiological astrocytes into neurotoxic reactive astrocytes classified as A1 phenotype. Recently, it has been established that IL-1α, TNF, and complement component 1q (C1q) released by activated microglia are sufficient and necessary to detonate the A1 phenotype (Figure 2a) [47]. Furthermore, evidence in vitro and in vivo shows that neurotoxic reactive astrocytes A1 can kill neurons through the secretion of neurotoxins not yet identified [47][48]. Thus, the neurotoxic role of reactive astrocytes A1 has severe implications for PD and other neurodegenerative diseases. It means that neurotoxic astrocytes lost their ability to promote neuronal survival, growth, synaptogenesis, and phagocytosis. Therefore, an effective therapy must also prevent the conversion of neurotoxic reactive astrocytes A1 and block their neurotoxic activity [47][48].
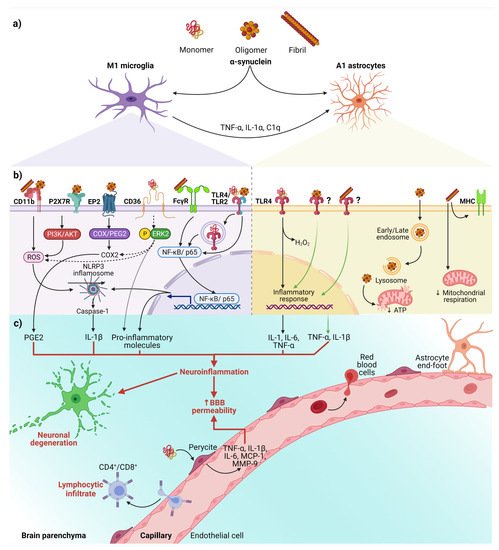
Figure 2. The interaction between α-syn and neuroinflammation in PD. (a) Activation of glial cells by pathological α-syn aggregates; (b) Signaling pathways in microglia and astrocytes triggered by interaction with the different aggregation patterns of α-syn; (c) Neuronal and BBB dysfunction triggered by the neuroinflammatory environment. Dash lines indicate the signaling pathway activated by CD36; Question marks and faded green lines indicate the proposed mechanism for oligomeric and fibrillar α-syn interaction with astrocytes, and thick red lines indicate the proinflammatory molecules that lead to dysfunction of the BBB and neural degeneration. Abbreviations: AKT, Protein kinase B; BBB, Blood-brain barrier; C1q, complement component 1q; CD, cluster of differentiation; COX, cyclooxygenase; EP2, E prostanoid receptor 2; ERK2, extracellular signal-regulated kinase 2; FcγR, the gamma chain subunit of Fc receptor; H2O2, hydrogen peroxide; IL, interleukin; MCP-1, Monocyte Chemoattractant Protein-1; MHC, Major Histocompatibility Complex; MMP-9, Matrix metallopeptidase 9; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; NLRP3, NLR family pyrin domain containing 3; P2X7R, P2X7 receptor; PGE2, Prostaglandin E2; PI3K, phosphoinositide 3-kinase; ROS, reactive oxygen species; TLR, Toll-like receptors; TNF-α, tumor necrosis factor-alpha. Created with BioRender.com.
Besides, the interaction between α-syn with glial cells depends on α-syn aggregation state and the receptors responsible for its uptake (Figure 2b). These receptors expressed in glial cells, through which α-syn interact to trigger a neuroinflammatory environment (Table 1 and Figure 2b,c), play a critical role in early PD, considering that the α-syn aggregation process from soluble oligomers to insoluble inclusions occurs in the early phase of the disease [49][50].
Table 1. Neuroinflammatory and neurotoxic effects triggered by pathological α-syn interaction with glial cells.
| α-Syn Aggregation Pattern | Glial Receptor/Mechanism | Signaling Pathway | Neuroinflammatory/Neurotoxic Effects | Ref. |
|---|---|---|---|---|
| Fibrils | NLRP3 a | α-syn acts as DAMP and activates the NLRP3 inflammasome. | Synthesis and release of IL-1β and cleaved caspase-1 that triggers pyroptosis *. | [49][51][52][53][54][55] |
| Monomers, oligomers, and fibrils | TLRs a,b,† | TLRs sense DAMPs, including α-syn, leading to nuclear translocation of NF-κB. | Release of pro-inflammatory cytokines (TNF-α and IL-6). Dual effect on the astrocyte: secretion of pro-inflammatory and/or neuroprotective factors. | [43][49][56][57][58][59][60][61] |
| Fibrils | FcγR a | Internalization in phagosomes and nuclear translocation of NF-kB p65. | Clearance of α-syn, triggering the release of pro-inflammatory molecules and neurodegeneration. | [49][62][63] |
| Oligomers and fibrils | CD11b a | NOX2 activation through a RhoA-dependent pathway. |
NOX2 activation mediates the chemoattractant ability of α-syn. Induction of superoxide production. |
[49][46][64] |
| Oligomers | EP2 a | The cyclooxygenase/prostaglandin E2 (COX/PGE2) pathway. | Activation of PHOX NADPH and increase in prostaglandin E2 levels, leading to neuronal toxicity. | [49][65][66][67] |
| Monomers | CD36 a | Phosphorylation of ERK2, a downstream kinase activated by CD36 ligation. | Neuronal death through the release of TNF-α and ROS and up-regulation of COX2, NOX2, and iNOS. | [49][38] |
| Oligomers | P2X7R a | Activation of the PI3K/AKT pathway. | Increase of oxidative stress by p47phox translocation and PHOX activation. | [49][68] |
| Fibrils | MHC b | Changes in the expression of HLA genes encoding MHC class I and II proteins. | Impairment of ATP-generating mitochondrial respiration. | [69] |
| Oligomers | Endocytosis b | Dysfunction in mitochondrial dynamics. | Neuronal death is mediated by cytokines release. | [70] |
Abbreviations: AKT, Protein kinase B; ATP, Adenosine triphosphate; CD, cluster of differentiation; COX, cyclooxygenase; DAMP, damage-associated molecular pattern; EP2, E prostanoid receptor 2; ERK2, extracellular signal-regulated kinase 2; FcγR, The gamma chain subunit of Fc receptor; HLA, human leukocyte antigen; IL, interleukin; iNOS, inducible nitric oxide synthase; MHC; major histocompatibility complex; NADPH, nicotine adenine dinucleotide phosphate; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; NLRP3, NLR family pyrin domain containing 3; NOX2, NADPH oxidase 2; P2X7R, P2X7 receptor; PGE2, Prostaglandin E2; PHOX, phagocytic oxidase; PI3K, phosphoinositide 3-kinase; ROS, reactive oxygen species; TLR, Toll-like receptors; TNF-α, tumor necrosis factor-alpha. * An inflammatory type of cell death that causes excessive cell swelling, membrane rupture, and cytokines release. a Microglia and b astrocytes. † Oligomers for TLR-2; monomers, oligomers, and fibrils for TLR-4.
On the other hand, an in vitro study demonstrated that monomeric α-syn activates pericytes (critical cells in BBB regulation) to release pro-inflammatory molecules and matrix metalloproteinase-9 (MMP-9) [71]. These findings suggest that pericytes could exacerbate the neuroinflammatory environment and cause the BBB rupture in PD patients (Figure 2c).
Finally, the infiltrated peripheral immune cells, especially CD4+ and CD8+ T lymphocytes, extend and worsen the PD pathology (Figure 2c) [72][73]. This complication shows the critical role BBB breakdown plays in PD, thus becoming a point to control by new therapeutics. Moreover, infiltrated T lymphocytes, recognizing pathological α-syn aggregates as an antigen presented by microglial class II major histocompatibility complex (MHC-II), arise the immune response [74]. The increased infiltration of Th17 cells and reactive Th1 cells differentiated from CD4+ lymphocytes in PD brains proves the immune response’s involvement in this neuropathology [73][75]. Therefore, these antecedents support the immune response as another mechanism of neuronal death and promote the development of effective immunotherapies.
In summary, we propose the following sequential cellular events that lead to neuroinflammation in early PD (Figure 2). First, misfolded α-syn binds to microglial receptors causing differentiation of the microglia into the M1 phenotype. Then, M1 microglia release TNF-α, IL1α, and C1q inducing the conversion of astrocytes into neurotoxic reactive astrocytes A1. Both, the M1 microglia and A1 astrocytes release pro-inflammatory cytokines to open the BBB and chemokines to attract CD4+ and CD8+ cells, thus completing an immune response against misfolded α-syn. Moreover, the action of neurotrophic factors and other protective molecules released by astrocytes A2 is overpassed by the neuroinflammatory events. Altogether, the unknown molecules released by neurotoxic reactive astrocytes A1, the pro-inflammatory cytokines, and the cellular and humoral response of professional immune cells converge to kill the DA neurons in the early stages of PD. Therefore, new antiparkinsonian therapies should prevent α-syn binding to its glial receptors, block microglial M1 and astrocytic A1 activities, strengthen BBB permeability and avoid activation of the immune response. However, present therapeutic effects are insufficient for an integral PD therapy since the structural and functional restoring of the nigrostriatal dopaminergic system is not accomplished.
References
- Burre, J.; Sharma, M.; Sudhof, T.C. Cell Biology and Pathophysiology of alpha-Synuclein. Cold Spring Harb. Perspect. Med. 2018, 8, a024091.
- Mori, F.; Tanji, K.; Yoshimoto, M.; Takahashi, H.; Wakabayashi, K. Demonstration of alpha-synuclein immunoreactivity in neuronal and glial cytoplasm in normal human brain tissue using proteinase K and formic acid pretreatment. Exp. Neurol. 2002, 176, 98–104.
- Richter-Landsberg, C.; Gorath, M.; Trojanowski, J.Q.; Lee, V.M. alpha-synuclein is developmentally expressed in cultured rat brain oligodendrocytes. J. Neurosci. Res. 2000, 62, 9–14.
- Lashuel, H.A.; Overk, C.R.; Oueslati, A.; Masliah, E. The many faces of alpha-synuclein: From structure and toxicity to therapeutic target. Nat. Rev. Neurosci. 2013, 14, 38–48.
- Emamzadeh, F.N. Alpha-synuclein structure, functions, and interactions. J. Res. Med. Sci. 2016, 21, 29.
- Meade, R.M.; Fairlie, D.P.; Mason, J.M. Alpha-synuclein structure and Parkinson’s disease—Lessons and emerging principles. Mol. Neurodegener. 2019, 14, 29.
- Gonzalez, N.; Arcos-Lopez, T.; Konig, A.; Quintanar, L.; Menacho Marquez, M.; Outeiro, T.F.; Fernandez, C.O. Effects of alpha-synuclein post-translational modifications on metal binding. J. Neurochem. 2019, 150, 507–521.
- Wang, C.; Zhao, C.; Li, D.; Tian, Z.; Lai, Y.; Diao, J.; Liu, C. Versatile Structures of alpha-Synuclein. Front. Mol. Neurosci. 2016, 9, 48.
- Twohig, D.; Nielsen, H.M. alpha-synuclein in the pathophysiology of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 23.
- Runfola, M.; De Simone, A.; Vendruscolo, M.; Dobson, C.M.; Fusco, G. The N-terminal Acetylation of alpha-Synuclein Changes the Affinity for Lipid Membranes but not the Structural Properties of the Bound State. Sci. Rep. 2020, 10, 204.
- Carta, A.R.; Boi, L.; Pisanu, A.; Palmas, M.F.; Carboni, E.; De Simone, A. Advances in modelling alpha-synuclein-induced Parkinson’s diseases in rodents: Virus-based models versus inoculation of exogenous preformed toxic species. J. Neurosci. Methods 2020, 338, 108685.
- Li, X.; Dong, C.; Hoffmann, M.; Garen, C.R.; Cortez, L.M.; Petersen, N.O.; Woodside, M.T. Early stages of aggregation of engineered alpha-synuclein monomers and oligomers in solution. Sci. Rep. 2019, 9, 1734.
- Killinger, B.A.; Melki, R.; Brundin, P.; Kordower, J.H. Endogenous alpha-synuclein monomers, oligomers and resulting pathology: Let’s talk about the lipids in the room. NPJ Parkinsons Dis. 2019, 5, 23.
- Alam, P.; Bousset, L.; Melki, R.; Otzen, D.E. alpha-synuclein oligomers and fibrils: A spectrum of species, a spectrum of toxicities. J. Neurochem. 2019, 150, 522–534.
- Bridi, J.C.; Hirth, F. Mechanisms of alpha-Synuclein Induced Synaptopathy in Parkinson’s Disease. Front. Neurosci. 2018, 12, 80.
- Vinueza-Gavilanes, R.; Inigo-Marco, I.; Larrea, L.; Lasa, M.; Carte, B.; Santamaria, E.; Fernandez-Irigoyen, J.; Bugallo, R.; Aragon, T.; Aldabe, R.; et al. N-terminal acetylation mutants affect alpha-synuclein stability, protein levels and neuronal toxicity. Neurobiol. Dis. 2020, 137, 104781.
- Ma, M.R.; Hu, Z.W.; Zhao, Y.F.; Chen, Y.X.; Li, Y.M. Phosphorylation induces distinct alpha-synuclein strain formation. Sci. Rep. 2016, 6, 37130.
- Cascella, R.; Chen, S.W.; Bigi, A.; Camino, J.D.; Xu, C.K.; Dobson, C.M.; Chiti, F.; Cremades, N.; Cecchi, C. The release of toxic oligomers from alpha-synuclein fibrils induces dysfunction in neuronal cells. Nat. Commun. 2021, 12, 1814.
- Dettmer, U. Rationally Designed Variants of alpha-Synuclein Illuminate Its in vivo Structural Properties in Health and Disease. Front. Neurosci. 2018, 12, 623.
- Villar-Pique, A.; Lopes da Fonseca, T.; Outeiro, T.F. Structure, function and toxicity of alpha-synuclein: The Bermuda triangle in synucleinopathies. J. Neurochem. 2016, 139 (Suppl. 1), 240–255.
- Eastwood, T.A.; Baker, K.; Brooker, H.R.; Frank, S.; Mulvihill, D.P. An enhanced recombinant amino-terminal acetylation system and novel in vivo high-throughput screen for molecules affecting alpha-synuclein oligomerisation. FEBS Lett. 2017, 591, 833–841.
- Mavroeidi, P.; Xilouri, M. Neurons and Glia Interplay in alpha-Synucleinopathies. Int. J. Mol. Sci. 2021, 22, 4994.
- Du, X.Y.; Xie, X.X.; Liu, R.T. The Role of alpha-Synuclein Oligomers in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 8645.
- Braak, H.; Del Tredici, K.; Bratzke, H.; Hamm-Clement, J.; Sandmann-Keil, D.; Rub, U. Staging of the intracerebral inclusion body pathology associated with idiopathic Parkinson’s disease (preclinical and clinical stages). J. Neurol. 2002, 249 (Suppl. 3), iii1–iii5.
- Kim, S.; Kwon, S.H.; Kam, T.I.; Panicker, N.; Karuppagounder, S.S.; Lee, S.; Lee, J.H.; Kim, W.R.; Kook, M.; Foss, C.A.; et al. Transneuronal Propagation of Pathologic alpha-Synuclein from the Gut to the Brain Models Parkinson’s Disease. Neuron 2019, 103, 627–641.
- Xia, Y.; Zhang, G.; Han, C.; Ma, K.; Guo, X.; Wan, F.; Kou, L.; Yin, S.; Liu, L.; Huang, J.; et al. Microglia as modulators of exosomal alpha-synuclein transmission. Cell Death Dis. 2019, 10, 174.
- Sorrentino, Z.A.; Vijayaraghavan, N.; Gorion, K.M.; Riffe, C.J.; Strang, K.H.; Caldwell, J.; Giasson, B.I. Physiological C-terminal truncation of alpha-synuclein potentiates the prion-like formation of pathological inclusions. J. Biol. Chem. 2018, 293, 18914–18932.
- Visanji, N.P.; Brooks, P.L.; Hazrati, L.N.; Lang, A.E. The prion hypothesis in Parkinson’s disease: Braak to the future. Acta Neuropathol. Commun. 2013, 1, 2.
- Heras-Garvin, A.; Stefanova, N. From Synaptic Protein to Prion: The Long and Controversial Journey of alpha-Synuclein. Front. Synaptic Neurosci. 2020, 12, 584536.
- Izco, M.; Blesa, J.; Verona, G.; Cooper, J.M.; Alvarez-Erviti, L. Glial activation precedes alpha-synuclein pathology in a mouse model of Parkinson’s disease. Neurosci. Res. 2021, 170, 330–340.
- Flores-Martinez, Y.M.; Fernandez-Parrilla, M.A.; Ayala-Davila, J.; Reyes-Corona, D.; Blanco-Alvarez, V.M.; Soto-Rojas, L.O.; Luna-Herrera, C.; Gonzalez-Barrios, J.A.; Leon-Chavez, B.A.; Gutierrez-Castillo, M.E.; et al. Acute Neuroinflammatory Response in the Substantia Nigra Pars Compacta of Rats after a Local Injection of Lipopolysaccharide. J. Immunol. Res. 2018, 2018, 1838921.
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms underlying inflammation in neurodegeneration. Cell 2010, 140, 918–934.
- Badanjak, K.; Fixemer, S.; Smajic, S.; Skupin, A.; Grunewald, A. The Contribution of Microglia to Neuroinflammation in Parkinson’s Disease. Int. J. Mol. Sci. 2021, 22, 4676.
- Doorn, K.J.; Moors, T.; Drukarch, B.; van de Berg, W.; Lucassen, P.J.; van Dam, A.M. Microglial phenotypes and toll-like receptor 2 in the substantia nigra and hippocampus of incidental Lewy body disease cases and Parkinson’s disease patients. Acta Neuropathol. Commun. 2014, 2, 90.
- Halliday, G.M.; Stevens, C.H. Glia: Initiators and progressors of pathology in Parkinson’s disease. Mov. Disord. 2011, 26, 6–17.
- Marques, O.; Outeiro, T.F. Alpha-synuclein: From secretion to dysfunction and death. Cell Death Dis. 2012, 3, e350.
- Lee, S.B.; Park, S.M.; Ahn, K.J.; Chung, K.C.; Paik, S.R.; Kim, J. Identification of the amino acid sequence motif of alpha-synuclein responsible for macrophage activation. Biochem. Biophys. Res. Commun. 2009, 381, 39–43.
- Su, X.; Maguire-Zeiss, K.A.; Giuliano, R.; Prifti, L.; Venkatesh, K.; Federoff, H.J. Synuclein activates microglia in a model of Parkinson’s disease. Neurobiol. Aging 2008, 29, 1690–1701.
- Couch, Y.; Alvarez-Erviti, L.; Sibson, N.R.; Wood, M.J.; Anthony, D.C. The acute inflammatory response to intranigral alpha-synuclein differs significantly from intranigral lipopolysaccharide and is exacerbated by peripheral inflammation. J. Neuroinflammation 2011, 8, 166.
- Lee, H.J.; Suk, J.E.; Patrick, C.; Bae, E.J.; Cho, J.H.; Rho, S.; Hwang, D.; Masliah, E.; Lee, S.J. Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J. Biol. Chem. 2010, 285, 9262–9272.
- Reynolds, A.D.; Stone, D.K.; Mosley, R.L.; Gendelman, H.E. Nitrated -synuclein-induced alterations in microglial immunity are regulated by CD4+ T cell subsets. J. Immunol. 2009, 182, 4137–4149.
- Benner, E.J.; Banerjee, R.; Reynolds, A.D.; Sherman, S.; Pisarev, V.M.; Tsiperson, V.; Nemachek, C.; Ciborowski, P.; Przedborski, S.; Mosley, R.L.; et al. Nitrated alpha-synuclein immunity accelerates degeneration of nigral dopaminergic neurons. PLoS ONE 2008, 3, e1376.
- Lee, H.J.; Kim, C.; Lee, S.J. Alpha-synuclein stimulation of astrocytes: Potential role for neuroinflammation and neuroprotection. Oxid. Med. Cell Longev. 2010, 3, 283–287.
- Subramaniam, S.R.; Federoff, H.J. Targeting Microglial Activation States as a Therapeutic Avenue in Parkinson’s Disease. Front. Aging Neurosci. 2017, 9, 176.
- Kim, S.; Cho, S.H.; Kim, K.Y.; Shin, K.Y.; Kim, H.S.; Park, C.H.; Chang, K.A.; Lee, S.H.; Cho, D.; Suh, Y.H. Alpha-synuclein induces migration of BV-2 microglial cells by up-regulation of CD44 and MT1-MMP. J. Neurochem. 2009, 109, 1483–1496.
- Wang, S.; Chu, C.H.; Stewart, T.; Ginghina, C.; Wang, Y.; Nie, H.; Guo, M.; Wilson, B.; Hong, J.S.; Zhang, J. alpha-Synuclein, a chemoattractant, directs microglial migration via H2O2-dependent Lyn phosphorylation. Proc. Natl. Acad. Sci. USA 2015, 112, E1926–E1935.
- Liddelow, S.A.; Barres, B.A. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 2017, 46, 957–967.
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Munch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487.
- Ferreira, S.A.; Romero-Ramos, M. Microglia Response During Parkinson’s Disease: Alpha-Synuclein Intervention. Front. Cell. Neurosci. 2018, 12, 247.
- Polymeropoulos, M.H.; Lavedan, C.; Leroy, E.; Ide, S.E.; Dehejia, A.; Dutra, A.; Pike, B.; Root, H.; Rubenstein, J.; Boyer, R.; et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 1997, 276, 2045–2047.
- Gordon, R.; Albornoz, E.A.; Christie, D.C.; Langley, M.R.; Kumar, V.; Mantovani, S.; Robertson, A.A.B.; Butler, M.S.; Rowe, D.B.; O’Neill, L.A.; et al. Inflammasome inhibition prevents alpha-synuclein pathology and dopaminergic neurodegeneration in mice. Sci. Transl. Med. 2018, 10, aah4066.
- Pike, A.F.; Varanita, T.; Herrebout, M.A.C.; Plug, B.C.; Kole, J.; Musters, R.J.P.; Teunissen, C.E.; Hoozemans, J.J.M.; Bubacco, L.; Veerhuis, R. alpha-Synuclein evokes NLRP3 inflammasome-mediated IL-1beta secretion from primary human microglia. Glia 2021, 69, 1413–1428.
- Lieberman, J.; Wu, H.; Kagan, J.C. Gasdermin D activity in inflammation and host defense. Sci. Immunol. 2019, 4, 4.
- Kovacs, S.B.; Miao, E.A. Gasdermins: Effectors of Pyroptosis. Trends Cell Biol. 2017, 27, 673–684.
- Wang, X.; Chi, J.; Huang, D.; Ding, L.; Zhao, X.; Jiang, L.; Yu, Y.; Gao, F. alpha-synuclein promotes progression of Parkinson’s disease by upregulating autophagy signaling pathway to activate NLRP3 inflammasome. Exp. Ther. Med. 2020, 19, 931–938.
- Kim, C.; Ho, D.H.; Suk, J.E.; You, S.; Michael, S.; Kang, J.; Joong Lee, S.; Masliah, E.; Hwang, D.; Lee, H.J.; et al. Neuron-released oligomeric alpha-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat. Commun. 2013, 4, 1562.
- Stefanova, N.; Fellner, L.; Reindl, M.; Masliah, E.; Poewe, W.; Wenning, G.K. Toll-like receptor 4 promotes alpha-synuclein clearance and survival of nigral dopaminergic neurons. Am. J. Pathol. 2011, 179, 954–963.
- Dzamko, N.; Gysbers, A.; Perera, G.; Bahar, A.; Shankar, A.; Gao, J.; Fu, Y.; Halliday, G.M. Toll-like receptor 2 is increased in neurons in Parkinson’s disease brain and may contribute to alpha-synuclein pathology. Acta Neuropathol. 2017, 133, 303–319.
- Rannikko, E.H.; Weber, S.S.; Kahle, P.J. Exogenous alpha-synuclein induces toll-like receptor 4 dependent inflammatory responses in astrocytes. BMC Neurosci. 2015, 16, 57.
- Fellner, L.; Irschick, R.; Schanda, K.; Reindl, M.; Klimaschewski, L.; Poewe, W.; Wenning, G.K.; Stefanova, N. Toll-like receptor 4 is required for alpha-synuclein dependent activation of microglia and astroglia. Glia 2013, 61, 349–360.
- Chavarria, C.; Rodriguez-Bottero, S.; Quijano, C.; Cassina, P.; Souza, J.M. Impact of monomeric, oligomeric and fibrillar alpha-synuclein on astrocyte reactivity and toxicity to neurons. Biochem. J. 2018, 475, 3153–3169.
- Cao, S.; Standaert, D.G.; Harms, A.S. The gamma chain subunit of Fc receptors is required for alpha-synuclein-induced pro-inflammatory signaling in microglia. J. Neuroinflamm. 2012, 9, 259.
- Cao, S.; Theodore, S.; Standaert, D.G. Fcgamma receptors are required for NF-kappaB signaling, microglial activation and dopaminergic neurodegeneration in an AAV-synuclein mouse model of Parkinson’s disease. Mol. Neurodegener. 2010, 5, 42.
- Hou, L.; Bao, X.; Zang, C.; Yang, H.; Sun, F.; Che, Y.; Wu, X.; Li, S.; Zhang, D.; Wang, Q. Integrin CD11b mediates alpha-synuclein-induced activation of NADPH oxidase through a Rho-dependent pathway. Redox Biol. 2018, 14, 600–608.
- Zhang, W.; Wang, T.; Pei, Z.; Miller, D.S.; Wu, X.; Block, M.L.; Wilson, B.; Zhang, W.; Zhou, Y.; Hong, J.S.; et al. Aggregated alpha-synuclein activates microglia: A process leading to disease progression in Parkinson’s disease. FASEB J. 2005, 19, 533–542.
- Jin, J.; Shie, F.S.; Liu, J.; Wang, Y.; Davis, J.; Schantz, A.M.; Montine, K.S.; Montine, T.J.; Zhang, J. Prostaglandin E2 receptor subtype 2 (EP2) regulates microglial activation and associated neurotoxicity induced by aggregated alpha-synuclein. J. Neuroinflammation 2007, 4, 2.
- Johansson, J.U.; Woodling, N.S.; Wang, Q.; Panchal, M.; Liang, X.; Trueba-Saiz, A.; Brown, H.D.; Mhatre, S.D.; Loui, T.; Andreasson, K.I. Prostaglandin signaling suppresses beneficial microglial function in Alzheimer’s disease models. J. Clin. Investig. 2015, 125, 350–364.
- Jiang, T.; Hoekstra, J.; Heng, X.; Kang, W.; Ding, J.; Liu, J.; Chen, S.; Zhang, J. P2X7 receptor is critical in alpha-synuclein–mediated microglial NADPH oxidase activation. Neurobiol. Aging 2015, 36, 2304–2318.
- Russ, K.; Teku, G.; Bousset, L.; Redeker, V.; Piel, S.; Savchenko, E.; Pomeshchik, Y.; Savistchenko, J.; Stummann, T.C.; Azevedo, C.; et al. TNF-alpha and alpha-synuclein fibrils differently regulate human astrocyte immune reactivity and impair mitochondrial respiration. Cell Rep. 2021, 34, 108895.
- Lindstrom, V.; Gustafsson, G.; Sanders, L.H.; Howlett, E.H.; Sigvardson, J.; Kasrayan, A.; Ingelsson, M.; Bergstrom, J.; Erlandsson, A. Extensive uptake of alpha-synuclein oligomers in astrocytes results in sustained intracellular deposits and mitochondrial damage. Mol. Cell. Neurosci. 2017, 82, 143–156.
- Dohgu, S.; Takata, F.; Matsumoto, J.; Kimura, I.; Yamauchi, A.; Kataoka, Y. Monomeric alpha-synuclein induces blood-brain barrier dysfunction through activated brain pericytes releasing inflammatory mediators in vitro. Microvasc. Res. 2019, 124, 61–66.
- Brochard, V.; Combadiere, B.; Prigent, A.; Laouar, Y.; Perrin, A.; Beray-Berthat, V.; Bonduelle, O.; Alvarez-Fischer, D.; Callebert, J.; Launay, J.M.; et al. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J. Clin. Investig. 2009, 119, 182–192.
- Sommer, A.; Marxreiter, F.; Krach, F.; Fadler, T.; Grosch, J.; Maroni, M.; Graef, D.; Eberhardt, E.; Riemenschneider, M.J.; Yeo, G.W.; et al. Th17 Lymphocytes Induce Neuronal Cell Death in a Human iPSC-Based Model of Parkinson’s Disease. Cell Stem Cell 2018, 23, 123–131.
- Subbarayan, M.S.; Hudson, C.; Moss, L.D.; Nash, K.R.; Bickford, P.C. T cell infiltration and upregulation of MHCII in microglia leads to accelerated neuronal loss in an alpha-synuclein rat model of Parkinson’s disease. J. Neuroinflamm. 2020, 17, 242.
- Kustrimovic, N.; Comi, C.; Magistrelli, L.; Rasini, E.; Legnaro, M.; Bombelli, R.; Aleksic, I.; Blandini, F.; Minafra, B.; Riboldazzi, G.; et al. Parkinson’s disease patients have a complex phenotypic and functional Th1 bias: Cross-sectional studies of CD4+ Th1/Th2/T17 and Treg in drug-naive and drug-treated patients. J. Neuroinflamm. 2018, 15, 205.
More
Information
Subjects:
Neurosciences
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Revisions:
3 times
(View History)
Update Date:
12 Nov 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





