
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ferdinando Nicoletti | + 2646 word(s) | 2646 | 2021-10-28 11:03:01 | | | |
| 2 | Peter Tang | Meta information modification | 2646 | 2021-10-29 06:05:37 | | |
Video Upload Options
The most common form of dementia is accounted for by Alzheimer′s disease (AD) that includes between 50% and 75% of the cases of dementia with a doubling of its prevalence every five years after the age of 65 years. Macrophage migration inhibitory factor (MIF) is a pleiotropic cytokine produced by several cells of the innate and adaptive immune system, as well as non-immune cells. Dismantling the exact role of MIF and its receptors in AD may offer novel diagnostic and therapeutic opportunities in AD.
1. Introduction
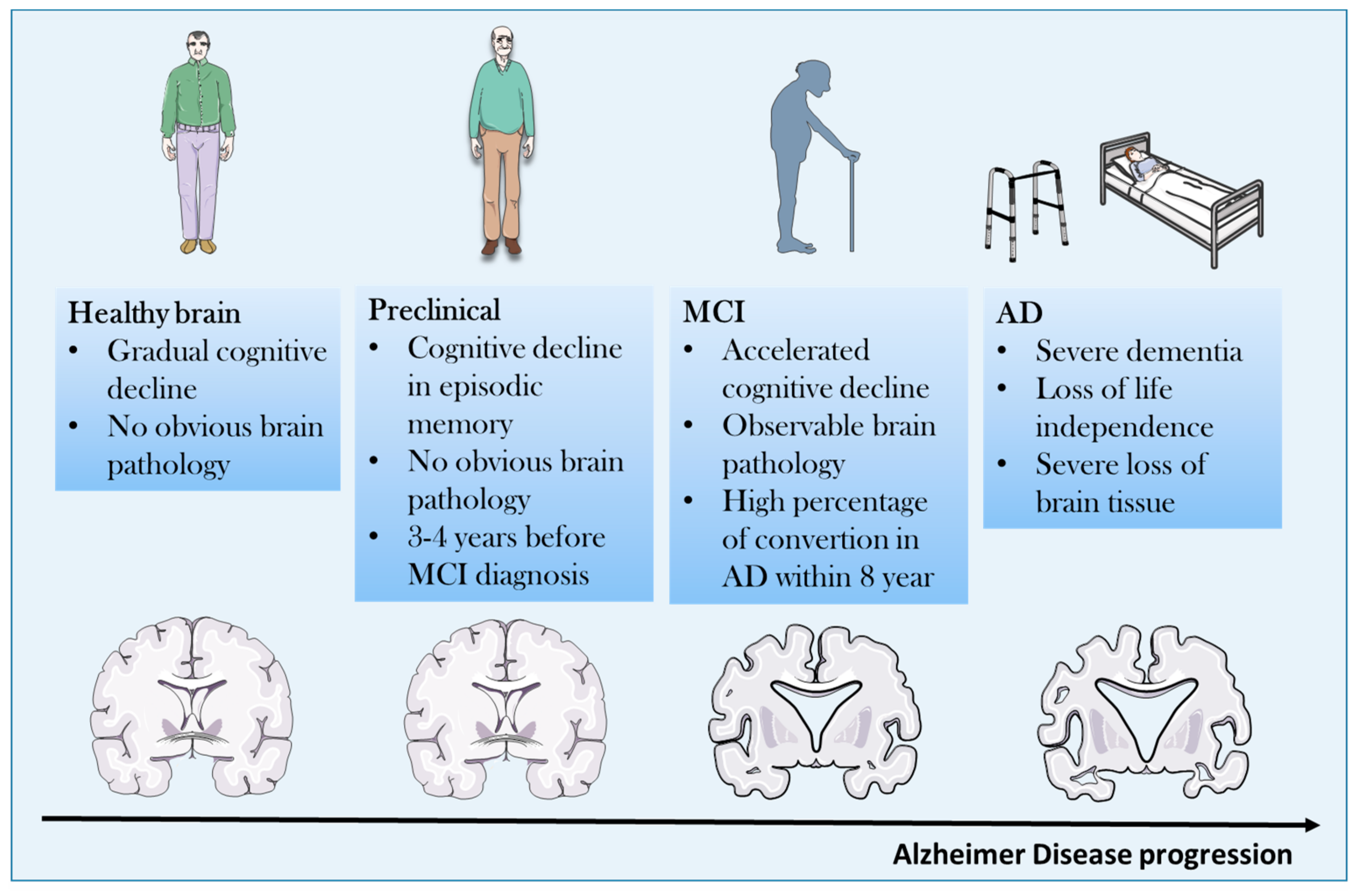
2. Pathogenetic Concepts
3. Neuroinflammation and AD
3.1. Innate Immune System and AD
3.2. Proinflammaory Cytokines and AD
3.3. Proinflammatory Cytokines as Biomarkers During AD Development and Progression
4. MIF: An Emerging Player in the Neuroinflammatory Hypothesis of AD
4.1. Biology, Physiology and Physiopathology of MIF
4.2. The Emerging Role of MIF Homologue, D-dopachrome Tautomerase (D-DT; MIF-2)
5. MIF in Neurodegenerative Diseases
MIF in PD and ALS
|
Disease |
Preclinical Data |
Human Data |
References |
|---|---|---|---|
|
Parkinson′s Diseases (PD) |
MIF reduces apoptosis and induces autophagy in an in vitro model of PD (SH-SY5Y cells exposed to MPP+) |
[47] |
|
|
MIF is upregulated in mouse model of PD (induced by i.p. injection of MPTP) |
[47] |
||
|
↑ serum levels |
[48] |
||
|
Amyotrophic Lateral Sclerosis (ALS) |
MIF inhibits mutant SOD1 misfolding in motor neuron-like cells |
||
|
Endogenous MIF knockdown in SOD1 mutant mice accelerates disease |
|||
|
MIF overexpression in the spinal cord improves ALS in SOD1 mutant mice |
6. MIF and AD
|
Preclinical Data |
Human Data |
References |
|---|---|---|
|
ISO-1 reduces Aβ-induced toxicity in vitro |
||
|
↑ MIF in CNS of AD and MCI patients |
||
|
MIF colocalizes with Aβ plaques in APP23 transgenic mice |
||
|
↑ MIF in plasma of AD and MCI patients |
[54] |
|
|
MIF deficiency attenuates tau hyperphosphorylation in astrocytes from APP/PS1 transgenic mice |
[55] |
|
|
MIF overexpression prevents Aβ toxicity in SH-SY5Y cells |
[56] |
|
|
APP23/MIF+/− mice show significant memory impairment |
[56] |
References
- Briggs, R.; Kennelly, S.P.; O’Neill, D. Drug treatments in Alzheimer’s disease. Clin. Med. 2016, 16, 247–253.
- Adlimoghaddam, A.; Neuendorff, M.; Roy, B.; Albensi, B.C. A review of clinical treatment considerations of donepezil in severe Alzheimer′s disease. CNS Neurosci. Ther. 2018, 24, 876–888.
- Lane, C.A.; Hardy, J.; Schott, J.M. Alzheimer′s disease. Eur. J. Neurol. 2018, 25, 59–70.
- Dubois, B.; Hampel, H.; Feldman, H.H.; Scheltens, P.; Aisen, P.; Andrieu, S.; Bakardjian, H.; Benali, H.; Bertram, L.; Blennow, K.; et al. Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria. Alzheimer’s Dement. 2016, 12, 292–323.
- Melis, R.J.; Haaksma, M.L.; Muniz-Terrera, G. Understanding and predicting the longitudinal course of dementia. Curr. Opin. Psychiatry 2019, 32, 123–129.
- Sigurdsson, E.M. Alzheimer′s therapy development: A few points to consider. Prog. Mol. Biol. Transl. Sci. 2019, 168, 205–217.
- Herradon, G.; Ramos-Alvarez, M.P.; Gramage, E. Connecting Metainflammation and Neuroinflammation Through the PTN-MK-RPTPβ/ζ Axis: Relevance in Therapeutic Development. Front. Pharmacol. 2019, 10, 377.
- Shau, K. ‘Type 3′ diabetes: A brain insulin-resistant state linked to Alzheimer′s disease. Pr. Diabetes 2017, 34, 187–188.
- Kim, B.; Sullivan, K.A.; Backus, C.; Feldman, E.L. Cortical Neurons Develop Insulin Resistance and Blunted Akt Signaling: A Potential Mechanism Contributing to Enhanced Ischemic Injury in Diabetes. Antioxidants Redox Signal. 2011, 14, 1829–1839.
- Stošić-Grujičić, S.; Saksida, T.; Miljković, Đ.; Stojanović, I. MIF and insulin: Lifetime companions from common genesis to common pathogenesis. Cytokine 2020, 125, 154792.
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, U.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405.
- Webers, A.; Heneka, M.T.; Gleeson, P.A. The role of innate immune responses and neuroinflammation in amyloid accumulation and progression of Alzheimer’s disease. Immunol. Cell Boil. 2019, 12301.
- Minter, M.R.; Taylor, J.M.; Crack, P.J. The contribution of neuroinflammation to amyloid toxicity in Alzheimer′s disease. J. Neurochem. 2016, 136, 457–474.
- Su, F.; Bai, F.; Zhang, Z. Inflammatory Cytokines and Alzheimer’s Disease: A Review from the Perspective of Genetic Polymorphisms. Neurosci. Bull. 2016, 32, 469–480.
- Patel, N.S.; Paris, D.; Mathura, V.; Quadros, A.N.; Crawford, F.C.; Mullan, M.J. Inflammatory cytokine levels correlate with amyloid load in transgenic mouse models of Alzheimer′s disease. J. Neuroinflammation 2005, 2.
- Berg, J.V.; Prokop, S.; Miller, K.R.; Obst, J.; E Kälin, R.; Lopategui-Cabezas, I.; Wegner, A.; Mair, F.; Schipke, C.G.; Peters, O.; et al. Inhibition of IL-12/IL-23 signaling reduces Alzheimer’s disease–like pathology and cognitive decline. Nat. Med. 2012, 18, 1812–1819.
- Brosseron, F.; Kolbe, C.-C.; Santarelli, F.; Carvalho, S.; Antonell, A.; Castro-Gomez, S.; Tacik, P.; Namasivayam, A.A.; Mangone, G.; Schneider, R.; et al. AETIONOMY study group Multicenter Alzheimer′s and Parkinson′s disease immune biomarker verification study. Alzheimers. Dement. 2019.
- Popp, J.; Oikonomidi, A.; Tautvydaitė, D.; Dayon, L.; Bacher, M.; Migliavacca, E.; Henry, H.; Kirkland, R.; Severin, I.; Wojcik, J.; et al. Markers of neuroinflammation associated with Alzheimer’s disease pathology in older adults. Brain, Behav. Immun. 2017, 62, 203–211.
- Bacher, M.; Deuster, O.; Aljabari, B.; Egensperger, R.; Neff, F.; Jessen, F.; Popp, J.; Noelker, C.; Reese, J.P.; Al-Abed, Y.; et al. The Role of Macrophage Migration Inhibitory Factor in Alzheimer’s Disease. Mol. Med. 2010, 16, 116–121.
- Peplow, P.V.; Martinez, B. Amelioration of Alzheimer’s disease pathology and cognitive deficits by immunomodulatory agents in animal models of Alzheimer’s disease. Neural Regen. Res. 2019, 14, 1158–1176.
- McManus, R.M.; Heneka, M.T. Role of neuroinflammation in neurodegeneration: New insights. Alzheimer’s Res. Ther. 2017, 9, 14.
- Wang, M.-M.; Miao, D.; Cao, X.-P.; Tan, L.; Tan, L. Innate immune activation in Alzheimer’s disease. Ann. Transl. Med. 2018, 6, 177.
- Rezai-Zadeh, K.; Gate, D.; Gowing, G.; Town, T. How to get from here to there: Macrophage recruitment in Alzheimer’s disease. Curr. Alzheimer Res. 2011, 8, 156–163.
- Joaquín Merino, J.; Muñetón-Gómez, V.; Alvárez, M.-I.; Toledano-Díaz, A. Effects of CX3CR1 and Fractalkine Chemokines in Amyloid Beta Clearance and p-Tau Accumulation in Alzheimer’s Disease (AD) Rodent Models: Is Fractalkine a Systemic Biomarker for AD? Curr. Alzheimer Res. 2016, 13, 403–412.
- Fingerle-Rowson, G.; Koch, P.; Bikoff, R.; Lin, X.; Metz, C.N.; Dhabhar, F.S.; Meinhardt, A.; Bucala, R. Regulation of Macrophage Migration Inhibitory Factor Expression by Glucocorticoids in Vivo. Am. J. Pathol. 2003, 162, 47–56.
- Calandra, T.; Bucala, R. Macrophage Migration Inhibitory Factor (MIF): A Glucocorticoid Counter-Regulator within the Immune System. Crit. Rev. Immunol. 1997, 17, 77–88.
- Jankauskas, S.S.; Wong, D.W.; Bucala, R.; Djudjaj, S.; Boor, P. Evolving complexity of MIF signaling. Cell. Signal. 2019, 57, 76–88.
- Shin, M.S.; Kang, Y.; Wahl, E.R.; Park, H.-J.; Lazova, R.; Leng, L.; Mamula, M.; Krishnaswamy, S.; Bucala, R.; Kang, I. Macrophage Migration Inhibitory Factor Regulates U1 Small Nuclear RNP Immune Complex-Mediated Activation of the NLRP3 Inflammasome. Arthritis Rheumatol. 2019, 71, 109–120.
- Lang, T.; Lee, J.P.W.; Elgass, K.; Pinar, A.A.; Tate, M.D.; Aitken, E.H.; Fan, H.; Creed, S.J.; Deen, N.S.; Traore, D.A.K.; et al. Macrophage migration inhibitory factor is required for NLRP3 inflammasome activation. Nat. Commun. 2018, 9, 2223.
- Cavalli, E.; Mazzon, E.; Basile, M.S.; Mangano, K.; Marco, D.; Bramanti, P.; Nicoletti, F.; Fagone, P.; Petralia, M.C.; Di Marco, R. Upregulated Expression of Macrophage Migration Inhibitory Factor, Its Analogue D-Dopachrome Tautomerase, and the CD44 Receptor in Peripheral CD4 T Cells from Clinically Isolated Syndrome Patients with Rapid Conversion to Clinical Defined Multiple Sclerosis. Medicina 2019, 55, 667.
- Benedek, G.; Meza-Romero, R.; Jordan, K.; Zhang, Y.; Nguyen, H.; Kent, G.; Li, J.; Siu, E.; Frazer, J.; Piecychna, M.; et al. MIF and D-DT are potential disease severity modifiers in male MS subjects. Proc. Natl. Acad. Sci. USA 2017, 114, E8421–E8429.
- Fagone, P.; Mazzon, E.; Cavalli, E.; Bramanti, A.; Petralia, M.C.; Mangano, K.; Al-Abed, Y.; Bramati, P.; Nicoletti, F. Contribution of the macrophage migration inhibitory factor superfamily of cytokines in the pathogenesis of preclinical and human multiple sclerosis: In silico and in vivo evidences. J. Neuroimmunol. 2018, 322, 46–56.
- Bilsborrow, J.B.; Doherty, E.; Tilstam, P.V.; Bucala, R. Macrophage migration inhibitory factor (MIF) as a therapeutic target for rheumatoid arthritis and systemic lupus erythematosus. Expert Opin. Ther. Targets 2019, 23, 733–744.
- Günther, S.; Fagone, P.; Jalce, G.; Atanasov, A.G.; Guignabert, C.; Nicoletti, F. Role of MIF and D-DT in immune-inflammatory, autoimmune, and chronic respiratory diseases: From pathogenic factors to therapeutic targets. Drug Discov. Today 2019, 24, 428–439.
- Lombardo, S.D.; Mazzon, E.; Mangano, K.; Basile, M.S.; Cavalli, E.; Mammana, S.; Fagone, P.; Nicoletti, F.; Petralia, M.C. Transcriptomic Analysis Reveals Involvement of the Macrophage Migration Inhibitory Factor Gene Network in Duchenne Muscular Dystrophy. Genes 2019, 10, 939.
- Petralia, M.C.; Mazzon, E.; Fagone, P.; Basile, M.S.; Lenzo, V.; Quattropani, M.C.; Bendtzen, K.; Nicoletti, F. Pathogenic contribution of the Macrophage migration inhibitory factor family to major depressive disorder and emerging tailored therapeutic approaches. J. Affect. Disord. 2020, 263, 15–24.
- O’Reilly, C.; Doroudian, M.; Mawhinney, L.; Donnelly, S.C. Targeting MIF in Cancer: Therapeutic Strategies, Current Developments, and Future Opportunities. Med. Res. Rev. 2016, 36, 440–460.
- Penticuff, J.C.; Woolbright, B.L.; Sielecki, T.M.; Weir, S.J.; Taylor, J.A. MIF family proteins in genitourinary cancer: Tumorigenic roles and therapeutic potential. Nat. Rev. Urol. 2019, 16, 318–328.
- Soumoy, L.; Kindt, N.; Ghanem, G.; Saussez, S.; Journe, F. Role of Macrophage Migration Inhibitory Factor (MIF) in Melanoma. Cancers 2019, 11, 529.
- Lechien, J.R.; Nassri, A.; Kindt, N.; Brown, D.N.; Journe, F.; Saussez, S. Role of macrophage migration inhibitory factor in head and neck cancer and novel therapeutic targets: A systematic review. Head Neck 2017, 39, 2573–2584.
- Mangano, K.; Mazzon, E.; Basile, M.S.; Di Marco, R.; Bramanti, P.; Mammana, S.; Petralia, M.C.; Fagone, P.; Nicoletti, F. Pathogenic role for macrophage migration inhibitory factor in glioblastoma and its targeting with specific inhibitors as novel tailored therapeutic approach. Oncotarget 2018, 9, 17951–17970.
- Presti, M.; Mazzon, E.; Basile, M.S.; Petralia, M.C.; Bramanti, A.; Colletti, G.; Bramanti, P.; Nicoletti, F.; Fagone, P. Overexpression of macrophage migration inhibitory factor and functionally-related genes, D-DT, CD74, CD44, CXCR2 and CXCR4, in glioblastoma. Oncol. Lett. 2018, 16, 2881–2886.
- Cavalli, E.; Mazzon, E.; Mammana, S.; Basile, M.S.; Lombardo, S.D.; Mangano, K.; Bramanti, P.; Nicoletti, F.; Fagone, P.; Petralia, M.C. Overexpression of Macrophage Migration Inhibitory Factor and Its Homologue D-Dopachrome Tautomerase as Negative Prognostic Factor in Neuroblastoma. Brain Sci. 2019, 9, 284.
- Qi, D.; Hu, X.; Wu, X.; Merk, M.; Leng, L.; Bucala, R.; Young, L.H. Cardiac macrophage migration inhibitory factor inhibits JNK pathway activation and injury during ischemia/reperfusion. J. Clin. Investig. 2009, 119, 3807–3816.
- Tilstam, P.V.; Qi, D.; Leng, L.; Young, L.; Bucala, R. MIF family cytokines in cardiovascular diseases and prospects for precision-based therapeutics. Expert Opin. Ther. Targets 2017, 21, 671–683.
- Merk, M.; Mitchell, R.A.; Endres, S.; Bucala, R. D-dopachrome tautomerase (D-DT or MIF-2): Doubling the MIF cytokine family. Cytokine 2012, 59, 10–17.
- Li, S.; Nie, K.; Zhang, Q.; Guo, M.; Qiu, Y.; Li, Y.; Gao, Y.; Wang, L. Macrophage Migration Inhibitory Factor Mediates Neuroprotective Effects by Regulating Inflammation, Apoptosis and Autophagy in Parkinson’s Disease. Neuroscience 2019, 416, 50–62.
- Nicoletti, A.; Fagone, P.; Donzuso, G.; Mangano, K.; Dibilio, V.; Caponnetto, S.; Bendtzen, K.; Zappia, M.; Nicoletti, F. Parkinson’s disease is associated with increased serum levels of macrophage migration inhibitory factor. Cytokine 2011, 55, 165–167.
- Israelson, A.; Ditsworth, D.; Sun, S.; Song, S.; Liang, J.; Hruska-Plochan, M.; McAlonis-Downes, M.; Abu-Hamad, S.; Zoltsman, G.; Shani, T.; et al. Macrophage migration inhibitory factor as a chaperone inhibiting accumulation of misfolded SOD1. Neuron 2015, 86, 218–232.
- Shvil, N.; Banerjee, V.; Zoltsman, G.; Shani, T.; Kahn, J.; Abu-Hamad, S.; Papo, N.; Engel, S.; Bernhagen, J.; Israelson, A. MIF inhibits the formation and toxicity of misfolded SOD1 amyloid aggregates: Implications for familial ALS. Cell Death Dis. 2018, 9, 107.
- Leyton-Jaimes, M.F.; Benaim, C.; Abu-Hamad, S.; Kahn, J.; Guetta, A.; Bucala, R.; Israelson, A. Endogenous macrophage migration inhibitory factor reduces the accumulation and toxicity of misfolded SOD1 in a mouse model of ALS. Proc. Natl. Acad. Sci. USA 2016, 113, 10198–10203.
- Leyton-Jaimes, M.F.; Kahn, J.; Israelson, A. AAV2/9-mediated overexpression of MIF inhibits SOD1 misfolding, delays disease onset, and extends survival in mouse models of ALS. Proc. Natl. Acad. Sci. USA 2019, 116, 14755–14760.
- Oyama, R.; Yamamoto, H.; Titani, K. Glutamine synthetase, hemoglobin α-chain, and macrophage migration inhibitory factor binding to amyloid β-protein: Their identification in rat brain by a novel affinity chromatography and in Alzheimer’s disease brain by immunoprecipitation. Biochim. et Biophys. Acta (BBA)-Protein Struct. Mol. Enzym. 2000, 1479, 91–102.
- Lee, K.S.; Chung, J.H.; Lee, K.H.; Shin, M.-J.; Oh, B.H.; Hong, C.H. Bioplex analysis of plasma cytokines in Alzheimer’s disease and mild cognitive impairment. Immunol. Lett. 2008, 121, 105–109.
- Li, S.-Q.; Yü, Y.; Han, J.-Z.; Wang, D.; Liu, J.; Qian, F.; Fan, G.-H.; Bucala, R.; Ye, R.D. Deficiency of macrophage migration inhibitory factor attenuates tau hyperphosphorylation in mouse models of Alzheimer’s disease. J. Neuroinflammation 2015, 12, 177.
- Zhang, S.; Zhao, J.; Zhang, Y.; Zhang, Y.; Cai, F.; Wang, L.; Song, W. Upregulation of MIF as a defense mechanism and a biomarker of Alzheimer’s disease. Alzheimer’s Res. Ther. 2019, 11, 54.




