Sepsis is a life-threatening organ dysfunction caused by a dysregulated host response to infection; the pathophysiology of sepsis is complex. The incidence of sepsis is steadily increasing, with worldwide mortality ranging between 30% and 50%. Current treatment approaches mainly rely on the timely and appropriate administration of antimicrobials and supportive therapies, but the search for pharmacotherapies modulating the host response has been unsuccessful. Chinese herbal medicines, i.e., Chinese patent medicines, Chinese herbal prescriptions, and single Chinese herbs, play an important role in the treatment of sepsis through multicomponent, multipathway, and multitargeting abilities and have been officially recommended for the management of COVID-19.
- Chinese herbal medicines
- sepsis
- antiseptic actions
- material basis
- Qingwen Baidu decoction
- Shengfu injection
- Shengmai
- Xuanbai Chengqi decoction
- XueBiJing injection
1. Introduction
Based on the characteristics of emergency medicine in China, the Preventing Sepsis Campaign in China (PSCC) was initiated in May 2018 [1][17]. It was advocated by experts that the prevention, diagnosis, and treatment of sepsis should be performed as early as possible to decrease morbidity and mortality, and the principle of the prevention of sepsis was introduced to prevent its occurrence. Several Chinese treatment guidelines for sepsis management and expert consensus—e.g., the Chinese guidelines for the emergency management of sepsis and septic shock 2018, the clinical practice guidelines on traditional Chinese medicine therapy alone or combined with antibiotics for sepsis, and the Chinese emergency medicine expert consensus on the diagnosis and treatment of sepsis complicated by disseminated intravascular coagulation—have been successively released for the management of sepsis [2][1][3][16,17,18]. In these treatment guidelines and expert agreements, CHMs are recommended as add-on therapies to complement the conventional treatment of sepsis, e.g., a XueBiJing injection (XBJ) for sepsis, a ShenFu injection (SF) for septic shock, the ShengMai formula (SMF) for sepsis with the qi and yin exhaustion pattern, the Xuanbai Chengqi decoction (XBCQ) for sepsis with acute respiratory distress syndrome (ARDS), the Qingwen Baidu decoction (QWBD) for sepsis with the internal exuberance of toxins and heat pattern, etc. [4][15]. The diagnosis and treatment protocol for COVID-19 (the revised eighth version) released by China’s National Health Commission also recommends the use of CHM in accordance with different degrees of severity of COVID-19 [5][19]. XueBiJing, ShenFu, and ShengMai injections are typical herbal injections officially recommended for the management of COVID-19 when patients with a severe case of the disease develop SIRS and/or MODS [5][19].
2. Chinese Patent Medicines - XueBiJing Injection
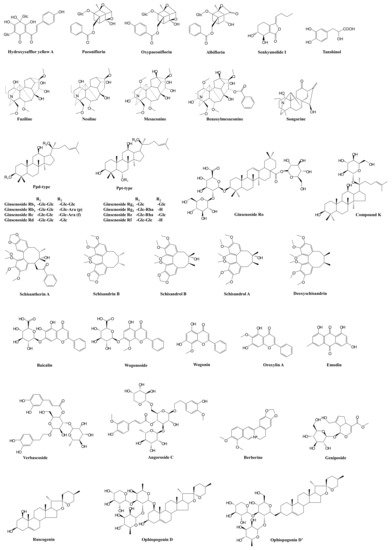
| Prescription | Component Herbs | Chemical Composition | Pharmacological Actions | Bioactive and Bioavailable Compounds | Potential Target Pathway | Potential DDI Target | References | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| XueBiJing injection | Carthamus tinctorius | flower (Honghua in Chinese), | Paeonia lactiflora | root (Chishao), | Ligusticum chuanxiong | rhizome (Chuanxiong), | Angelica sinensis | root (Danggui), and | Salvia miltiorrhiza | root (Danshen) | Flavonoids, monoterpene glycosides, catechols, phthalides, organic acids, etc. | Exhibit anti-inflammatory, anticoagulant, endothelium-protective, immunoregulatory, antioxidant, and organ-protective activities; inhibit ox-LDL-induced apoptosis; improve microcirculation and myocardial ischemia/reperfusion injury | Hydroxysafflor yellow A | TLR4/NF-κB; NLRP3; Rac1/Akt; NF-κB/ICAM-1 | — | [45][47][48][51] | [59,61,62,76] | ||||||||||||||||||||||
| Paeoniflorin Oxypaeoniflorin Albiflorin |
SIRT1; IRAK1-NF-κB; IκB; PI3K/Akt; TLR2; Sirt1/Foxo1 | — | [19][43][46][52][53][54] | [33,57,60,77,78,79] | |||||||||||||||||||||||||||||||||||
| Senkyunolide I | p-Erk1/2; Nrf2/HO-1; Caspase 3; MAPK; TLRs | As victim: UGT2B15 |
[15][19][50][55] | [29,33,64,80] | |||||||||||||||||||||||||||||||||||
| Tanshinol | cAMP-PKA | As victim: OAT1/2 |
[15][44] | [29,58] | |||||||||||||||||||||||||||||||||||
| ShenFu injection | Panax ginseng | steamed root (Hongshen) and processed | Aconitum carmichaelii | root (Fuzi) | Ginsenosides, aconitum alkaloids, organic acids, etc. | Regulate oxidative stress and inflammatory responses, inhibit HMGB1-mediated severe inflammatory response, restore endothelial integrity, attenuate the proinflammatory response, enhance innate immunity, preserve adaptive immunity, alleviate neuropathic pain | Ginsenosides Rb | 1 | , Rc, Rb | 2 | , Rf, Rd, Rg | 1 | , etc. | TLR4; PXR/NF-κB; TLRs/IRAK-1; TBK-1/IκB kinase ε/IRF-3; p38/ATF-2 | As substrate: OATP1B3 (for Ginsenosides Rg | 1 | , Rf) As perpetrator: OATP1B1/1B3 (for Ginsenosides Rb | 1 | , Rc, Rb | 2 | , Rd) | [56][57][58][]68] | [81,82 | 59][ | ,83 | [62] | ,84 | 60][61[63] | ,85 | [64] | ,86 | [ | ,87 | 65 | ,88 | ][66][67] | ,89 | [ | ,90,91,92,93] |
| Benzoylmesaconine Fuziline Mesaconine Neoline Songorine |
TLR4/NF-κB; Nrf2 | As victim: P-gp (for benzoylmesaconine) |
[69][70][71] | [94 | [ | ,95 | 72 | ,96 | ] | ,97] | |||||||||||||||||||||||||||||
| ShengMai formula | Panax ginseng | root (Renshen), | Ophiopogon japonicus | root (Maidong), and | Schisandra chinensis | fruit (Wuweizi) | Ginsenosides, lignans, steroidal saponins, and homoisoflavanones | Exhibit anti-inflammatory or antioxidant, hepatoprotective activities | Ginsenosides Rb₁, Rb | 2 | , Rc, Rd, Re, Rg | 1 | , Rh | 1 | , Compound K, Rf, and Rg | 2 | TLR4; PXR/NF-κB; TLRs/IRAK-1; TBK-1/IκB kinase ε/IRF-3; p38/ATF-2 | As substrate: OATP1B1/1B3 (for Ginsenoside Rg | 2 | ) OATP1B3 (for Ginsenosides Rg | 1 | , Rf, Re) As perpetrator: OATP1B1/1B3 (for Ginsenosides Rb | 1 | , Rc, Rb | 2 | , Rd) NTCP (for Rg1) CYP3A (for Rd) |
[56][57][58][59][60][61][62][63][64][65][66][67][68][73][74][75][76] | [81,82,83,84,85,86,87,88,89,90,91,92,93,98,99,100,101] | |||||||||||
| Ophiopogonin D Ophiopogonin D’ Ruscogenin |
PPARα; NF-κB/IκBα; SIRT1; TLR4; TLR4/NF-κB/MyD88 | As perpetrator: CYP3A4, 2C9, and 2E1 (for Ophiopogonin D) UGT1A6/1A8 (for Ophiopogonin D) UGT1A6/1A10 (for Ophiopogonin D’) NTCP (for Ophiopogonin D’) CYP3A (for Ophiopogonin D) As victim: OATP1B1/1B3 (for Ophiopogonin D) |
[67][76][77][78][79][80][81] | [92,101,102,103,104,105,106] | |||||||||||||||||||||||||||||||||||
| Schisandrol A Schisandrol B Schizandrin A Schizandrin B Deoxyschisandrin |
iNOS; COX-2; PGE2; MAPK; TLR4/NF-κB/MyD88 | As perpetrator: NTCP (for Schizandrin A) | [75][82][83][84][85] | [100,107,108,109,110] | |||||||||||||||||||||||||||||||||||
| Qingwen Baidu decoction |
Rehmannia glutinosa | root (Dihuang), | Rhinoceros unicornis | horn (Xijiao), | Coptidis chinensis | rhizome (Huanglian), | Gardenia jasminoides | fruit (Zhizi), | Platycodon grandiflorum | root (Jiegeng), | Scutellaria baicalensis | root (Huangqin), | Anemarrhena asphodeloides | rhizome (Zhimu), | Paeonia lactiflora | root (Chishao), | Scrophularia ningpoensis | root (Xuanshen), | Forsythia suspense | fruit (Lianqiao), | Lophatherum gracile | stem and leaf(Danzhuye), | Glycyrrhiza uralensis | root and rhizome (Gancao), | Paeonia suffruticosa | root cortex (Danpi), and | Gypsum Fibrosum | (Shigao) | Alkaloids, iridoids, flavonoids, etc. | Reduce LPS-induced intestinal damage; treat inflammation; alleviate LPS-induced acute kidney injury; alleviate liver injury in sepsis; exhibit anti-inflammatory, antioxidant, and cardioprotective effects | Berberine | TLRs; NF-κB; STAT3; Wnt/β-catenin; PI3K/Akt; MAPK/JNK/p38/ERK | As perpetrator: CYP3A4, CYP2D6 As victim: P-gp |
[86][87][88][89][90][91][92] | [111,112,113,114,115,116,117] | ||||
| Geniposide Genipin |
NF-κB; MAPK; PPARγ; AMPK; NLRP3; AKT-mTOR | — | [93][94] | 119 | [95][96] | ,120 | [97] | ,121 | [98] | [118,,122,123] | |||||||||||||||||||||||||||||
| Baicalin | iNOS; COX-2; NF-κB; HMGB1 | As perpetrator: CYP1A2/3A/2E1, OATP1B1, P-gp | [99][100][101][102][103] | [124,125,126,127,128] | |||||||||||||||||||||||||||||||||||
| Wogonoside Wogonin |
TLR4; NF-κB; Nrf2; NLRP3 | As perpetrator: CYP1A2 (for Wogonin) | [104][105][106][107][108] | [129,130,131,132,133] | |||||||||||||||||||||||||||||||||||
| Oroxylin A | JAK/STAT; IRF2BP2-NFAT1; NF-κB | As perpetrator: CYP1A2, OATP1B1, OAT1/3 and BCRP | [108][109][110][111][112][113][114][115] | [133,134,135,136,137,138,139,140] | |||||||||||||||||||||||||||||||||||
| Verbascoside | iNOS | — | [116][117][118] | [141,142,143] | |||||||||||||||||||||||||||||||||||
| XuanBai Chengqi decoction |
Rheum palmatum | rhizome and root (Dahuang), | Gypsum Fibrosum | (Shigao), | Prunus armeniaca | seed (Kuxingren), and | Trichosanthes kirilowii | fruit (Gualou) | Anthraquinones, etc. | Attenuate LPS-induced microcirculatory disturbance | Emodin | TLR4/NF-κB/ICAM-1; JAK1/STAT3; MAPK; cAMP-PKA; NLRP3; PPARγ | As victim: CYP1A2, UGT1A8/1A10/12B7 | [119][120][121][122][123][][125][126] | [144,145,146,147 | 124 | ,148,149,150,151] |
