
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Chen Cheng | + 3494 word(s) | 3494 | 2021-10-21 05:19:44 | | | |
| 2 | Amina Yu | -15 word(s) | 3479 | 2021-11-01 10:02:17 | | | | |
| 3 | Amina Yu | -15 word(s) | 3479 | 2021-11-01 10:02:52 | | |
Video Upload Options
Sepsis is a life-threatening organ dysfunction caused by a dysregulated host response to infection; the pathophysiology of sepsis is complex. The incidence of sepsis is steadily increasing, with worldwide mortality ranging between 30% and 50%. Current treatment approaches mainly rely on the timely and appropriate administration of antimicrobials and supportive therapies, but the search for pharmacotherapies modulating the host response has been unsuccessful. Chinese herbal medicines, i.e., Chinese patent medicines, Chinese herbal prescriptions, and single Chinese herbs, play an important role in the treatment of sepsis through multicomponent, multipathway, and multitargeting abilities and have been officially recommended for the management of COVID-19.
1. Introduction
Based on the characteristics of emergency medicine in China, the Preventing Sepsis Campaign in China (PSCC) was initiated in May 2018 [1]. It was advocated by experts that the prevention, diagnosis, and treatment of sepsis should be performed as early as possible to decrease morbidity and mortality, and the principle of the prevention of sepsis was introduced to prevent its occurrence. Several Chinese treatment guidelines for sepsis management and expert consensus—e.g., the Chinese guidelines for the emergency management of sepsis and septic shock 2018, the clinical practice guidelines on traditional Chinese medicine therapy alone or combined with antibiotics for sepsis, and the Chinese emergency medicine expert consensus on the diagnosis and treatment of sepsis complicated by disseminated intravascular coagulation—have been successively released for the management of sepsis [2][1][3]. In these treatment guidelines and expert agreements, CHMs are recommended as add-on therapies to complement the conventional treatment of sepsis, e.g., a XueBiJing injection (XBJ) for sepsis, a ShenFu injection (SF) for septic shock, the ShengMai formula (SMF) for sepsis with the qi and yin exhaustion pattern, the Xuanbai Chengqi decoction (XBCQ) for sepsis with acute respiratory distress syndrome (ARDS), the Qingwen Baidu decoction (QWBD) for sepsis with the internal exuberance of toxins and heat pattern, etc. [4]. The diagnosis and treatment protocol for COVID-19 (the revised eighth version) released by China’s National Health Commission also recommends the use of CHM in accordance with different degrees of severity of COVID-19 [5]. XueBiJing, ShenFu, and ShengMai injections are typical herbal injections officially recommended for the management of COVID-19 when patients with a severe case of the disease develop SIRS and/or MODS [5].
2. Chinese Patent Medicines - XueBiJing Injection
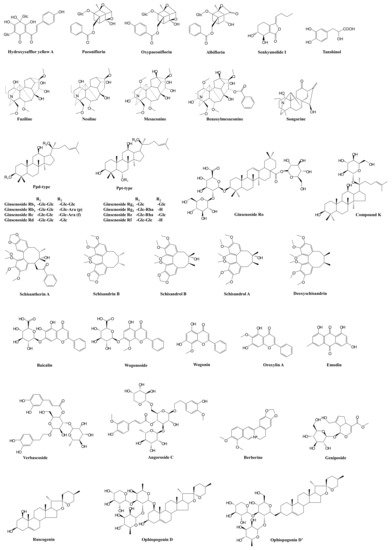
| Prescription | Component Herbs | Chemical Composition | Pharmacological Actions | Bioactive and Bioavailable Compounds | Potential Target Pathway | Potential DDI Target | References |
|---|---|---|---|---|---|---|---|
| XueBiJing injection | Carthamus tinctorius flower (Honghua in Chinese), Paeonia lactiflora root (Chishao), Ligusticum chuanxiong rhizome (Chuanxiong), Angelica sinensis root (Danggui), and Salvia miltiorrhiza root (Danshen) | Flavonoids, monoterpene glycosides, catechols, phthalides, organic acids, etc. | Exhibit anti-inflammatory, anticoagulant, endothelium-protective, immunoregulatory, antioxidant, and organ-protective activities; inhibit ox-LDL-induced apoptosis; improve microcirculation and myocardial ischemia/reperfusion injury | Hydroxysafflor yellow A | TLR4/NF-κB; NLRP3; Rac1/Akt; NF-κB/ICAM-1 | — | [45][47][48][51] |
| Paeoniflorin Oxypaeoniflorin Albiflorin |
SIRT1; IRAK1-NF-κB; IκB; PI3K/Akt; TLR2; Sirt1/Foxo1 | — | [19][43][46][52][53][54] | ||||
| Senkyunolide I | p-Erk1/2; Nrf2/HO-1; Caspase 3; MAPK; TLRs | As victim: UGT2B15 |
[15][19][50][55] | ||||
| Tanshinol | cAMP-PKA | As victim: OAT1/2 |
[15][44] | ||||
| ShenFu injection | Panax ginseng steamed root (Hongshen) and processed Aconitum carmichaelii root (Fuzi) | Ginsenosides, aconitum alkaloids, organic acids, etc. | Regulate oxidative stress and inflammatory responses, inhibit HMGB1-mediated severe inflammatory response, restore endothelial integrity, attenuate the proinflammatory response, enhance innate immunity, preserve adaptive immunity, alleviate neuropathic pain | Ginsenosides Rb1, Rc, Rb2, Rf, Rd, Rg1, etc. | TLR4; PXR/NF-κB; TLRs/IRAK-1; TBK-1/IκB kinase ε/IRF-3; p38/ATF-2 | As substrate: OATP1B3 (for Ginsenosides Rg1, Rf) As perpetrator: OATP1B1/1B3 (for Ginsenosides Rb1, Rc, Rb2, Rd) |
[56][57][58][59][60][61][62][63][64][65][66][67][68] |
| Benzoylmesaconine Fuziline Mesaconine Neoline Songorine |
TLR4/NF-κB; Nrf2 | As victim: P-gp (for benzoylmesaconine) |
[69][70][71][72] | ||||
| ShengMai formula | Panax ginseng root (Renshen), Ophiopogon japonicus root (Maidong), and Schisandra chinensis fruit (Wuweizi) | Ginsenosides, lignans, steroidal saponins, and homoisoflavanones | Exhibit anti-inflammatory or antioxidant, hepatoprotective activities | Ginsenosides Rb₁, Rb2, Rc, Rd, Re, Rg1, Rh1, Compound K, Rf, and Rg2 | TLR4; PXR/NF-κB; TLRs/IRAK-1; TBK-1/IκB kinase ε/IRF-3; p38/ATF-2 | As substrate: OATP1B1/1B3 (for Ginsenoside Rg2) OATP1B3 (for Ginsenosides Rg1, Rf, Re) As perpetrator: OATP1B1/1B3 (for Ginsenosides Rb1, Rc, Rb2, Rd) NTCP (for Rg1) CYP3A (for Rd) |
[56][57][58][59][60][61][62][63][64][65][66][67][68][73][74][75][76] |
| Ophiopogonin D Ophiopogonin D’ Ruscogenin |
PPARα; NF-κB/IκBα; SIRT1; TLR4; TLR4/NF-κB/MyD88 | As perpetrator: CYP3A4, 2C9, and 2E1 (for Ophiopogonin D) UGT1A6/1A8 (for Ophiopogonin D) UGT1A6/1A10 (for Ophiopogonin D’) NTCP (for Ophiopogonin D’) CYP3A (for Ophiopogonin D) As victim: OATP1B1/1B3 (for Ophiopogonin D) |
[67][76][77][78][79][80][81] | ||||
| Schisandrol A Schisandrol B Schizandrin A Schizandrin B Deoxyschisandrin |
iNOS; COX-2; PGE2; MAPK; TLR4/NF-κB/MyD88 | As perpetrator: NTCP (for Schizandrin A) | [75][82][83][84][85] | ||||
| Qingwen Baidu decoction |
Rehmannia glutinosa root (Dihuang), Rhinoceros unicornis horn (Xijiao), Coptidis chinensis rhizome (Huanglian), Gardenia jasminoides fruit (Zhizi), Platycodon grandiflorum root (Jiegeng), Scutellaria baicalensis root (Huangqin), Anemarrhena asphodeloides rhizome (Zhimu), Paeonia lactiflora root (Chishao), Scrophularia ningpoensis root (Xuanshen), Forsythia suspense fruit (Lianqiao), Lophatherum gracile stem and leaf(Danzhuye), Glycyrrhiza uralensis root and rhizome (Gancao), Paeonia suffruticosa root cortex (Danpi), and Gypsum Fibrosum (Shigao) | Alkaloids, iridoids, flavonoids, etc. | Reduce LPS-induced intestinal damage; treat inflammation; alleviate LPS-induced acute kidney injury; alleviate liver injury in sepsis; exhibit anti-inflammatory, antioxidant, and cardioprotective effects | Berberine | TLRs; NF-κB; STAT3; Wnt/β-catenin; PI3K/Akt; MAPK/JNK/p38/ERK | As perpetrator: CYP3A4, CYP2D6 As victim: P-gp |
[86][87][88][89][90][91][92] |
| Geniposide Genipin |
NF-κB; MAPK; PPARγ; AMPK; NLRP3; AKT-mTOR | — | [93][94][95][96][97][98] | ||||
| Baicalin | iNOS; COX-2; NF-κB; HMGB1 | As perpetrator: CYP1A2/3A/2E1, OATP1B1, P-gp | [99][100][101][102][103] | ||||
| Wogonoside Wogonin |
TLR4; NF-κB; Nrf2; NLRP3 | As perpetrator: CYP1A2 (for Wogonin) | [104][105][106][107][108] | ||||
| Oroxylin A | JAK/STAT; IRF2BP2-NFAT1; NF-κB | As perpetrator: CYP1A2, OATP1B1, OAT1/3 and BCRP | [108][109][110][111][112][113][114][115] | ||||
| Verbascoside | iNOS | — | [116][117][118] | ||||
| XuanBai Chengqi decoction |
Rheum palmatum rhizome and root (Dahuang), Gypsum Fibrosum (Shigao), Prunus armeniaca seed (Kuxingren), and Trichosanthes kirilowii fruit (Gualou) | Anthraquinones, etc. | Attenuate LPS-induced microcirculatory disturbance | Emodin | TLR4/NF-κB/ICAM-1; JAK1/STAT3; MAPK; cAMP-PKA; NLRP3; PPARγ | As victim: CYP1A2, UGT1A8/1A10/12B7 | [119][120][121][122][123][124][125][126] |
3. Chinese Herbal Prescriptions-ShengMai Formula
References
- Yu, X.-Z.; Yao, Y.-M.; Zhou, R.-B. Chinese Guidelines for Emergency Management of Sepsis and Septic Shock 2018. J. Clin. Emerg. 2018, 38, 741–756.
- Zhao, G.-Z.; Chen, R.-B.; Li, B.; Guo, Y.-H.; Xie, Y.-M.; Liao, X.; Yang, Y.-F.; Chen, T.-F.; Di, H.-R.; Shao, F.; et al. Clinical practice guideline on traditional Chinese medicine therapy alone or combined with antibiotics for sepsis. Ann. Transl. Med. 2018, 43, 4776–4881.
- Wang, L.-J.; Chia, Y.-F. Chinese Emergency Medicine Expert Consensus on Diagnosis and Treatment of Sepsis Complicated with Disseminated Intravascular Coagulation. Chin. Crit. Care Med. 2017, 29, 577–580.
- Wang, Z.; Yu, X.; Chen, Y.; Lv, C.; Zhao, X. Chinese expert consensus on early prevention and intervention of sepsis. Asian Pac. J. Trop. Med. 2020, 13, 335–349.
- Chinese National Health Commission and Chinese State Administration of Traditional Chinese Medicine. Diagnosis and Treatment of Adults with Coronavirus Disease 2019 (COVID-19) (The Revised Eighth Version). 2021. Available online: http://www.gov.cn/zhengce/zhengceku/2021-04/15/5599795/files/e9ce837932e6434db998bdbbc5d36d32.pdf (accessed on 28 August 2021).
- Li, C.; Wang, P.; Zhang, L.; Li, M.; Lei, X.; Liu, S.; Feng, Z.; Yao, Y.; Chang, B.; Liu, B.; et al. Efficacy and safety of Xuebijing injection (a Chinese patent) for sepsis: A meta-analysis of randomized controlled trials. J. Ethnopharmacol. 2018, 224, 512–521.
- Chen, G.; Gao, Y.; Jiang, Y.; Yang, F.; Li, S.; Tan, D.; Ma, Q. Efficacy and Safety of Xuebijing Injection Combined with Ulinastatin as Adjunctive Therapy on Sepsis: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2018, 9, 743.
- Gao, J.; Kong, L.; Liu, S.; Feng, Z.; Shen, H.; Liu, Q. A prospective multicenter clinical study of Xuebijing injection in the treatment of sepsis and multiple organ dysfunction syndrome. Chin. Crit. Care Med. 2015, 27, 465–470.
- Chen, Y.-X.; Li, C.-S. The Effectiveness of XueBiJing Injection in Therapy of Sepsis: A Multicenter Clinical Study. China J. Emerg. Med. 2013, 22, 130–135.
- Wu, Y.; Zhang, J.; Qi, L. Clinical efficacy and safety of Xuebijing injection on sepsis: A Meta-analysis. Chin. Crit. Care Med. 2020, 32, 691–695.
- Song, Y.; Yao, C.; Yao, Y.; Han, H.; Zhao, X.; Yu, K.; Liu, L.; Xu, Y.; Liu, Z.; Zhou, Q.; et al. XueBiJing Injection Versus Placebo for Critically Ill Patients with Severe Community-Acquired Pneumonia. Crit. Care Med. 2019, 47, e735–e743.
- Luo, Z.; Chen, W.; Xiang, M.; Wang, H.; Xiao, W.; Xu, C.; Li, Y.; Min, J.; Tu, Q. The preventive effect of Xuebijing injection against cytokine storm for severe patients with COVID-19: A prospective randomized controlled trial. Eur. J. Integr. Med. 2021, 42, 101305.
- Zheng, R.; Wang, H.; Liu, Z.; Wang, X.; Li, J.; Lei, X.; Fan, Y.; Liu, S.; Feng, Z.; Shang, H. A real-world study on adverse drug reactions to Xuebijing injection: Hospital intensive monitoring based on 93 hospitals (31,913 cases). Ann. Transl. Med. 2019, 7, 117.
- Wang, C.; Shi, Q.-P.; Ding, F.; Jiang, X.-D.; Tang, W.; Yu, M.-L.; Cheng, J.-Q. Reevaluation of the post-marketing safety of Xuebijing injection based on real-world and evidence-based evaluations. Biomed. Pharmacother. 2019, 109, 1523–1531.
- Li, J.; Olaleye, O.E.; Yu, X.; Jia, W.; Yang, J.; Lu, C.; Liu, S.; Yu, J.; Duan, X.; Wang, Y.; et al. High degree of pharmacokinetic compatibility exists between the five-herb medicine XueBiJing and antibiotics comedicated in sepsis care. Acta Pharm. Sin. B 2019, 9, 1035–1049.
- Zuo, L.; Zhou, L.; Xu, T.; Li, Z.; Liu, L.; Shi, Y.; Kang, J.; Gao, G.; Du, S.; Sun, Z.; et al. Antiseptic Activity of Ethnomedicinal Xuebijing Revealed by the Metabolomics Analysis Using UHPLC-Q-Orbitrap HRMS. Front. Pharmacol. 2018, 9, 300.
- Xu, T.; Zhou, L.; Shi, Y.; Liu, L.; Zuo, L.; Jia, Q.; Du, S.; Kang, J.; Zhang, X.; Sun, Z. Metabolomics approach in lung tissue of septic rats and the interventional effects of Xuebijing injection using UHPLC-Q-Orbitrap-HRMS. J. Biochem. 2018, 164, 427–435.
- Chen, X.; Feng, Y.; Shen, X.; Pan, G.; Fan, G.; Gao, X.; Han, J.; Zhu, Y. Anti-sepsis protection of Xuebijing injection is mediated by differential regulation of pro- and anti-inflammatory Th17 and T regulatory cells in a murine model of polymicrobial sepsis. J. Ethnopharmacol. 2018, 211, 358–365.
- Jiang, M.; Zhou, M.; Han, Y.; Xing, L.; Zhao, H.; Dong, L.; Bai, G.; Luo, G. Identification of NF-κB Inhibitors in Xuebijing injection for sepsis treatment based on bioactivity-integrated UPLC-Q/TOF. J. Ethnopharmacol. 2013, 147, 426–433.
- Zhou, W.; Lai, X.; Wang, X.; Yao, X.; Wang, W.; Li, S. Network pharmacology to explore the anti-inflammatory mechanism of Xuebijing in the treatment of sepsis. Phytomedicine 2021, 85, 153543.
- Li, A.; Li, J.; Bao, Y.; Yuan, D.; Huang, Z. Xuebijing injection alleviates cytokine-induced inflammatory liver injury in CLP-induced septic rats through induction of suppressor of cytokine signaling 1. Exp. Ther. Med. 2016, 12, 1531–1536.
- Li, T.; Qian, Y.; Miao, Z.; Zheng, P.; Shi, T.; Jiang, X.; Pan, L.; Qian, F.; Yang, G.; An, H.; et al. Xuebijing Injection Alleviates Pam3CSK4-Induced Inflammatory Response and Protects Mice from Sepsis Caused by Methicillin-Resistant Staphylococcus aureus. Front. Pharmacol. 2020, 11, 104.
- Liu, J.; Wang, Z.; Lin, J.; Li, T.; Guo, X.; Pang, R.; Dong, L.; Duan, M. Xuebijing injection in septic rats mitigates kidney injury, reduces cortical microcirculatory disorders, and suppresses activation of local inflammation. J. Ethnopharmacol. 2021, 276, 114199.
- Geng, P.; Zhang, H.; Xiong, J.; Wang, Y.; Ling, B.; Wang, H.; Tan, D.; Wang, D.; Zhang, J. XueBiJing Injection Attenuates Hydrogen Sulfide-induced Endothelial Barrier Dysfunction by Upregulating Claudin-5 Expression. Chin. Crit. Care Med. 2020, 32, 443–448.
- Jiang, Y.; Zou, L.; Liu, S.; Liu, X.; Chen, F.; Liu, X.; Zhu, Y. GC/MS-based metabonomics approach reveals effects of Xuebijing injection in CLP induced septic rats. Biomed. Pharmacother. 2019, 117, 109163.
- Tianyu, Z.; Liying, G. Identifying the molecular targets and mechanisms of xuebijing injection for the treatment of COVID-19 via network parmacology and molecular docking. Bioengineered 2021, 12, 2274–2287.
- Zhang, B.; Zhang, D.; Lv, J.-T.; Sa, R.-N.; Ma, B.-B.; Zhang, X.-M.; Lin, Z.-J. Molecular insight into the therapeutic promise of xuebijing injection against coronavirus disease 2019. World J. Tradit. Chin. Med. 2020, 6, 203–215.
- Zuo, L.-H.; Zhou, L.; Shi, Y.-Y.; Li, Z.-L.; Liu, L.-W.; Jiang, X.-F.; Wang, D.; Yan, S.-X.; Sun, Z.; Zhang, X.-J. Mechanism of XueBiJing Injection in Anti-acute Lung Injury Based on Network Pharmacology. Chin. Tradit. Herb. Drugs 2018, 49, 3541–3549.
- Feng, Y.-Y.; Xie, Y.-Y.; Wang, Y.-P.; Lian, Q.; Wang, Y.-M.; Luo, G.-A.; Wang, S.-M. Molecular Mechanism of XueBiJing Injection in Treatment of Sepsis according to Drug-target-pathway Network. Acta Pharmaceut Sin. 2017, 52, 556–562.
- Sun, Z.; Zuo, L.; Sun, T.; Tang, J.; Ding, D.; Zhou, L.; Kang, J.; Zhang, X. Chemical profiling and quantification of XueBiJing injection, a systematic quality control strategy using UHPLC-Q Exactive hybrid quadrupole-orbitrap high-resolution mass spectrometry. Sci. Rep. 2017, 7, 1–15.
- Li, D.; Cao, X.-X.; Pu, W.-L.; Sun, L.-L.; Ren, X.-L. Research on Quality Control Method of XueBiJing Injection. Mod. Chin. Med. 2018, 20, 1157–1160.
- Zuo, L.; Sun, Z.; Hu, Y.; Sun, Y.; Xue, W.; Zhou, L.; Zhang, J.; Bao, X.; Zhu, Z.; Suo, G.; et al. Rapid determination of 30 bioactive constituents in XueBiJing injection using ultra high performance liquid chromatography-high resolution hybrid quadrupole-orbitrap mass spectrometry coupled with principal component analysis. J. Pharm. Biomed. Anal. 2017, 137, 220–228.
- Huang, H.; Wang, J.; Fu, J.Z.; Wang, L.Q.; Zhao, H.Z.; Song, S.Y.; Ji, L.X.; Jiang, M.; Bai, G.; Luo, G.A. Simultaneous determination of thirteen main components and identification of eight major metabolites in Xuebijing Injection by UPLC/Q-TOF. J. Anal. Chem. 2013, 68, 348–356.
- Chen, Y.; Li, Y.; Chen, X.; Wang, L.; Sun, C.; Yan, W.; Liu, X. Development and Validation of a HPLC Method for the Determination of Five Bioactive Compounds in the “Xuebijing” Injection. Anal. Lett. 2010, 43, 2456–2464.
- Zhang, N.; Cheng, C.; Olaleye, O.E.; Sun, Y.; Li, L.; Huang, Y.; Du, F.; Yang, J.; Wang, F.; Shi, Y.; et al. Pharmacokinetics-Based Identification of Potential Therapeutic Phthalides from XueBiJing, a Chinese Herbal Injection Used in Sepsis Management. Drug Metab. Dispos. 2018, 46, 823–834.
- Li, X.; Cheng, C.; Wang, F.; Huang, Y.; Jia, W.; Olaleye, O.; Li, M.; Li, Y.; Li, C. Pharmacokinetics of catechols in human subjects intravenously receiving XueBiJing injection, an emerging antiseptic herbal medicine. Drug Metab. Pharmacokinet. 2016, 31, 95–98.
- Cheng, C.; Lin, J.-Z.; Li, L.; Yang, J.-L.; Jia, W.-W.; Huang, Y.-H.; Du, F.-F.; Wang, F.-Q.; Li, M.-J.; Li, Y.-F.; et al. Pharmacokinetics and disposition of monoterpene glycosides derived from Paeonia lactiflora roots (Chishao) after intravenous dosing of antiseptic XueBiJing injection in human subjects and rats. Acta Pharmacol. Sin. 2016, 37, 530–544.
- Zuo, L.; Sun, Z.; Wang, Z.; Ding, D.; Xu, T.; Liu, L.; Gao, L.; Du, S.; Kang, J.; Zhang, X. Tissue distribution profiles of multiple major bioactive components in rats after intravenous administration of Xuebijing injection by UHPLC-Q-Orbitrap HRMS. Biomed. Chromatogr. 2019, 33, e4400.
- Ouyang, H.-Z.; He, J. Simultaneous determination of nine constituents of Xuebijing Injection in rat plasma and their pharmacokinetics by LC-MS/MS. China J. Chin. Mater. Med. 2018, 43, 3553–3561.
- Zuo, L.; Zhong, Q.; Wang, Z.; Sun, Z.; Zhou, L.; Li, Z.; Xu, T.; Shi, Y.; Tang, J.; Du, S.; et al. Simultaneous determination and pharmacokinetic study of twelve bioactive compounds in rat plasma after intravenous administration of Xuebijing injection by UHPLC-Q-Orbitrap HRMS. J. Pharm. Biomed. Anal. 2017, 146, 347–353.
- Chen, X.-M.; Wang, X.-W.; Luo, J.; Jia, P.; Wang, X.-Y.; Xiao, C.-N.; Wang, S.-X.; Liu, Q.-S.; Zheng, X.-H. Pharmacokinetic Studies of XueBiJing Injection in Rats. Chin. J. Pharm. Anal. 2012, 32, 744–748.
- Sheng, C.; Peng, W.; Xia, Z.; Wang, Y. Plasma and cerebrospinal fluid pharmacokinetics of hydroxysafflor yellow A in patients with traumatic brain injury after intravenous administration of Xuebijing using LC-MS/MS method. Xenobiotica 2019, 50, 545–551.
- Wang, Y.; Che, J.; Zhao, H.; Tang, J.; Shi, G. Paeoniflorin attenuates oxidized low-density lipoprotein-induced apoptosis and adhesion molecule expression by autophagy enhancement in human umbilical vein endothelial cells. J. Cell. Biochem. 2019, 120, 9291–9299.
- Xu, J.; Yang, L.; Dong, L. Tanshinol upregulates the expression of aquaporin 5 in lung tissue of rats with sepsis. Oncol. Lett. 2018, 16, 3290–3296.
- Sun, Y.; Xu, D.-P.; Qin, Z.; Wang, P.-Y.; Hu, B.-H.; Yu, J.-G.; Zhao, Y.; Cai, B.; Chen, Y.-L.; Lu, M.; et al. Protective cerebrovascular effects of hydroxysafflor yellow A (HSYA) on ischemic stroke. Eur. J. Pharmacol. 2018, 818, 604–609.
- Ji, L.; Hou, X.; Liu, W.; Deng, X.; Jiang, Z.; Huang, K.; Li, R. Paeoniflorin inhibits activation of the IRAK1-NF-κB signaling pathway in peritoneal macrophages from lupus-prone MRL/lpr mice. Microb. Pathog. 2018, 124, 223–229.
- Bai, J.; Zhao, J.; Cui, D.; Wang, F.; Song, Y.; Cheng, L.; Gao, K.; Wang, J.; Li, L.; Li, S.; et al. Protective effect of hydroxysafflor yellow A against acute kidney injury via the TLR4/NF-κB signaling pathway. Sci. Rep. 2018, 8, 1–11.
- Xu, X.; Guo, Y.; Zhao, J.; Wang, N.; Ding, J.; Liu, Q. Hydroxysafflor Yellow A Inhibits LPS-Induced NLRP3 Inflammasome Activation via Binding to Xanthine Oxidase in Mouse RAW264.7 Macrophages. Mediat. Inflamm. 2016, 2016, 1–11.
- Wang, L.; Liu, Z.; Dong, Z.; Pan, J.; Ma, X. Effects of Xuebijing injection on microcirculation in septic shock. J. Surg. Res. 2016, 202, 147–154.
- Hu, Y.; Duan, M.; Liang, S.; Wang, Y.; Feng, Y. Senkyunolide I protects rat brain against focal cerebral ischemia–reperfusion injury by up-regulating p-Erk1/2, Nrf2/HO-1 and inhibiting caspase 3. Brain Res. 2015, 1605, 39–48.
- Jin, M.; Sun, C.-Y.; Zang, B.-X. Hydroxysafflor yellow A attenuate lipopolysaccharide-induced endothelium inflammatory injury. Chin. J. Integr. Med. 2015, 22, 36–41.
- Wang, K.; Hu, W. Oxypaeoniflorin improves myocardial ischemia/reperfusion injury by activating the Sirt1/Foxo1 signaling pathway. Acta Biochim. Pol. 2020, 67, 239–245.
- Zhang, Q.; Zhou, J.; Huang, M.; Bi, L.; Zhou, S. Paeoniflorin Reduced BLP-Induced Inflammatory Response by Inhibiting the NF-κB Signal Transduction in Pathway THP-1 Cells. Cent. Eur. J. Immunol. 2014, 39, 461–467.
- Li, G.; Seo, C.-S.; Lee, K.-S.; Kim, H.-J.; Chang, H.-W.; Jung, J.-S.; Song, D.-K.; Son, J.-K. Protective constituents against sepsis in mice from the root cortex ofPaeonia suffruticosa. Arch. Pharmacal Res. 2004, 27, 1123–1126.
- Xie, J.; Zhao, Z.-Z.; Li, P.; Zhu, C.-L.; Guo, Y.; Wang, J.; Deng, X.-M.; Wang, J.-F. Senkyunolide I Protects against Sepsis-Associated Encephalopathy by Attenuating Sleep Deprivation in a Murine Model of Cecal Ligation and Puncture. Oxidative Med. Cell. Longev. 2021, 2021, 1–11.
- Su, F.; Xue, Y.; Wang, Y.; Zhang, L.; Chen, W.; Hu, S. Protective Effect of Ginsenosides Rg1 and Re on Lipopolysaccharide-Induced Sepsis by Competitive Binding to Toll-Like Receptor 4. Antimicrob. Agents Chemother. 2015, 59, 5654–5663.
- Lee, W.; Ku, S.-K.; Jeong, T.C.; Lee, S.; Bae, J.-S. Ginsenosides Inhibit HMGB1-induced Inflammatory Responses in HUVECs and in Murine Polymicrobial Sepsis. Bull. Korean Chem. Soc. 2014, 35, 2955–2962.
- Zou, Y.; Tao, T.; Tian, Y.; Zhu, J.; Cao, L.; Deng, X.; Li, J. Ginsenoside Rg1 improves survival in a murine model of polymicrobial sepsis by suppressing the inflammatory response and apoptosis of lymphocytes. J. Surg. Res. 2013, 183, 760–766.
- Zhang, L.; Zhu, M.; Li, M.; Du, Y.; Duan, S.; Huang, Y.; Lu, Y.; Zhang, J.; Wang, T.; Fu, F. Ginsenoside Rg1 attenuates adjuvant-induced arthritis in rats via modulation of PPAR-γ/NF-κB signal pathway. Oncotarget 2017, 8, 55384–55393.
- Huang, Q.; Wang, T.; Wang, H.-Y. Ginsenoside Rb2 enhances the anti-inflammatory effect of ω-3 fatty acid in LPS-stimulated RAW264.7 macrophages by upregulating GPR120 expression. Acta Pharmacol. Sin. 2016, 38, 192–200.
- Zhang, J.; Cao, L.; Wang, H.; Cheng, X.; Wang, L.; Zhu, L.; Yan, T.; Xie, Y.; Wu, Y.; Zhao, M.; et al. Ginsenosides Regulate PXR/NF-κB Signaling and Attenuate Dextran Sulfate Sodium–Induced Colitis. Drug Metab. Dispos. 2015, 43, 1181–1189.
- Sun, R.-J.; Li, Y.-N.; Chen, W.; Zhang, F.; Li, T.-S. Total Ginsenosides Synergize with Ulinastatin against Septic Acute Lung Injury and Acute Respiratory Distress Syndrome. Int. J. Clin. Exp. Pathol. 2015, 8, 7385–7390.
- Zhang, Y.; Sun, K.; Liu, Y.-Y.; Zhang, Y.-P.; Hu, B.-H.; Chang, X.; Yan, L.; Pan, C.-S.; Li, Q.; Fan, J.-Y.; et al. Ginsenoside Rb1 ameliorates lipopolysaccharide-induced albumin leakage from rat mesenteric venules by intervening in both trans- and paracellular pathway. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, G289–G300.
- Joh, E.-H.; Lee, I.-A.; Jung, I.-H.; Kim, D.-H. Ginsenoside Rb1 and its metabolite compound K inhibit IRAK-1 activation—The key step of inflammation. Biochem. Pharmacol. 2011, 82, 278–286.
- Yu, T.; Yang, Y.; Kwak, Y.-S.; Song, G.G.; Kim, M.-Y.; Rhee, M.H.; Cho, J.Y. Ginsenoside Rc from Panax ginseng exerts anti-inflammatory activity by targeting TANK-binding kinase 1/interferon regulatory factor-3 and p38/ATF-2. J. Ginseng Res. 2017, 41, 127–133.
- Jiang, R.; Dong, J.; Li, X.; Du, F.; Jia, W.; Xu, F.; Wang, F.; Yang, J.; Niu, W.; Li, C. Molecular mechanisms governing different pharmacokinetics of ginsenosides and potential for ginsenoside-perpetrated herb–drug interactions on OATP1B3. Br. J. Pharmacol. 2015, 172, 1059–1073.
- Olaleye, O.; Niu, W.; Du, F.-F.; Wang, F.-Q.; Xu, F.; Pintusophon, S.; Lu, J.-L.; Yang, J.-L.; Li, C. Multiple circulating saponins from intravenous ShenMai inhibit OATP1Bs in vitro: Potential joint precipitants of drug interactions. Acta Pharmacol. Sin. 2019, 40, 833–849.
- Pintusophon, S.; Niu, W.; Duan, X.-N.; Olaleye, O.E.; Huang, Y.-H.; Wang, F.-Q.; Li, Y.-F.; Yang, J.-L.; Li, C. Intravenous formulation of Panax notoginseng root extract: Human pharmacokinetics of ginsenosides and potential for perpetrating drug interactions. Acta Pharmacol. Sin. 2019, 40, 1351–1363.
- You, Q.; Wang, J.; Jiang, L.; Chang, Y.; Li, W. Aqueous extract of Aconitum carmichaelii Debeaux attenuates sepsis-induced acute lung injury via regulation of TLR4/NF-ΚB pathway. Trop. J. Pharm. Res. 2020, 19, 533–539.
- Tanimura, Y.; Yoshida, M.; Ishiuchi, K.; Ohsawa, M.; Makino, T. Neoline is the active ingredient of processed aconite root against murine peripheral neuropathic pain model, and its pharmacokinetics in rats. J. Ethnopharmacol. 2019, 241, 111859.
- Li, Y.; Feng, Y.-F.; Liu, X.-T.; Li, Y.-C.; Zhu, H.-M.; Sun, M.-R.; Li, P.; Liu, B.; Yang, H. Songorine promotes cardiac mitochondrial biogenesis via Nrf2 induction during sepsis. Redox Biol. 2021, 38, 101771.
- Ma, J.; Kan, H.; Ma, Y.; Men, L.; Pi, Z.; Liu, Z.; Liu, Z. Qualitative and quantitative analysis of drug interactions: Fritillary mediating the transport of alkaloids in caco-2 cells by p-glycoprotein. Chem. Res. Chin. Univ. 2014, 30, 731–737.
- Park, J.-S.; Shin, J.A.; Jung, J.-S.; Hyun, J.-W.; Van Le, T.K.; Kim, D.-H.; Park, E.-M.; Kim, H.-S. Anti-Inflammatory Mechanism of Compound K in Activated Microglia and Its Neuroprotective Effect on Experimental Stroke in Mice. J. Pharmacol. Exp. Ther. 2011, 341, 59–67.
- Yang, C.-S.; Ko, S.-R.; Cho, B.-G.; Shin, N.-M.; Yuk, J.-M.; Li, S.; Kim, J.-M.; Evans, R.M.; Jung, J.-S.; Song, N.-K.; et al. The ginsenoside metabolite compound K, a novel agonist of glucocorticoid receptor, induces tolerance to endotoxin-induced lethal shock. J. Cell. Mol. Med. 2008, 12, 1739–1753.
- Zhan, T.; Yao, N.; Wu, L.; Lu, Y.; Liu, M.; Liu, F.; Xiong, Y.; Xia, C. The major effective components in Shengmai Formula interact with sodium taurocholate co-transporting polypeptide. Phytomedicine 2019, 59, 152916.
- Xia, C.; Sun, J.; Wang, G.; Shang, L.; Zhang, X.; Zhang, R.; Wang, X.; Hao, H.; Xie, L. Differential effect of Shenmai injection, a herbal preparation, on the cytochrome P450 3A-mediated 1′-hydroxylation and 4-hydroxylation of midazolam. Chem. Interact. 2009, 180, 440–448.
- Huang, X.; Wang, Y.; Zhang, Z.; Wang, Y.; Chen, X.; Wang, Y.; Gao, Y. Ophiopogonin D and EETs ameliorate Ang II-induced inflammatory responses via activating PPARα in HUVECs. Biochem. Biophys. Res. Commun. 2017, 490, 123–133.
- Wang, Y.; Chen, Y.; Wang, H.; Cheng, Y.; Zhao, X. Specific Turn-On Fluorescent Probe with Aggregation-Induced Emission Characteristics for SIRT1 Modulator Screening and Living-Cell Imaging. Anal. Chem. 2015, 87, 5046–5049.
- Sun, Q.; Chen, L.; Gao, M.; Jiang, W.; Shao, F.; Li, J.; Wang, J.; Kou, J.; Yu, B. Ruscogenin inhibits lipopolysaccharide-induced acute lung injury in mice: Involvement of tissue factor, inducible NO synthase and nuclear factor (NF)-κB. Int. Immunopharmacol. 2012, 12, 88–93.
- Ji, X.; Ding, B.; Wu, X.; Liu, F.; Yang, F. In vitro study on the effect of ophiopogonin D on the activity of cytochrome P450 enzymes. Xenobiotica 2021, 51, 262–267.
- Jiang, L.-P.; Zhao, J.; Cao, Y.-F.; Hong, M.; Sun, D.-X.; Sun, X.-Y.; Yin, J.; Zhu, Z.-T.; Fang, Z.-Z. The Inhibition of the Components from Shengmai Injection towards UDP-Glucuronosyltransferase. Evid.-Based Complement. Altern. Med. 2014, 2014, 1–9.
- Liu, S.; Lu, B. Schisantherin-A Alleviates Lipopolysaccharide-Induced Inflammation and Apoptosis in WI-38 Cells. Curr. Top. Nutraceutical Res. 2021, 19, 421–426.
- Bae, S.; Kim, J.; Choi, Y.; Lee, S.; Gong, J.; Choi, Y.-W.; Hwang, D. Novel Function of α-Cubebenoate Derived from Schisandra chinensis as Lipogenesis Inhibitor, Lipolysis Stimulator and Inflammasome Suppressor. Molecules 2020, 25, 4995.
- Xu, J.-J.; Lu, C.-J.; Liu, Z.-J.; Zhang, P.; Guo, H.-L.; Wang, T.-T. Schizandrin B Protects LPS-Induced Sepsis via TLR4/NF-κB/MyD88 Signaling Pathway. Am. J. Transl. Res. 2018, 10, 1155–1163.
- Kook, M.; Lee, S.K.; Kim, S.D.; Lee, H.Y.; Hwang, J.S.; Choi, Y.W.; Bae, Y.-S. Anti-septic activity of α-cubebenoate isolated from Schisandra chinensis. BMB Rep. 2015, 48, 336–341.
- Wang, Y.; Du, P.; Jiang, D. Berberine functions as a negative regulator in lipopolysaccharide -induced sepsis by suppressing NF-κB and IL-6 mediated STAT3 activation. Pathog. Dis. 2020, 78, 47.
- He, Y.; Yuan, X.; Zuo, H.; Sun, Y.; Feng, A. Berberine Exerts a Protective Effect on Gut-Vascular Barrier via the Modulation of the Wnt/Beta-Catenin Signaling Pathway During Sepsis. Cell. Physiol. Biochem. 2018, 49, 1342–1351.
- Li, G.-X.; Wang, X.-M.; Jiang, T.; Gong, J.-F.; Niu, L.-Y.; Li, N. Berberine Prevents Intestinal Mucosal Barrier Damage During Early Phase of Sepsis in Rat through the Toll-Like Receptors Signaling Pathway. Korean J. Physiol. Pharmacol. 2015, 19, 1–7.
- Gao, M.-Y.; Chen, L.; Yang, L.; Yu, X.; Kou, J.-P.; Yu, B.-Y. Berberine inhibits LPS-induced TF procoagulant activity and expression through NF-κB/p65, Akt and MAPK pathway in THP-1 cells. Pharmacol. Rep. 2014, 66, 480–484.
- Zhang, Q.; Piao, X.-L.; Lu, T.; Wang, D.; Kim, S.W. Preventive effect of Coptis chinensis and berberine on intestinal injury in rats challenged with lipopolysaccharides. Food Chem. Toxicol. 2011, 49, 61–69.
- Kumar, A.; Ekavali, K.C.; Mukherjee, M.; Pottabathini, R.; Dhull, D.K. Current knowledge and pharmacological profile of berberine: An update. Eur. J. Pharmacol. 2015, 761, 288–297.
- Lee, S.Y.; Jang, H.; Lee, J.-Y.; Ma, J.Y.; Oh, S.J.; Kim, S.K. Inhibitory effects of Hwang-Ryun-Hae-Dok-Tang on cytochrome P450 in human liver microsomes. Xenobiotica 2014, 45, 131–138.
- Song, P.; Shen, D.-F.; Meng, Y.-Y.; Kong, C.-Y.; Zhang, X.; Yuan, Y.-P.; Yan, L.; Tang, Q.-Z.; Ma, Z.-G. Geniposide protects against sepsis-induced myocardial dysfunction through AMPKα-dependent pathway. Free. Radic. Biol. Med. 2020, 152, 186–196.
- Liu, J.; Zhao, N.; Shi, G.; Wang, H. Geniposide ameliorated sepsis-induced acute kidney injury by activating PPARγ. Aging 2020, 12, 22744–22758.
- Cho, H.; Kim, S.; Choi, J.; Lee, S. Genipin alleviates sepsis-induced liver injury by restoring autophagy. Br. J. Pharmacol. 2016, 173, 980–991.
- Liu, H.; Chen, Y.-F.; Li, F.; Zhang, H.-Y. Fructus Gardenia (Gardenia jasminoides J. Ellis) phytochemistry, pharmacology of cardiovascular, and safety with the perspective of new drugs development. J. Asian Nat. Prod. Res. 2012, 15, 94–110.
- Yang, X.; Cai, Q.; He, J.; Chu, X.; Wei, M.; Feng, X.; Xie, X.; Huo, M.; Liu, J.; Wei, J.; et al. Geniposide, an Iridoid Glucoside Derived from Gardenia jasminoides, Protects against Lipopolysaccharide-induced Acute Lung Injury in Mice. Planta Med. 2012, 78, 557–564.
- Zheng, X.; Yang, D.; Liu, X.; Wang, N.; Li, B.; Cao, H.; Lu, Y.; Wei, G.; Zhou, H.; Zheng, J. Identification of a new anti-LPS agent, geniposide, from Gardenia jasminoides Ellis, and its ability of direct binding and neutralization of lipopolysaccharide in vitro and in vivo. Int. Immunopharmacol. 2010, 10, 1209–1219.
- Duan, X.-Y.; Sun, Y.; Zhao, Z.-F.; Shi, Y.-Q.; Ma, X.-Y.; Tao, L.; Liu, M.-W. Baicalin attenuates LPS-induced alveolar type II epithelial cell A549 injury by attenuation of the FSTL1 signaling pathway via increasing miR-200b-3p expression. Innate Immun. 2021, 27, 294–312.
- Kuo, S.-W.; Su, W.-L.; Chou, T.-C. Baicalin improves the survival in endotoxic mice and inhibits the inflammatory responses in LPS-treated RAW 264.7 macrophages. Eur. J. Inflamm. 2020, 18, 18.
- Zhu, Y.; Fu, Y.; Lin, H. Baicalin Inhibits Renal Cell Apoptosis and Protects Against Acute Kidney Injury in Pediatric Sepsis. Med. Sci. Monit. 2016, 22, 5109–5115.
- Chen, H.-M.; Lou, S.-F.; Hsu, J.-H.; Chen, T.-J.; Cheng, T.-L.; Chiu, C.-C.; Yeh, J.-L. Baicalein Inhibits HMGB1 Release and MMP-2/-9 Expression in Lipopolysaccharide-induced Cardiac Hypertrophy. Am. J. Chin. Med. 2014, 42, 785–797.
- Noh, K.; Kang, Y.; Nepal, M.R.; Jeong, K.S.; Oh, D.G.; Kang, M.J.; Lee, S.; Kang, W.; Jeong, H.G.; Jeong, T.C. Role of Intestinal Microbiota in Baicalin-Induced Drug Interaction and Its Pharmacokinetics. Molecules 2016, 21, 337.
- Dai, J.; Guo, W.; Tan, Y.; Niu, K.; Zhang, J.; Liu, C.; Yang, X.; Tao, K.; Chen, Z.; Dai, J. Wogonin alleviates liver injury in sepsis through Nrf2-mediated NF-κB signalling suppression. J. Cell. Mol. Med. 2021, 25, 5782–5798.
- Gao, Y.-Z.; Zhao, L.-F.; Ma, J.; Xue, W.-H.; Zhao, H. Protective mechanisms of wogonoside against Lipopolysaccharide/D-galactosamine-induced acute liver injury in mice. Eur. J. Pharmacol. 2016, 780, 8–15.
- Lee, J.Y.; Park, W. Anti-Inflammatory Effect of Wogonin on RAW 264.7 Mouse Macrophages Induced with Polyinosinic-Polycytidylic Acid. Molecules 2015, 20, 6888–6900.
- Zhang, L.; Ren, Y.; Yang, C.; Guo, Y.; Zhang, X.; Hou, G.; Guo, X.; Sun, N.; Liu, Y. Wogonoside Ameliorates Lipopolysaccharide-Induced Acute Lung Injury in Mice. Inflammation 2014, 37, 2006–2012.
- Shao, Y.-X.; Zhao, P.; Li, Z.; Liu, M.; Liu, P.; Huang, M.; Luo, H.-B. The molecular basis for the inhibition of human cytochrome P450 1A2 by oroxylin and wogonin. Eur. Biophys. J. 2012, 41, 297–306.
- Zhang, Z.; Wang, Y.; Shan, Y.; Zhou, R.; Yin, W. Oroxylin A alleviates immunoparalysis of CLP mice by degrading CHOP through interacting with FBXO15. Sci. Rep. 2020, 10, 1–15.
- Tseng, T.-L.; Chen, M.-F.; Hsu, Y.-H.; Lee, T.J. OroxylinA reverses lipopolysaccharide-induced adhesion molecule expression and endothelial barrier disruption in the rat aorta. Toxicol. Appl. Pharmacol. 2020, 400, 115070.
- Li, H.-F.; Wu, Y.-L.; Tseng, T.-L.; Chao, S.-W.; Lin, H.; Chen, H.-H. Inhibition of miR-155 potentially protects against lipopolysaccharide-induced acute lung injury through the IRF2BP2-NFAT1 pathway. Am. J. Physiol. Physiol. 2020, 319, C1070–C1081.
- Lee, J.Y.; Park, W. Anti-inflammatory effects of oroxylin A on RAW 264.7 mouse macrophages induced with polyinosinic-polycytidylic acid. Exp. Ther. Med. 2016, 12, 151–156.
- Ku, S.-K.; Han, M.-S.; Lee, M.Y.; Lee, Y.-M.; Bae, J.-S. Inhibitory effects of oroxylin A on endothelial protein C receptor shedding in vitro and in vivo. BMB Rep. 2014, 47, 336–341.
- Tseng, T.-L.; Chen, M.-F.; Tsai, M.-J.; Hsu, Y.-H.; Chen, C.-P.; Lee, T.J.F. Oroxylin-A Rescues LPS-Induced Acute Lung Injury via Regulation of NF-κB Signaling Pathway in Rodents. PLoS ONE 2012, 7, e47403.
- Ren, G.; Qin, Z.; Yang, N.; Chen, H.; Fu, K.; Lu, C.; Lu, Y.; Li, N.; Zhang, Y.; Chen, X.; et al. Interactions between Oroxylin A with the solute carrier transporters and ATP-binding cassette transporters: Drug transporters profile for this flavonoid. Chem. Interact. 2020, 324, 109097.
- Li, Y.; Yu, H.; Jin, Y.; Li, M.; Qu, C. Verbascoside Alleviates Atopic Dermatitis-Like Symptoms in Mice via Its Potent Anti-Inflammatory Effect. Int. Arch. Allergy Immunol. 2018, 175, 220–230.
- Campo, G.; Marchesini, J.; Bristot, L.; Monti, M.; Gambetti, S.; Pavasini, R.; Pollina, A.; Ferrari, R. The in vitro effects of verbascoside on human platelet aggregation. J. Thromb. Thrombolysis 2012, 34, 318–325.
- Speranza, L.; Franceschelli, S.; Pesce, M.; Reale, M.; Menghini, L.; Vinciguerra, I.; De Lutiis, M.A.; Felaco, M.; Grilli, A. Antiinflammatory effects in THP-1 cells treated with verbascoside. Phytother. Res. 2010, 24, 1398–1404.
- Li, A.; Dong, L.; Duan, M.-L.; Sun, K.; Liu, Y.-Y.; Wang, M.-X.; Deng, J.-N.; Fan, J.-Y.; Wang, B.-E.; Han, J.-Y. Emodin Improves Lipopolysaccharide-Induced Microcirculatory Disturbance in Rat Mesentery. Microcirculation 2013, 20, 617–628.
- Dai, S.; Ye, B.; Chen, L.; Hong, G.; Zhao, G.; Lu, Z. Emodin alleviates LPS -induced myocardial injury through inhibition of NLRP3 inflammasome activation. Phytother. Res. 2021, 35, 5203–5213.
- Ding, Y.; Liu, P.; Chen, Z.-L.; Zhang, S.-J.; Wang, Y.-Q.; Cai, X.; Luo, L.; Zhou, X.; Zhao, L. Emodin Attenuates Lipopolysaccharide-Induced Acute Liver Injury via Inhibiting the TLR4 Signaling Pathway in vitro and in vivo. Front. Pharmacol. 2018, 9, 962.
- Zhu, T.; Zhang, W.; Feng, S.-J.; Yu, H.-P. Emodin suppresses LPS-induced inflammation in RAW264.7 cells through a PPARγ-dependent pathway. Int. Immunopharmacol. 2016, 34, 16–24.
- Yin, J.-T.; Wan, B.; Liu, D.-D.; Wan, S.-X.; Fu, H.-Y.; Wan, Y.; Zhang, H.; Chen, Y. Emodin alleviates lung injury in rats with sepsis. J. Surg. Res. 2016, 202, 308–314.
- Chen, Y.-K.; Xu, Y.-K.; Zhang, H.; Yin, J.-T.; Fan, X.; Liu, D.-D.; Fu, H.-Y.; Wan, B. Emodin alleviates jejunum injury in rats with sepsis by inhibiting inflammation response. Biomed. Pharmacother. 2016, 84, 1001–1007.
- Sun, Y.-N.; Sun, L.-J.; Liu, S.-Q.; Song, J.; Cheng, J.-J.; Liu, J. Effect of Emodin on Aquaporin 5 Expression in Rats with Sepsis-Induced Acute Lung Injury. J. Tradit. Chin. Med. 2015, 35, 679–684.
- Wang, D.; Wang, X.-H.; Yu, X.; Cao, F.; Cai, X.; Chen, P.; Li, M.; Feng, Y.; Li, H.; Wang, X. Pharmacokinetics of Anthraquinones from Medicinal Plants. Front. Pharmacol. 2021, 12, 638993.
- Fu, S.; Zhang, J.; Gao, X.; Xia, Y.; Ferrelli, R.M.; Fauci, A.; Guerra, R.; Hu, L. Clinical practice of traditional Chinese medicines for chronic heart failure. Hear. Asia 2010, 2, 24–27.
- Huang, X.; Duan, X.; Wang, K.; Wu, J.; Zhang, X. Shengmai injection as an adjunctive therapy for the treatment of chronic obstructive pulmonary disease: A systematic review and meta-analysis. Complement. Ther. Med. 2019, 43, 140–147.
- Ma, D.-H.; Xi, R.; Sun, J. Adverse Reaction Characteristics and Influencing Factors of Shengmai Injections. Pharm. Clin. Res. 2016, 24, 324–327.
- Ha, Y.-X.; Wang, X.-P.; Huang, P.; Zhuang, R.; Xu, X.-L.; Li, B.; Liu, Q.-Q. Effect of Shengmai Injection on Septic Shock, a Systematic Review and Meta-Analysis. J. Emerg. Tradit. Chin. Med. 2019, 28, 1893–1898.
- Lu, J.; Yu, Y.; Wang, X.-J.; Chai, R.-P.; Lyu, X.-K.; Deng, M.-H.; Hu, M.-G.; Qi, Y.; Chen, X. Mechanism of Shengmai Injection on Anti-Sepsis and Protective Activities of Intestinal Mucosal Barrier in Mice. Chin. J. Integr. Med. 2021, 2021, 1–6.
- Cao, Y.; Han, X.; Pan, H.; Jiang, Y.; Peng, X.; Xiao, W.; Rong, J.; Chen, F.; He, J.; Zou, L.; et al. Emerging protective roles of shengmai injection in septic cardiomyopathy in mice by inducing myocardial mitochondrial autophagy via caspase-3/Beclin-1 axis. Inflamm. Res. 2020, 69, 41–50.
- Chai, R.-P.; Zhang, Y.-H.; Lu, J.; Wang, T.-J.; Chen, X. Research on Mechanism of Shengmai Injection in the Treatment of Sepsis Based on Metabolomics. China J. Pharm. Econ. 2019, 14, 30–34.
- Zheng, B.-H.; Zhan, S.-Y.; Zhou, H.-Y.; Feng, Y.-H.; Fang, M.-T.; Zheng, Y.-X.; Liu, G.-Q.; Li, M.-J. Research Progress on Material Composition, Pre-clinical Pharmacokinetic and Pharmacodynamic Studies of Shengmai Injection. Chin. Tradit. Herb. Drugs 2020, 51, 5360–5371.
- Zheng, C.; Hao, H.; Wang, X.; Wu, X.; Wang, G.; Sang, G.; Liang, Y.; Xie, L.; Xia, C.; Yao, X. Diagnostic fragment-ion-based extension strategy for rapid screening and identification of serial components of homologous families contained in traditional Chinese medicine prescription using high-resolution LC-ESI- IT-TOF/MS: Shengmai injection as an example. J. Mass Spectrom. 2008, 44, 230–244.
- Zhao, C.; Liu, H.; Miao, P.; Wang, H.; Yu, H.; Wang, C.; Li, Z. A Strategy for Selecting "Q-Markers" of Chinese Medical Preparation via Components Transfer Process Analysis with Application to the Quality Control of Shengmai Injection. Molecules 2019, 24, 1811.
- Wu, F.; Sun, H.; Wei, W.; Han, Y.; Wang, P.; Dong, T.; Yan, G.; Wang, X. Rapid and global detection and characterization of the constituents in ShengMai San by ultra-performance liquid chromatography-high-definition mass spectrometry. J. Sep. Sci. 2011, 34, 3194–3199.
- Cheng, Y.-Y.; Tsai, T.-H. Analysis of Sheng-Mai-San, a Ginseng-Containing Multiple Components Traditional Chinese Herbal Medicine Using Liquid Chromatography Tandem Mass Spectrometry and Physical Examination by Electron and Light Microscopies. Molecules 2016, 21, 1159.
- Zheng, Y.; Fan, C.; Liu, M.; Chen, Y.; Lu, Z.; Xu, N.; Huang, H.; Zeng, H.; Liu, S.; Cao, H.; et al. Overall quality control of the chemical and bioactive consistency of ShengMai Formula. J. Pharm. Biomed. Anal. 2020, 189, 113411.
- Li, X.; Chen, H.; Jia, W.; Xie, G. A Metabolomics-Based Strategy for the Quality Control of Traditional Chinese Medicine: Shengmai Injection as a Case Study. Evid.-Based Complement. Altern. Med. 2013, 2013, 1–8.
- Li, F.; Cheng, T.-F.; Dong, X.; Li, P.; Yang, H. Global analysis of chemical constituents in Shengmai injection using high performance liquid chromatography coupled with tandem mass spectrometry. J. Pharm. Biomed. Anal. 2016, 117, 61–72.
- Lu, J.; Lyu, X.; Chai, R.; Yu, Y.; Deng, M.; Zhan, X.; Dong, Z.; Chen, X. Investigation of the Mechanism of Shengmai Injection on Sepsis by Network Pharmacology Approaches. Evid.-Based Complement. Altern. Med. 2020, 2020, 1–11.
- Zhang, Y.; Hu, Y.; Li, M.; Wang, J.; Guo, G.; Li, F.; Yu, B.; Kou, J. The Traditional Chinese Medicine Compound, GRS, Alleviates Blood–Brain Barrier Dysfunction. Drug Des. Dev. Ther. 2020, 14, 933–947.
- Zhan, S.; Shao, Q.; Fan, X.; Li, Z. Development of a sensitive LC-MS/MS method for simultaneous quantification of eleven constituents in rat serum and its application to a pharmacokinetic study of a Chinese medicine Shengmai injection. Biomed. Chromatogr. 2015, 29, 275–284.
- Zhan, S.-Y.; Shao, Q.; Fan, X.-H.; Li, Z.; Cheng, Y.-Y. Tissue distribution and excretion of herbal components after intravenous administration of a Chinese medicine (Shengmai injection) in rat. Arch. Pharmacal Res. 2014, 1–12.
- Han, Y.; Sun, H.; Zhang, A.; Wu, F.; Wei, W.; Dong, T.; Wang, X. Characterization and Pharmacokinetic Study of Multiple Constituents from Shengmai San. In Serum Pharmacochemistry of Traditional Chinese Medicine; Wang, X., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 103–117.
- Han, Y.; Wu, F.; Zhang, A.; Sun, H.; Wei, W.; Wang, X. Characterization of multiple constituents in rat plasma after oral administration of Shengmai San using ultra-performance liquid chromatography coupled with electrospray ionization/quadrupole-time-of-flight high-definition mass spectrometry. Anal. Methods 2015, 7, 830–837.
- Qiang, T.-T.; Li, Y.-P.; Wang, X.-L. Inhibitory Effect of Shengmai Injection on CYP450 Enzyme and Transporter in Vitro. Chin. Tradit. Herb. Drugs 2021, 52, 3568–3575.
- Chiang, T.-Y.; Wang, H.-J.; Wang, Y.-C.; Tan, E.C.-H.; Lee, I.-J.; Yun, C.-H.; Ueng, Y.-F. Effects of Shengmai San on key enzymes involved in hepatic and intestinal drug metabolism in rats. J. Ethnopharmacol. 2021, 271, 113914.
- Liang, Y.; Zhou, Y.; Zhang, J.; Rao, T.; Zhou, L.; Xing, R.; Wang, Q.; Fu, H.; Hao, K.; Xie, L.; et al. Pharmacokinetic Compatibility of Ginsenosides and Schisandra Lignans in Shengmai-san: From the Perspective of P-Glycoprotein. PLoS ONE 2014, 9, e98717.




