
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Greta Elovsson | + 2637 word(s) | 2637 | 2021-10-11 11:31:14 | | | |
| 2 | Vicky Zhou | Meta information modification | 2637 | 2021-10-30 03:08:09 | | |
Video Upload Options
Drosophila melanogaster has proved to be a dynamic model organism that can produce high-quality data in a short time frame. One of the fly’s most prominent feature is the possibility to perform genetic alterations through the well-known Gal4/UAS expression system, thus making it possible to express target proteins in a specific cell type or tissue.
1. Introduction
Neurodegenerative diseases, such as Alzheimer’s disease (AD), are associated with proteotoxicity, which is caused by protein aggregation and results in extensive neuronal damage in the brain. Neurodegeneration and the disturbance of essential functions in the cell manifest in cognitive impairments and premature death [1][2]. A main proteotoxic contributor in AD is the amyloid-β (Aβ) peptide, where the propensity to aggregate differs between different variants [3]. Aβ misfolds and aggregates into pre-fibrillar assemblies that are highly associated with toxicity. They then progressively merge into mature fibrils [1][4]. There is an urgent need to find disease-modifying treatments and, therefore, reliable and powerful methods to pin down fundamental components underlying the mechanisms responsible for proteotoxicity are needed. Drosophila offers a number of advantages as a model system for studying diseases where: (i) a variety of phenotypic markers are available for identifying detrimental effects due to proteotoxicity, (ii) the lifespan of Drosophila makes it possible to investigate age-related diseases on a reasonable time scale (days to weeks, as opposed to months and years, in mouse model systems), (iii) the system is amenable to large drug screens since the flies proliferate well and are relatively inexpensive and easy to work with and (vi) there are extensive tools that allow disease-related genes and molecular pathways to be genetically and pharmacologically manipulated in order to find out both the function of their orthologs in vivo, and how these genes are involved in the pathogenesis of different diseases, which can generate in vivo data that are translatable to mammalian system [5][6].
2. Drosophila as Model Organism for Drug Screen against Aβ Proteotoxicity
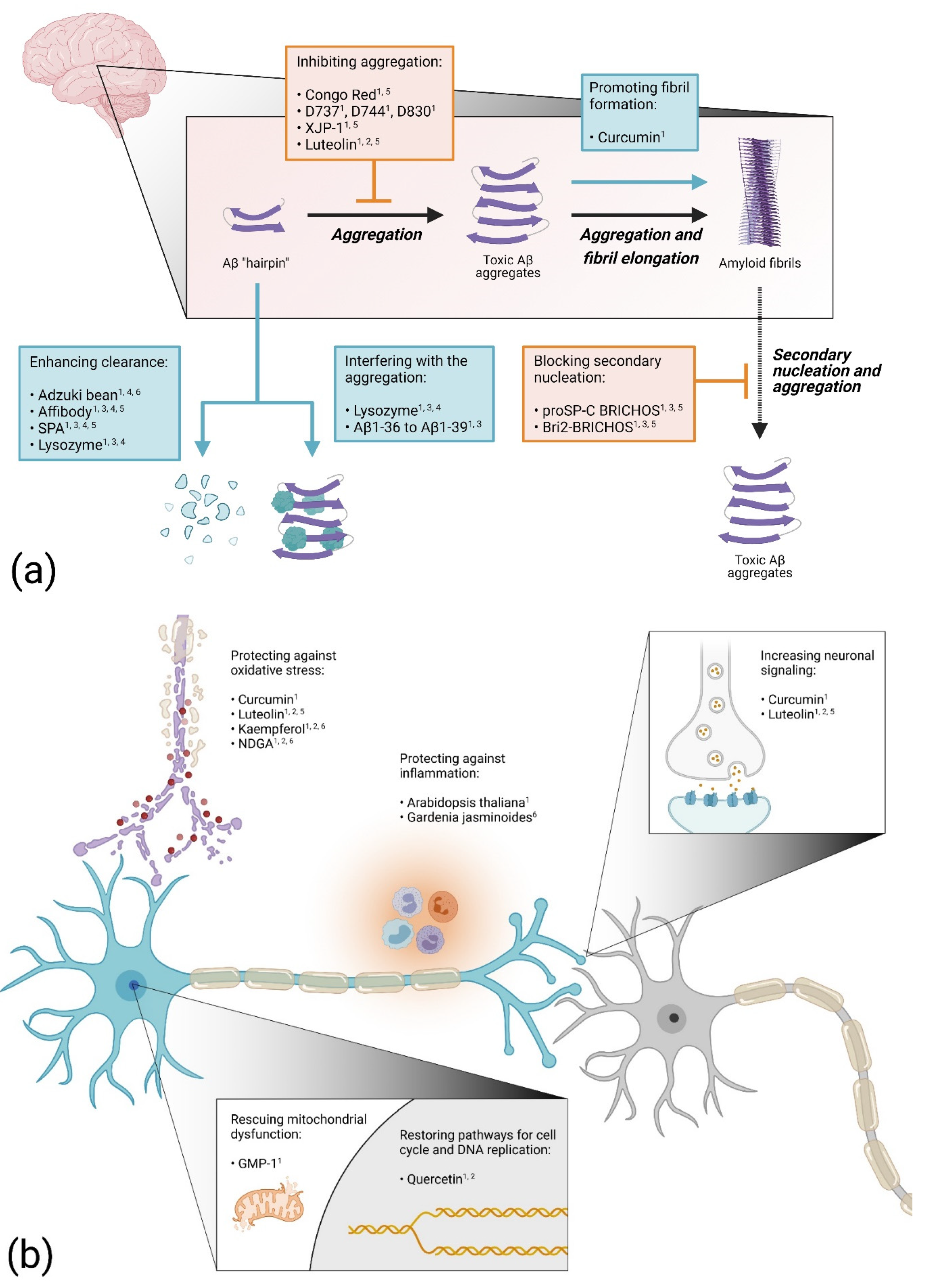
2.1. Blocking Aβ Aggregation
2.2. Enhancing Aβ Aggregation
2.3. Increasing Protein Clearance
2.4. Proteins and Peptides as Drug Candidates
2.5. Targeting Inflammatory Processes
2.6. Preventing Oxidative Stress
2.7. Preventing Mitochondrial Dysfunction
3. Conclusions
References
- Takahashi, R.H.; Nagao, T.; Gouras, G.K. Plaque Formation and the Intraneuronal Accumulation of β-Amyloid in Alzheimer’s Disease. Pathol. Int. 2017, 67, 185–193.
- Alzheimer’s Association. 2016 Alzheimer’s Disease Facts and Figures. Alzheimers Dement. J. Alzheimers Assoc. 2016, 12, 459–509.
- Roher, A.E.; Kokjohn, T.A.; Clarke, S.G.; Sierks, M.R.; Maarouf, C.L.; Serrano, G.E.; Sabbagh, M.S.; Beach, T.G. APP/Aβ Structural Diversity and Alzheimer’s Disease Pathogenesis. Neurochem. Int. 2017, 110, 1–13.
- Thal, D.R.; Walter, J.; Saido, T.C.; Fändrich, M. Neuropathology and Biochemistry of Aβ and Its Aggregates in Alzheimer’s Disease. Acta Neuropathol. 2015, 129, 167–182.
- Yamaguchi, M.; Yoshida, H. Drosophila as a Model Organism. Adv. Exp. Med. Biol. 2018, 1076, 1–10.
- Mhatre, S.D.; Paddock, B.E.; Saunders, A.J.; Marenda, D.R. Invertebrate Models of Alzheimer’s Disease. J. Alzheimers Dis. 2013, 33, 3–16.
- Crowther, D.C.; Kinghorn, K.J.; Miranda, E.; Page, R.; Curry, J.A.; Duthie, F.A.I.; Gubb, D.C.; Lomas, D.A. Intraneuronal Aβ, Non-Amyloid Aggregates and Neurodegeneration in a Drosophila Model of Alzheimer’s Disease. Neuroscience 2005, 132, 123–135.
- McKoy, A.F.; Chen, J.; Schupbach, T.; Hecht, M.H. A Novel Inhibitor of Amyloid β (Aβ) Peptide Aggregation. J. Biol. Chem. 2012, 287, 38992–39000.
- McKoy, A.F.; Chen, J.; Schupbach, T.; Hecht, M.H. Structure-Activity Relationships for a Series of Compounds That Inhibit Aggregation of the Alzheimer’s Peptide, Aβ 42. Chem. Biol. Drug Des. 2014, 84, 505–512.
- Uras, G.; Manca, A.; Zhang, P.; Markus, Z.; Mack, N.; Allen, S.; Bo, M.; Xu, S.; Xu, J.; Georgiou, M.; et al. In Vivo Evaluation of a Newly Synthesized Acetylcholinesterase Inhibitor in a Transgenic Drosophila Model of Alzheimer’s Disease. Front. Neurosci. 2021, 15, 691222.
- Caesar, I.; Jonson, M.; Nilsson, K.P.R.; Thor, S.; Hammarström, P. Curcumin Promotes A-Beta Fibrillation and Reduces Neurotoxicity in Transgenic Drosophila. PLoS ONE 2012, 7, e31424.
- Akinyemi, A.J.; Oboh, G.; Ogunsuyi, O.; Abolaji, A.O.; Udofia, A. Curcumin-Supplemented Diets Improve Antioxidant Enzymes and Alter Acetylcholinesterase Genes Expression Level in Drosophila melanogaster Model. Metab. Brain Dis. 2018, 33, 369–375.
- Luheshi, L.M.; Hoyer, W.; de Barros, T.P.; van Dijk Härd, I.; Brorsson, A.-C.; Macao, B.; Persson, C.; Crowther, D.C.; Lomas, D.A.; Ståhl, S.; et al. Sequestration of the Aβ Peptide Prevents Toxicity and Promotes Degradation In Vivo. PLoS Biol. 2010, 8, e1000334.
- Miyazaki, H.; Okamoto, Y.; Motoi, A.; Watanabe, T.; Katayama, S.; Kawahara, S.; Makabe, H.; Fujii, H.; Yonekura, S. Adzuki Bean (Vigna angularis) Extract Reduces Amyloid-β Aggregation and Delays Cognitive Impairment in Drosophila Models of Alzheimer’s Disease. Nutr. Res. Pract. 2019, 13, 64.
- Kruppa, A.J.; Ott, S.; Chandraratna, D.S.; Irving, J.A.; Page, R.M.; Speretta, E.; Seto, T.; Camargo, L.M.; Marciniak, S.J.; Lomas, D.A.; et al. Suppression of Aβ Toxicity by Puromycin-Sensitive Aminopeptidase Is Independent of Its Proteolytic Activity. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2013, 1832, 2115–2126.
- Helmfors, L.; Boman, A.; Civitelli, L.; Nath, S.; Sandin, L.; Janefjord, C.; McCann, H.; Zetterberg, H.; Blennow, K.; Halliday, G.; et al. Protective Properties of Lysozyme on β-Amyloid Pathology: Implications for Alzheimer Disease. Neurobiol. Dis. 2015, 83, 122–133.
- Sandin, L.; Bergkvist, L.; Nath, S.; Kielkopf, C.; Janefjord, C.; Helmfors, L.; Zetterberg, H.; Blennow, K.; Li, H.; Nilsberth, C.; et al. Beneficial Effects of Increased Lysozyme Levels in Alzheimer’s Disease Modelled in Drosophila melanogaster. FEBS J. 2016, 283, 3508–3522.
- Moore, B.D.; Martin, J.; de Mena, L.; Sanchez, J.; Cruz, P.E.; Ceballos-Diaz, C.; Ladd, T.B.; Ran, Y.; Levites, Y.; Kukar, T.L.; et al. Short Aβ Peptides Attenuate Aβ42 Toxicity in Vivo. J. Exp. Med. 2018, 215, 283–301.
- Hermansson, E.; Schultz, S.; Crowther, D.; Linse, S.; Winblad, B.; Westermark, G.; Johansson, J.; Presto, J. The Chaperone Domain BRICHOS Prevents Amyloid β-Peptide CNS Toxicity in Drosophila melanogaster. Dis. Model. Mech. 2014, 7, 659–665.
- Poska, H.; Haslbeck, M.; Kurudenkandy, F.R.; Hermansson, E.; Chen, G.; Kostallas, G.; Abelein, A.; Biverstål, H.; Crux, S.; Fisahn, A.; et al. Dementia-Related Bri2 BRICHOS Is a Versatile Molecular Chaperone That Efficiently Inhibits Aβ42 Toxicity in Drosophila. Biochem. J. 2016, 473, 3683–3704.
- Cohen, S.I.A.; Arosio, P.; Presto, J.; Kurudenkandy, F.R.; Biverstål, H.; Dolfe, L.; Dunning, C.; Yang, X.; Frohm, B.; Vendruscolo, M.; et al. A Molecular Chaperone Breaks the Catalytic Cycle That Generates Toxic Aβ Oligomers. Nat. Struct. Mol. Biol. 2015, 22, 207–213.
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a Central Mechanism in Alzheimer’s Disease. Alzheimers Dement. Transl. Res. Clin. Interv. 2018, 4, 575–590.
- Mattioli, R.; Francioso, A.; d’Erme, M.; Trovato, M.; Mancini, P.; Piacentini, L.; Casale, A.; Wessjohann, L.; Gazzino, R.; Costantino, P.; et al. Anti-Inflammatory Activity of A Polyphenolic Extract from Arabidopsis Thaliana in In Vitro and In Vivo Models of Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 708.
- Kong, Y.; Li, K.; Fu, T.; Wan, C.; Zhang, D.; Song, H.; Zhang, Y.; Liu, N.; Gan, Z.; Yuan, L. Quercetin Ameliorates Aβ Toxicity in Drosophila AD Model by Modulating Cell Cycle-Related Protein Expression. Oncotarget 2016, 7, 67716–67731.
- Beg, T.; Jyoti, S.; Naz, F.; Rahul; Ali, F.; Ali, S.K.; Reyad, A.M.; Siddique, Y.H. Protective Effect of Kaempferol on the Transgenic Drosophila Model of Alzheimer’s Disease. CNS Neurol. Disord. Drug Targets 2018, 17, 421–429.
- Ali, F.; Rahul; Jyoti, S.; Naz, F.; Ashafaq, M.; Shahid, M.; Siddique, Y.H. Therapeutic Potential of Luteolin in Transgenic Drosophila Model of Alzheimer’s Disease. Neurosci. Lett. 2019, 692, 90–99.
- Ma, W.-W.; Tao, Y.; Wang, Y.-Y.; Peng, I.-F. Effects of Gardenia Jasminoides Extracts on Cognition and Innate Immune Response in an Adult Drosophila Model of Alzheimer’s Disease. Chin. J. Nat. Med. 2017, 15, 899–904.
- Fedorova, M.; Bollineni, R.C.; Hoffmann, R. Protein Carbonylation as a Major Hallmark of Oxidative Damage: Update of Analytical Strategies: PROTEIN CARBONYLATION: AN ANALYTICAL UPDATE. Mass Spectrom. Rev. 2014, 33, 79–97.
- Siddique, Y.H.; Ali, F. Protective Effect of Nordihydroguaiaretic Acid (NDGA) on the Transgenic Drosophila Model of Alzheimer’s Disease. Chem. Biol. Interact. 2017, 269, 59–66.
- Lee, J.; Kim, Y.; Liu, T.; Hwang, Y.J.; Hyeon, S.J.; Im, H.; Lee, K.; Alvarez, V.E.; McKee, A.C.; Um, S.-J.; et al. SIRT3 Deregulation Is Linked to Mitochondrial Dysfunction in Alzheimer’s Disease. Aging Cell 2018, 17, e12679.
- Chen, Y.-G. Research Progress in the Pathogenesis of Alzheimer’s Disease. Chin. Med. J. 2018, 131, 1618–1624.
- Pavlov, P.F.; Hutter-Paier, B.; Havas, D.; Windisch, M.; Winblad, B. Development of GMP-1 a Molecular Chaperone Network Modulator Protecting Mitochondrial Function and Its Assessment in Fly and Mice Models of Alzheimer’s Disease. J. Cell. Mol. Med. 2018, 22, 3464–3474.
- Speretta, E.; Jahn, T.R.; Tartaglia, G.G.; Favrin, G.; Barros, T.P.; Imarisio, S.; Lomas, D.A.; Luheshi, L.M.; Crowther, D.C.; Dobson, C.M. Expression in Drosophila of Tandem Amyloid β Peptides Provides Insights into Links between Aggregation and Neurotoxicity. J. Biol. Chem. 2012, 287, 20748–20754.




