| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Anna Maria Rychter | + 2922 word(s) | 2922 | 2020-08-03 11:15:48 | | | |
| 2 | Bruce Ren | Meta information modification | 2922 | 2020-08-08 03:12:43 | | | | |
| 3 | Bruce Ren | Meta information modification | 2922 | 2020-08-08 03:13:10 | | |
Video Upload Options
Although many preventive and treatment approaches have been proposed, cardiovascular disease (CVD) remains one of the leading causes of deaths worldwide. Current epidemiological data require the specification of new causative factors, as well as the development of improved diagnostic tools to provide better cardiovascular management. Excessive accumulation of adipose tissue among patients suffering from obesity not only constitutes one of the main risk factors of CVD development but also alters adipokines. Increased attention is devoted to bioactive adipokines, which are also produced by the adipose tissue. The retinol-binding protein 4 (RBP4) has been associated with numerous CVDs and is presumably associated with an increased cardiovascular risk. With this in mind, exploring the role of RBP4, particularly among patients with obesity, could be a promising direction and could lead to better CVD prevention and management in this patient group.
1. Introduction
Cardiovascular disease (CVD) constitutes the most common cause of death in European countries, accounting for 2.2 million deaths in females (47% of all-cause of deaths) and 1.9 million deaths in males (39% of all-cause of deaths) [1][2][3][4]. Additionally, it is responsible for 37% and 34% of all years lost (measured by potential years of life lost, PYLL) among females and males, respectively [1]. Not only is CVD a health issue, but it also involves a significant socio-economic impact [1]. It is estimated that by 2030 the total cost of CVD will rise to USD 1044 billion [5]. Currently, more than half (55%) of the costs is derived from direct healthcare (report on Accident and Emergency departments, medications; inpatient-, outpatient-and primary care) and 45% of the costs originate from the informal care and productivity loss due to morbidity and mortality [4]. The major risk factors of CVD development have been identified in the Framingham Heart Study and INTERHEART case-control study [6][7]. Eight risk factors and health behaviors (hypertension, dyslipidemia, diabetes, obesity, smoking, alcohol, diet, sedentary lifestyle) are the World Health Organization’s (WHO) targets for reduction by 2025 [8]. Obesity is a serious health problem and, if the current trends continue, in the next ten years, almost 40% and 20% of the global adult population will be suffering from overweight and obesity, respectively [9]. Currently, it is estimated that over 3 million patients worldwide die due to excessive body weight [10]. Moreover, obesity may influence cardiovascular risk by means of the presence of obesity-related comorbidities, hemodynamic repercussions, body fat mass content and distribution [11][12][13]. All-cause mortality increased log-linearly throughout the overweight range, with the hazard ratio (HR) of 1.39 per 5 kg/m2 [14]. Excessive accumulation of adipose tissue, particularly visceral fat (VF), contributes to a higher prevalence of hypertension, dyslipidemia, and glucose intolerance, which lead to CVD development [15][16][17]. Adipose tissue also regulates many systemic and pathological processes due to the secretion of bioactive proteins—adipokines [18]. Most of them—e.g., tumor necrosis factor-α (TNF-α); IL-6: interleukin (IL-6)—have pro-inflammatory properties and have been associated with vascular and atherothrombotic complications in atherosclerosis, since they influence the function of endothelial cells, arterial smooth muscle cells, and macrophages in the vessel walls [19][20][21][22][23]. However, few of them may have a protective effect in CVD (e.g., adiponectin). In fact, among patients with obesity, the secretion of adipokines is frequently abnormal [24]. We have characterized one of the novel adipokines—retinol-binding protein 4 (RBP4), with a particular emphasis on its role in obesity and CVD development. However, there is an ongoing controversy with regard to any RBP4 role in both inflammation and CVD prediction. Thus, more reviews and research studies are still necessary.
2. Role and Structure of RBP4
Retinol-binding protein 4 (RBP4), presented in Figure 1, belongs to the lipocalin family and has a tertiary structure known as the ‘lipocalin fold’ which facilitates the binding of small hydrophobic molecules, such as lipids [19]. RBP4 is synthesized and secreted in the liver (mostly) and other tissues, such as adipose tissue [24]. It transports vitamin A (retinol) from the liver to target tissues and constitutes a major regulator of circulating levels of retinol [25]. RBP4 is transferred within the bloodstream in combination with transthyretin (TTR), which prevents kidney filtration and catabolism of RBP4 [26]. The receptor proteins for RBP4 are STRA6 (stimulated by retinoic acid gene 6) and RBPR-2 (RBP4-receptor 2) [26]. In fact, urinary excretion of RBP4 may be a useful marker for the detection of renal dysfunction [27][28]. In patients with chronic kidney disease (CKD), higher serum RBP4 levels were associated with a higher rate of cardiovascular events and higher mortality, which suggests that RBP4 levels may indicate an increased risk of cardiovascular risk within this group [29]. In the Bobbert et al. study, serum RBP4 levels were also higher in diabetic patients compared to nondiabetic individuals, although not in terms of the levels of retinol and transthyretin [30]. Nevertheless, it should be emphasized that RBP4, retinol, and retinoic acid can differentially affect CVD and metabolic diseases [31]. Therefore, vitamin A metabolism should be taken into consideration when investigating the role of RBP4 since it can act by itself or affect retinol metabolism and retinoic acid signaling [32]. Additionally, vitamin A deficiency reduces serum RBP4 levels, and, hence, it is essential to evaluate not only the metabolism of vitamin A but also its dietary intake when assessing RBP4 concentrations [33]. A decrease in adipose tissue GLUT4 expression, which is the major glucose transporter protein mediating glucose uptake, leads to increased serum RBP4 levels associated with the induction of insulin resistance in the liver and muscle [34]. It has been shown that moderate weight reduction lowers serum RBP4 levels in nondiabetic subjects [27]. However, new evidence suggests that RBP4 plays a more significant role in the lipid metabolism than in insulin resistance [35]. One of the questions regarding RBP4 is which of its serum concentrations are normal, and which are pathological. In healthy individuals, normal serum RBP4 ranges from 10 to 50 µg/mL (without vitamin A deficiency), but among individuals with Type 2 diabetes (T2DM), CVD or obesity, it may reach up to 150 µg/mL [26][30][33][34][36][37][38][39]. In the study led by Farjo et al., the most substantial influence on pro-inflammatory molecules was achieved with RBP4 serum concentrations of 100 µg/mL [40]. However, lower concentrations in the range of 10 to 25 µg/mL were also shown to be enough to influence pro-inflammatory molecules, which suggest that endothelial cells may be responsive to even small elevations in serum RBP4 concentrations [33][40][41]. RBP4 is also known as a negative acute-phase reactant, and hospitalization may decrease its serum levels [33]. Therefore, the choice of the assay employed in the measurement of serum RBP4 levels should also be careful. As Graham et al. presented in their study, quantitative Western blotting is the most reliable method for assaying serum RBP4 elevations associated with insulin resistance [39]. However, other measurement methods—e.g., ELISA (enzyme-linked immunosorbent assay), EIA (enzyme immunoassay)—are also widely used in various populations [42]. Additionally, RBP4 concentrations were found to be different among men and women. Some research studies indicated that RBP4 was significantly higher in men than in women, including adolescent boys and girls, whereas no such association was observed in other studies [35][43][44]. This could be explained by different amounts and distribution of the adipose tissue (including liver fat), the influence of the sex hormones, and iron metabolism [44][45][46]. RBP4 levels were also markedly different between premenopausal and postmenopausal healthy women, with higher levels among the second group [47]. The summary of measurement methods and serum RBP4 range in the selected studies, according to CVD risk assessment, are listed in Table 1.
Figure 1. Retinol-binding protein 4 (RBP4).
Table 1. The summary of measurement methods and serum retinol-binding protein 4 (RBP4) range in studies assessing cardiovascular (CV) risk.
|
Authors |
Study Population |
Groups, Sex (Group Size, n) |
Age (Years) |
BMI (kg/m2) |
CV Risk Assessment Method |
CV Risk |
RBP4 Measurement Method (Unit) |
RBP4 Specimen |
Serum RBP4 Range |
Relation between RBP4 and CV Risk |
|
Feng et al. 2015 [48] |
T2DM |
498 F; 578 M (1076) |
62.80 ± 13.60 |
27.50 ± 4.20 |
cIMT (mm) |
G 1 (332): no abnormalities |
ELISA (mg/L) |
serum |
G 1 32.10 ± 10.3 |
+ |
|
27.90 ± 3.40 |
G 2 (386): ≥ 1 |
G 2 38.20 ± 8.30 |
||||||||
|
27.60 ± 3.60 |
G 3 (358): ≥ 1.5 |
G 3 46.90 ± 7.60 |
||||||||
|
Xiao et al. 2013 [19] |
T2DM |
140 F; 144 M (284) |
35.00–70.00 |
25.10 ± 2.80 |
cIMT (mm) fIMT (mm) iIMT (mm) |
subAS (78) cIMT 0.94 ± 0.34 fIMT 0.97 ± 0.33 iIMT 1.13 ± 0.28 |
ELISA with monoclonal antibodies (mg/L)
|
serum |
37.1 (32.3–40.8) |
+ |
|
24.50 ± 2.80 |
Non-subAS cIMT 0.70 ± 0.11 fIMT 0.70 ± 0.11 iIMT 0.76 ± 0.10 |
23.2 (20.1–29.2) |
+ |
|||||||
|
Won et al. 2012 [49] |
Healthy |
175 F; 116 M (291) |
40.00 ± 11.00 |
27.00 ± 2.60 |
The Framingham Risk Score |
MetS (57) Framingham risk: 2.0, 0.0 to >30.0 Framingham score: 9.0, −7.0 to 17.0 |
EIA (µg/mL) |
plasma |
MetS 65.1 ± 26.8 |
+ |
|
23.60 ± 3.00 |
Non-MetS (234) Framingham risk: 0.5, 0.0 to 20.0 Framingham score: 3.0, −9.0 to 18.0 |
Non-MetS 52.2 ± 20.0 |
||||||||
|
Su et al. 2020 [29] |
CKD |
58 F; 111 M (169) |
59.50–78.00 |
27.40 ± 2.90 |
CV events |
(total 80) CV events: 41 CV mortality: 10 |
ELISA (mg/L) |
serum |
>33.86 |
+ (higher rates of CV events than RBP4 < 33.86) |
|
25.90 ± 2.10 |
(total 89) CV events: 11 CV mortality: 4 |
<33.86 |
+ |
|||||||
|
Solini et al. 2009 [38] |
HYP |
35 F |
47.40 ± 5.00 |
25.00 ± 1.60 |
cIMT (mm) |
0.54 ± 0.15 |
ELISA (µg/mL) |
plasma |
Median value 38.75 |
+ |
|
CTL |
35 F |
46.90 ± 6.30 |
25.70 ± 1.40 |
0.5 ± 0.13 |
Median value 10.00 |
None |
||||
|
Mansouri et al. 2012 [50] |
T2DM |
53 F; 48 M (101) |
53.60 ± 8.40 |
27.70 ± 4.10 |
cIMT (mm) |
0.8 ± 0.2 |
ELISA (µg/mL) |
serum |
71.9 ± 35.6 |
None |
|
Bobbert et al. 2010 [30] |
T2DM and non-T2DM |
52 F; 44 M (96) |
55.00 ± 1.30 |
30.80 ± 0.70 |
cIMT (mm) |
0.72 ± 0.02 |
ELISA (µmol/L) |
serum |
1.89 ± 0.05 |
+ |
|
Chu et al. 2011 [51] |
T2DM with CKD |
86 (sex NM) |
70.00 ± 11.00 |
26.20 ± 6.20 |
cIMT (mm) |
0.75 ± 0.16 |
ELISA (µg/mL) |
serum |
44.8 ± 6.4 |
None |
|
T2DM without CKD |
153 (sex NM) |
60.00 ± 12.00 |
26.30 ± 5.90 |
0.69 ± 0.14 |
39.5 ± 4.9 |
None |
||||
|
Li et al. 2020 [52] |
CHF |
227 F; 707 M (934) |
≥60 |
22.49–26.67 |
MACE (Multivariable Cox regression)
|
- |
ELISA (µg/mL) |
serum |
46.66 ± 12.38 |
+ |
|
Bachmayer et al. 2013 [53] |
Patients with obesity |
65 F; 27 M (92) |
43.00 ± 10.00 |
50.00 ± 7.00 |
Endothelial dysfunction: CRAE (µm); CRVE (µm); AVR |
CRAE 178 ± 19 |
ELISA (ng/mL) |
NM |
24,773 ± 14,025 |
None |
|
CRVE 221 ± 24 |
None |
|||||||||
|
AVR 0.81 ± 0.09 |
None |
F—female, M—men, ± SDs, T2DM—Type 2 diabetes mellitus, G—group, ELISA—enzyme-linked immuno-absorbent assay, CV—cardiovascular, IMT—intima-media thickness, cIMT—carotid intima-media thickness, fIMT—femoral intima-media thickness, iIMT—common iliac intima-media thickness, subAS—subclinical atherosclerosis, EIA—enzyme immunoassay, MetS—metabolic syndrome, CKD—chronic kidney disease, HYP—hypertensive, CTL—normotensive, +—positive, NM—not mentioned, CHF—chronic heart failure, MACE—major adverse cardiac event(s) (cardiovascular death and rehospitalization due to the deterioration of CHF), CRAE, CRVE—central retinal artery/vein equivalent, AVR—arterio–venous-ratio.
3. RBP4 Gene—Structure and Polymorphism vs. CVD in Obesity
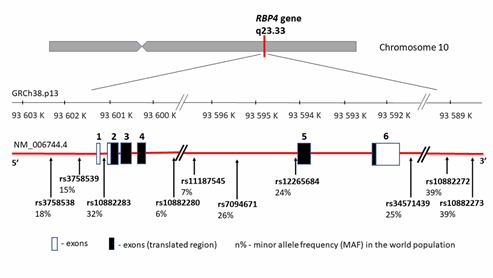
RBP4 protein is encoded by the same name gene—RBP4 (MIM 180250) located on chromosome 10 (10q23.33) between coordinates 93,591,694 and 93,601,744 bp according to human genome reference assembly GRCh38.p13 (Figure 2). It encompasses 10,050 bp of genomic DNA and consists of 6 exons, including five coding fragments. Transcript length is 1070 bp, and the translation product consists of 201 amino acids.
Since RBP4 may be correlated with conditions related to T2DM, obesity, or CVD, the RBP4 gene may constitute the gene carrying obesity-related implications [54]. Additionally, the RBP4 gene location is close to a region linked with an increased risk of T2DM and elevated fasting blood glucose levels [55][56][57]. Thus, it has been proposed that functional RBP4 gene polymorphisms influence a higher obesity incidence, insulin resistance, hyperinsulinemia, T2DM, and artery thickness [26]. These hypotheses were confirmed by research carried out in recent years by several research teams. The most significant RBP4 gene variants connected with CVD and its markers are summarized in Table 2 and presented in Figure 2, with the distribution of each locus and the minor allele frequency (MAF) occurrence in the world population based on 1000 Genomes Project data (phase 3; https://www.internationalgenome.org/). It is worth noting that all of the listed variants are located outside the coding gene region and that there can be a potential relationship between the regulation and the change in gene expression levels.
Figure 2. RBP4 gene structure, chromosome location, and cardiovascular disease (CVD) variants distribution. rs—number of the reference sequence in the National Center of Biotechnological Information database.
Table 2. RBP4 gene variants investigated as risk factors of cardiovascular diseases in obesity.
|
Variant |
Genetic Location |
Study Group |
Pathophysiology Association |
Reference |
|
|
n (Total) |
Diagnosis |
||||
|
rs10882280 |
g.6681G > T c.355+837G > T (intronic) |
1422 F; 414 M (1836) |
healthy (metabolic, cardiovascular, or endocrine disease excluded) |
Higher high-density lipoprotein level associated with minor allele T (p = 0.043) and C (p = 0.042), respectively |
Shea et al. 2010 [58] |
|
rs11187545 |
g.8889T > C c.355+3045T > C (intronic) |
||||
|
rs10882283 |
g.5030T > G c. −55T > G (5’ UTR variant) |
457 F; 477 M (934); 716 CTL |
T2DM |
G-allele associated with a higher body-mass index and waist-to-hip ratio values (p < 0.05). |
Kovacs et al. 2007 [59] |
|
rs10882273 |
g.27484T > C c.*1539T > C (3′ UTR variant) |
457 F; 477 M (934); 716 CTL |
T2DM |
C-allele associated with an increased BMI, plasma insulin, and circulating free fatty acid concentrations (p < 0.05) |
Kovacs et al. 2007 [59]
|
|
1787 F; 1423 M (3210) |
Chinese Hans population 50–70 years old |
Higher body-mass index values. Higher insulin and free fatty acids levels. Association with plasma RBP4 levels (p = 0.005). |
Wu et al. 2009 [60] |
||
|
rs10882272 |
g.26761T > C c.*816T > C (3′ UTR variant) |
593 F; 454 M (947) |
French-Canadian founder population 12–18 years old |
Association with circulating retinol levels. Modulation between vitamin A intake and abdominal adiposity. |
Goodwin et al. 2015 [61] |
|
5 006 |
Caucasian cohorts from Finland, USA, and Italy |
Association with circulating retinol levels. |
Mondul et al. 2011 [62] |
||
|
rs3758538 |
g.3944A > C c.697–1781A > C (upstream transcript variant) |
97 with obesity; 83 normal-weight |
Spanish Caucasian children |
Association with triglycerides levels and plasma RBP4 levels. C allele associated with obesity and higher BMI z-score. |
Codõner-Franch et al. 2016 [54] |
|
1787 F; 1423 M (3210) |
Chinese Hans population 50–70 years old |
Association with hypertriglyceridemia and plasma RBP4 levels. |
Wu et al. 2009 [60] |
||
|
rs3758539 |
g.4406G > A c.697-2243G > A (upstream transcript variant) |
97 cases 83 CTL |
Obesity, Spanish Caucasian children |
Association with triglycerides levels in children. |
Codõner-Franch et al. 2016 [54] |
|
66 F; 63 M (129) 192 CTL |
Obesity, cohort from Iran
|
Association with an increased susceptibility for obesity and an increased BMI. |
Shajarian et al. 2015 [55] |
||
|
rs12265684 |
g.12177G > A c.356-25G > A (intronic) |
97 cases 83 CTL |
Obesity, Spanish Caucasian children |
Association with triglycerides levels and blood pressure. |
Codõner-Franch et al. 2016 [54] |
|
rs34571439 |
g.14684T > G c.697-12521A > C (upstream transcript variant) |
Association with triglycerides and plasma RBP4 levels as well as plasma C-reactive protein values. |
|||
|
rs7094671 |
g.10377C > T c.356-1825C > T (intronic) |
297 M; 217 M CTL |
CAD, Chinese patients |
G allele associated with a higher risk of CAD |
Wan et al. 2014 [62] |
rs—number of the reference sequence in the National Center of Biotechnological Information database, UTR—untranslated region, F—female, M—men, CTL—controls, T2DM—Type 2 diabetes mellitus, CAD—coronary artery disease.
4. RBP4, Obesity, and Metabolic Syndrome
As mentioned before, the secretion of adipokines is frequently abnormal among patients with obesity. Adipocyte hypertrophy, ectopic fat accumulation, and adipose tissue inflammation may cause adverse adipokine secretion, which, in turn, can be associated with a number of health consequences, including metabolic, inflammatory, or cardiovascular diseases [63]. However, in several studies, RBP4 levels were higher among individuals with obesity in comparison to control groups. In other studies, no such correlation has been found. In the Korek et al. study, RBP4 levels did not correlate with BMI or fat mass and did not differ between individuals with obesity and those without obesity—RBP4 levels in both groups were 33.93 ± 4.46 and 32.53 ± 2.53 µg/mL, respectively [64]. Similar results were reported by other authors [65][66][67][68]. On the other hand, certain studies demonstrate increased RBP4 concentrations among individuals with obesity, as well as the association between RBP4 and BMI [36][69]. Therefore, it has been suggested that RPB4 concentrations may not be related necessarily to obesity itself, but to the location of the adipose tissue. The expression seems to be higher in visceral (VF) than in the subcutaneous tissue (SF); thus, RBP4 levels are more closely associated with VF levels and appear to constitute the best indicator of intra-abdominal adipose mass [35][70][71]. RBP4 may be the mechanistic link between the visceral adiposity and increased cardiovascular risk associated with this type of adipose tissue [49][71]. In the study by Lee et al., RBP4 levels were correlated with visceral fat areas, but not with the total body fat (wt.%), and, as the authors suggested, RBP4 could be the link between visceral obesity and atherosclerotic vascular changes [72]. Furthermore, RBP4 levels may also be prone to weight loss. In fact, serum RBP4 levels decreased considerably by 25.5% after weight reduction—almost 11% of weight loss in the course of a 16-week program [27]. However, it is essential to point out that in addition to a reduced caloric intake by 600 kcal/day, sibutramine was also used. Interestingly, the statistically significant increase in RBP4 levels was also observed in patients undergoing bariatric surgery—RBP4 levels (ng/mL) were 22,456.5 ± 13,158.8 and 31,342.2 ± 8172.5 in pre-and post-bariatric periods, respectively [68]. In two different studies, RBP4 levels decreased significantly following bariatric surgery [69][70]. According to Zachariah et al., participants with serum (log-transformed) RBP4 levels at the 4th quartile presented a 75% higher risk of developing the metabolic syndrome when compared to patients in the 1st quartile [73]. Moreover, in the study by Karamfilova et al., RBP4 levels ≥55 mcg/mL were associated with a 3.1 higher risk of developing metabolic syndrome [74]. Other studies also have confirmed the relationship between the components of metabolic syndrome and RBP4 levels [65][66]. Additionally, RBP4 can also be a predictor for the diagnosis of metabolic syndrome and weight regain [70][74][75]. In fact, Vink et al. demonstrated that RBP4 was a predictor of weight regain—stronger in men and individuals following a low-calorie diet than in women and individuals following a very-low-calorie diet [75].
Possible explanations regarding different concentrations or changes in RBP4 may include both ethnic and age differences (e.g., presence of renal dysfunction) [66]. Despite different data, there is still a strong association between RBP4 and obesity.
References
- Adam Timmis; Nicholas Townsend; Chris P Gale; Aleksandra Torbica; Maddalena Lettino; Steffen E Petersen; Elias A Mossialos; Aldo Pietro Maggioni; Dzianis Kazakiewicz; Heidi T May; et al.Delphine De SmedtMarcus FlatherLiesl ZuhlkeJohn BeltrameRadu HuculeciLuigi TavazziGerhard HindricksJeroen BaxBarbara CasadeiStephan AchenbachF. Lucy WrightPanos VardasLezha MimozaGoda ArtanDemiraj AurelMohammed ChettibiNaima HammoudiHamayak SisakianSergey PepoyanBernhard MetzlerPeter SiostrzonekFranz WeidingerTofig JahangirovFarid AliyevYasmin RustamovaNikolay ManakAliaksandr MrochakPatrizio LancellottiAgnès PasquetMarc ClaeysZumreta KušljugićLarisa Dizdarević HudićElnur SmajićMariya Petkova TokmakovaPlamen Marinov GatzovDavor MilicicMijo BergovecChristos ChristouHera Heracleous MoustraTheodoros ChristodoulidesAles LinhartMilos TaborskyHenrik Steen HansenLene HolmvangSteen Dalby KristensenMagdy AbdelhamidKhaled ShokryPriit KampusMargus ViigimaaEssi RyödiMatti NiemeläTuomas T RissanenJean-Yves Le HeuzeyMartine GilardA AladashviliA GamkrelidzeMaia KereselidzeA ZeiherH KatusK BestehornCostas TsioufisJohn GoudevenosZoltán CsanádiDávid BeckerKálmán TóthÞórdís Jóna HrafnkelsdóttirJames CrowleyPeter KearneyBarbra DaltonDoron ZahgerArik WolakDomenico GabrielliCiro IndolfiStefano UrbinatiGulnara ImantayevaSalim BerkinbayevGani BajraktariArtan AhmetiGezim BerishaMirrakhimov ErkinAbilova SaamayAndrejs ErglisIveta BajareSanda JegereMalek MohammedAntoine SarkisGeorges SaadehRuta ZvirblyteGintare SakalyteRimvydas SlapikasKhaled EllafiFathi El GhamariCristiana BanuJean BeisselTiziana FeliceSandra C ButtigiegRobert G XuerebMihail PopoviciAneta BoskovicMiroslav RabrenovicSamir ZtotSaadia Abir-KhalilA C Van RossumB J M MulderM W ElsendoornElizabeta Srbinovska-KostovskaJorgo KostovBosevski MarjanTerje SteigenOle Christian MjølstadPiotr PonikowskiAdam WitkowskiPiotr JankowskiVictor Machado GilJorge MimosoSérgio BaptistaDragos VinereanuOvidiu ChioncelBogdan A PopescuEvgeny ShlyakhtoRaphael OganovMarina FoscoliMarco ZavattaAna Djordjevic DikicBranko BeleslinMina Radosavljevic RadovanovicPeter HlivákRobert HatalaGabriela KaliskáMiran KendaZlatko FrasManuel AnguitaÁngel CequierJavier MuñizStefan JamesBengt JohanssonPyotr PlatonovMichael Johannes ZellwegerGiovanni B PedrazziniDavid CarballoHussam Eddin ShebliSamer KabbaniLeila AbidFaouzi AddadEngin BozkurtMeral KayıkçıoğluMustafa Kemal ErolVolodymyr KovalenkoElena NesukayAndrew WraggPeter LudmanSimon RayRavshanbek KurbanovDennis BoatengGhislain DavalVíctor De Benito RubioDavid SebastiaoPaola Thellung De CourtelaryIsabel BardinetEuropean Society Of Cardiology European Society of Cardiology: Cardiovascular Disease Statistics 2019. European Heart Journal 2019, 41, 12-85, 10.1093/eurheartj/ehz859.
- Francois Mach; Colin Baigent; Alberico L. Catapano; Konstantinos C Koskinas; Manuela Casula; Lina Badimon; M John Chapman; Guy G De Backer; Victoria Delgado; Brian A Ference; et al.Ian M GrahamAlison HallidayUlf LandmesserBorislava MihaylovaTerje R PedersenGabriele RiccardiDimitrios J RichterMarc S SabatineMarja-Riitta TaskinenLale TokgozogluOlov WiklundChristian MuellerHeinz DrexelVictor AboyansAlberto CorsiniWolfram DoehnerMichel FarnierBruna GiganteMeral KayikciogluGoran KrstacicEkaterini LambrinouBasil S LewisJosep MasipPhilippe MoulinSteffen PetersenAnna Sonia PetronioMassimo Francesco PiepoliXavier PintóLorenz RäberKausik K RayŽeljko ReinerWalter F RiesenMarco RoffiJean-Paul SchmidEvgeny ShlyakhtoIain A SimpsonErik StroesIsabella SudanoAlexandros D TselepisMargus ViigimaaCecile VindisAlexander VonbankMichal VrablikMislav VrsalovicJosé Luis ZamoranoJean-Philippe ColletStephan WindeckerVeronica DeanDonna FitzsimonsChris P GaleDiederick GrobbeeSigrun HalvorsenGerhard HindricksBernard IungPeter JüniHugo A KatusChristophe LeclercqMaddalena LettinoBela MerkelyMiguel Sousa-UvaRhian M TouyzDjamaleddine NiboucheParounak H ZelveianPeter SiostrzonekRuslan NajafovPhilippe Van De BorneBelma PojskicArman PostadzhiyanLambros KyprisJindřich ŠpinarMogens Lytken LarsenHesham Salah EldinTimo E StrandbergJean FerrièresRusudan AgladzeUlrich LaufsLoukianos RallidisLászló BajnokThorbjörn GudjónssonVincent MaherYaakov HenkinMichele Massimo GuliziaAisulu MussagaliyevaGani BajraktariAlina KerimkulovaGustavs LatkovskisOmar HamouiRimvydas SlapikasLaurent VisserPhilip DingliVictoria IvanovAneta BoskovicMbarek NazziFrank VisserenIrena MitevskaKjetil RetterstølPiotr JankowskiRicardo Fontes-CarvalhoDan GaitaMarat EzhovMarina FoscoliVojislav GigaDaniel PellaZlatko FrasLeopoldo Perez De IslaEmil HagströmRoger LehmannLeila AbidOner OzdoganOlena MitchenkoRiyaz S PatelEsc Scientific Document Group 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. European Heart Journal 2019, 41, 111-188, 10.1093/eurheartj/ehz455.
- Nick Townsend; Lauren Wilson; Prachi Bhatnagar; Kremlin Wickramasinghe; Mike Rayner; Melanie Nichols; Cardiovascular disease in Europe: epidemiological update 2016. European Heart Journal 2016, 37, 3232-3245, 10.1093/eurheartj/ehw334.
- Wilkins E, Wilson L, Wickramasinghe K, Bhatnagar P, Leal J, Luengo-Fernandez R, Burns R, Rayner M, Townsend N(2017). European Cardiovascular Disease Statistics 2017. European Heart Network, Brussels.
- The cost of CVD . Champion Advocates Programme. Retrieved 2020-8-7
- Syed Saad Mahmood; Daniel Levy; Ramachandran S. Vasan; Thomas J. Wang; The Framingham Heart Study and the epidemiology of cardiovascular disease: a historical perspective.. The Lancet 2013, 383, 999-1008, 10.1016/S0140-6736(13)61752-3.
- S. Yusuf; S. Hawken; S. Ounpuu; Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. ACC Current Journal Review 2004, 13, 15-16, 10.1016/j.accreview.2004.11.072.
- NCDs | Know the NCD targets . World Health Organization. Retrieved 2020-8-7
- Kristy Breuhl Smith; Michael Seth Smith; Obesity Statistics. Primary Care: Clinics in Office Practice 2016, 43, 121-135, 10.1016/j.pop.2015.10.001.
- Jana Krzysztoszek; Ida Laudańska-Krzemińska; Michal Bronikowski; Assessment of epidemiological obesity among adults in EU countries.. Annals of Agricultural and Environmental Medicine 2018, 26, 341-349, 10.26444/aaem/97226.
- Anna Maria Rychter; Alicja Ewa Ratajczak; Agnieszka Zawada; Agnieszka Dobrowolska; Iwona Krela-Kaźmierczak; Non-Systematic Review of Diet and Nutritional Risk Factors of Cardiovascular Disease in Obesity. Nutrients 2020, 12, 814, 10.3390/nu12030814.
- Robert W Caldwell; Mechanisms of obesity-induced metabolic and vascular dysfunctions. Frontiers in Bioscience 2019, 24, 890-934, 10.2741/4758.
- Chrysi Koliaki; Stavros Liatis; Alexander Kokkinos; Obesity and cardiovascular disease: revisiting an old relationship. Metabolism 2019, 92, 98-107, 10.1016/j.metabol.2018.10.011.
- Emanuele Di Angelantonio; Shilpa N Bhupathiraju; David Wormser; Pei Gao; Stephen Kaptoge; Amy Berrington De Gonzalez; Benjamin J. Cairns; Rachel Huxley; Chandra L Jackson; Grace Joshy; et al.Sarah LewingtonJoann MansonNeil MurphyAlpa V PatelJonathan M SametMark WoodwardWei ZhengMaigen ZhouNarinder BansalAurelio BarricarteBrian CarterJames R. CerhanGeorge Davey SmithXianghua FangOscar H. FrancoJane GreenJim HalseyJanet S HildebrandKeum Ji JungRosemary KordaDale F McLerranSteven C. MooreLinda Marie O'keeffeEllie PaigeAnna RamondGillian K ReevesBetsy RollandCarlotta SacerdoteNaveed SattarEleni SofianopoulouJune StevensMichael ThunHirotsugu UeshimaLing YangYoung Duk YunPeter WilleitEmily BanksValerie BeralZhengming ChenSusan M GapsturMarc J GunterPatricia HartgeSun Ha JeeTai Hing LamRichard PetoJohn D. PotterWalter C WillettSimon G ThompsonJohn DaneshFrank B HuJoann E MansonRory Collins Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents.. The Lancet 2016, 388, 776-86, 10.1016/S0140-6736(16)30175-1.
- Paul Poirier; Thomas D. Giles; George A. Bray; Yuling Hong; Judith S. Stern; F. Xavier Pi-Sunyer; Robert H Eckel; Obesity and Cardiovascular Disease: Pathophysiology, Evaluation, and Effect of Weight Loss. Circulation 2006, 113, 898-918, 10.1161/circulationaha.106.171016.
- Paul Poirier; Frcpc; Obesity and Cardiovascular Disease. Circulation 2006, 114, e564-e564, 10.1161/circulationaha.106.646455.
- Eugenia E Calle; Carmen Rodriguez; Kimberly Walker-Thurmond; Michael J. Thun; Overweight, Obesity, and Mortality from Cancer in a Prospectively Studied Cohort of U.S. Adults. New England Journal of Medicine 2003, 348, 1625-1638, 10.1056/nejmoa021423.
- José J. Fuster; Noriyuki Ouchi; Noyan Gokce; K. Walsh; Obesity-Induced Changes in Adipose Tissue Microenvironment and Their Impact on Cardiovascular Disease.. Circulation Research 2016, 118, 1786-807, 10.1161/CIRCRESAHA.115.306885.
- Yang Xiao; Aimin Xu; Xiaoyan Hui; Pengcheng Zhou; Xing Li; Hui Zhong; Weili Tang; Gan Huang; Zhiguang Zhou; Circulating Lipocalin-2 and Retinol-Binding Protein 4 Are Associated with Intima-Media Thickness and Subclinical Atherosclerosis in Patients with Type 2 Diabetes. PLOS ONE 2013, 8, e66607, 10.1371/journal.pone.0066607.
- Harman S Mattu; Harpal S Randeva; Role of adipokines in cardiovascular disease. Journal of Endocrinology 2012, 216, T17-T36, 10.1530/joe-12-0232.
- Rei Shibata; Noriyuki Ouchi; Koji Ohashi; Toyoaki Murohara; The role of adipokines in cardiovascular disease. Journal of Cardiology 2017, 70, 329-334, 10.1016/j.jjcc.2017.02.006.
- Sandrine Morel; Brenda R. Kwak; Françoise Rohner-Jeanrenaud; Sabine Steffens; Filippo Molica; Adipokines at the crossroad between obesity and cardiovascular disease. Thrombosis and Haemostasis 2015, 113, 553-566, 10.1160/th14-06-0513.
- George Ntaios; Nikolaos K. Gatselis; Konstantinos Makaritsis; George Dalekos; Adipokines as mediators of endothelial function and atherosclerosis. Atherosclerosis 2013, 227, 216-221, 10.1016/j.atherosclerosis.2012.12.029.
- Korek, Emila; Krauss, Hanna; Nowe adipokiny o potencjalnym znaczeniu w patogenezie otyłości i zaburzeń metabolicznych. Postępy Higieny Medycyny Doświadczalnej 2015, 69, 799-810.
- Alison M. Mondul; Kai Yu; William Wheeler; Hong Zhang; Stephanie J. Weinstein; Jacqueline M. Major; Marilyn C. Cornelis; Satu Mannisto; Aditi Hazra; Ann W. Hsing; et al.Kevin B. JacobsHeather EliassenToshiko TanakaDouglas J. RedingSara HendricksonLuigi FerrucciJarmo VirtamoDavid J. HunterStephen J. ChanockPeter KraftDemetrius Albanes Genome-wide association study of circulating retinol levels. Human Molecular Genetics 2011, 20, 4724-4731, 10.1093/hmg/ddr387.
- Majerczyk, Marcin; Olszanecka-Glinianowicz, Magdalena; Puzianowska, Monika; Chudek, Jerzy; Białko wiążące retinol typu 4 (RBP4) jako czynnik i marker uszkodzenia naczyń związany z insulinoopornością. Postępy Higieny Medycyny Doświadczalnej 2016, 70, 1267-1275.
- Ji-Won Lee; Hye-Ree Lee; Jae-Yong Shim; Jee-Aee Im; Duk-Chul Lee; Abdominal Visceral Fat Reduction Is Associated with Favorable Changes of Serum Retinol Binding Protein-4 in Nondiabetic Subjects. Endocrine Journal 2008, 55, 811-818, 10.1507/endocrj.k08e-030.
- A. Cabré; Iolanda Lazaro; Josefa Girona; J. Manzanares; F. Marimón; Núria Plana; M. Heras; L. Masana; Retinol-binding protein 4 as a plasma biomarker of renal dysfunction and cardiovascular disease in type 2 diabetes. Journal of Internal Medicine 2007, 262, 496-503, 10.1111/j.1365-2796.2007.01849.x.
- Yuhao Su; Ying Huang; Ying Jiang; Meilan Zhu; The Association between Serum Retinol-Binding Protein 4 Levels and Cardiovascular Events in Patients with Chronic Kidney Disease.. Laboratory Medicine 2020, lmz104, 1-7, 10.1093/labmed/lmz104.
- Thomas Bobbert; J Raila; Franziska Schwarz; Knut Mai; Andrea Henze; Andreas F. H. Pfeiffer; F. J. Schweigert; Joachim Spranger; Relation between retinol, retinol-binding protein 4, transthyretin and carotid intima media thickness. Atherosclerosis 2010, 213, 549-551, 10.1016/j.atherosclerosis.2010.07.063.
- Thomas Olsen; Rune Blomhoff; Retinol, Retinoic Acid, and Retinol-Binding Protein 4 are Differentially Associated with Cardiovascular Disease, Type 2 Diabetes, and Obesity: An Overview of Human Studies. Advances in Nutrition: An International Review Journal 2019, 11, 644-666, 10.1093/advances/nmz131.
- Giuliano Generoso; Marcio S. Bittencourt; Vitamin A: An enhanced vision of the relationship between apolipoproteins and cardiovascular risk?. Atherosclerosis 2017, 265, 256-257, 10.1016/j.atherosclerosis.2017.08.020.
- Kohzo Takebayashi; Mariko Suetsugu; Sadao Wakabayashi; Yoshimasa Aso; Toshihiko Inukai; Retinol Binding Protein-4 Levels and Clinical Features of Type 2 Diabetes Patients. The Journal of Clinical Endocrinology & Metabolism 2007, 92, 2712-2719, 10.1210/jc.2006-1249.
- Irina Kowalska; Agnieszka Adamska; Agnieszka Nikołajuk; Monika Karczewska-Kupczewska; Marek Straczkowski; Elżbieta Otziomek; Maria Górska; Serum Retinol Binding Protein 4 Is Related to Insulin Resistance and Nonoxidative Glucose Metabolism in Lean and Obese Women with Normal Glucose Tolerance. The Journal of Clinical Endocrinology & Metabolism 2008, 93, 2786-2789, 10.1210/jc.2008-0077.
- Milagros Rocha; Celia Bañuls; Lorena Bellod; Susana Rovira-Llopis; Carlos Morillas; Eva Solá; Víctor Manuel Víctor; A Hernández-Mijares; Association of Serum Retinol Binding Protein 4 with Atherogenic Dyslipidemia in Morbid Obese Patients. PLOS ONE 2013, 8, e78670, 10.1371/journal.pone.0078670.
- Timothy E. Graham; Qin Yang; Matthias Blüher; Ann Hammarstedt; Theodore P. Ciaraldi; Robert R. Henry; Christopher J. Wason; Andreas Oberbach; Per-Anders Jansson; Ulf Smith; et al.Barbara B. Kahn Retinol-Binding Protein 4 and Insulin Resistance in Lean, Obese, and Diabetic Subjects. New England Journal of Medicine 2006, 354, 2552-2563, 10.1056/nejmoa054862.
- Erik Ingelsson; J Sundström; Håkan Melhus; Karl Michaëlsson; Christian Berne; Ramachandran S. Vasan; Ulf Riserus; Rune Blomhoff; Lars Lind; Johan Ärnlöv; et al. Circulating retinol-binding protein 4, cardiovascular risk factors and prevalent cardiovascular disease in elderly. Atherosclerosis 2009, 206, 239-244, 10.1016/j.atherosclerosis.2009.02.029.
- Anna Solini; Eleonora Santini; Stephanie Madec; Chiara Rossi; Elza Muscelli; Retinol-Binding Protein-4 in Women With Untreated Essential Hypertension. American Journal of Hypertension 2009, 22, 1001-1006, 10.1038/ajh.2009.116.
- T. E. Graham; C. J. Wason; M. Blüher; B. B. Kahn; Shortcomings in methodology complicate measurements of serum retinol binding protein (RBP4) in insulin-resistant human subjects. Diabetologia 2007, 50, 814-823, 10.1007/s00125-006-0557-0.
- Krysten Farjo; Rafal A. Farjo; Stacey Halsey; Gennadiy Moiseyev; Jian-Xing Ma; Retinol-Binding Protein 4 Induces Inflammation in Human Endothelial Cells by an NADPH Oxidase- and Nuclear Factor Kappa B-Dependent and Retinol-Independent Mechanism. Molecular and Cellular Biology 2012, 32, 5103-5115, 10.1128/MCB.00820-12.
- Z-Z Li; X-Z Lu; J-B Liu; L Chen; Serum Retinol-Binding Protein 4 Levels in Patients with Diabetic Retinopathy. Journal of International Medical Research 2010, 38, 95-99, 10.1177/147323001003800111.
- Fateme Zabetian-Targhi; Mohammad J Mahmoudi; Nima Rezaei; Maryam Mahmoudi; Retinol binding protein 4 in relation to diet, inflammation, immunity, and cardiovascular diseases.. Advances in Nutrition: An International Review Journal 2015, 6, 748-62, 10.3945/an.115.008292.
- Jiahua Fan; Songping Yin; Diaozhu Lin; Yangqing Liu; Nixuan Chen; Xinxiu Bai; Qiuyi Ke; Jia Shen; Lili You; Xiuhong Lin; et al.Feng LiFengyi HeLi YanChaogang ChenMin Xia Association of Serum Retinol-Binding Protein 4 Levels and the Risk of Incident Type 2 Diabetes in Subjects With Prediabetes. Diabetes Care 2019, 42, 1574-1581, 10.2337/dc19-0265.
- Chin-Jung Lin; Nain-Feng Chu; Yi-Jen Hung; Jin-Biou Chang; Chih-Tsueng He; Fone-Ching Hsiao; Chang-Hsun Hsieh; The Association of Retinol-Binding Protein 4 With Metabolic Syndrome and Obesity in Adolescents. Clinical Pediatrics 2012, 52, 16-23, 10.1177/0009922812459948.
- José Manuel Fernández-Real; José Maria Moreno-Navarrete; Wifredo Ricart; Circulating Retinol-Binding Protein-4 Concentration Might Reflect Insulin Resistance–Associated Iron Overload. Diabetes 2008, 57, 1918-1925, 10.2337/db08-0041.
- Rocio Mateo-Gallego; Laura LaCalle; Sofia Pérez-Calahorra; Victoria Marco-Benedí; Valle Recasens; Noelia Padrón; Itziar Lamiquiz-Moneo; Lucía Baila-Rueda; Estíbaliz Jarauta; Pilar Calmarza; et al.Ana CenarroPilar De Miguel-Etayo Efficacy of repeated phlebotomies in hypertriglyceridemia and iron overload: A prospective, randomized, controlled trial. Journal of Clinical Lipidology 2018, 12, 1190-1198, 10.1016/j.jacl.2018.06.017.
- Chiying An; Han Wang; Xiaomin Liu; Yanbo Li; Ying Su; Xinyuan Gao; Wai Jiang; Serum retinol-binding protein 4 is elevated and positively associated with insulin resistance in postmenopausal women.. Endocrine Journal 2009, 56, 987-996, 10.1507/endocrj.k09e-096.
- Shangyong Feng; Yan Zhu; Caifeng Yan; Yan Wang; Zhenweng Zhang; Retinol binding protein 4 correlates with and is an early predictor of carotid atherosclerosis in type 2 diabetes mellitus patients. The Journal of Biomedical Research 2015, 29, 451-455, 10.7555/jbr.29.20140087.
- Jong Chul Won; Cheol-Young Park; Sang Woo Oh; Sung Woo Park; Increased plasma levels of retinol-binding protein 4 with visceral obesity is associated with cardiovascular risk factors. Journal of Diabetes Investigation 2012, 3, 457-463, 10.1111/j.2040-1124.2012.00213.x.
- Masoume Mansouri; Ramin Heshmat; Ozra Tabatabaei-Malazy; Farshad Sharifi; Zohre Badamchizadeh; Sudabeh Alatab; K. Omidfar; Hossein Fakhrzadeh; Bagher Larijani; The association of carotid intima media thickness with retinol binding protein-4 and total and high molecular weight adiponectin in type 2 diabetic patients. Journal of Diabetes & Metabolic Disorders 2012, 11, 2-2, 10.1186/2251-6581-11-2.
- Chih-Hsun Chu; Hing-Chung Lam; Jenn-Kuen Lee; Chih-Chen Lu; Chun-Chin Sun; Hsin-Ju Cheng; Mei-Chun Wang; Ming-Ju Chuang; Elevated serum retinol-binding protein 4 concentrations are associated with chronic kidney disease but not with the higher carotid intima-media thickness in type 2 diabetic subjects.. Endocrine Journal 2011, 58, 841-847, 10.1507/endocrj.ej11-0028.
- Xiu‐Zhen Li; Kang‐Zhen Zhang; Jian‐Jun Yan; Li Wang; Yue Wang; Xi‐Yu Shen; Hui‐Xian Sun; Li Liu; Can Zhao; Hui‐Wei He; et al.Lian‐Sheng WangWei GaoXiang Lu Serum retinol‐binding protein 4 as a predictor of cardiovascular events in elderly patients with chronic heart failure. ESC Heart Failure 2020, 7, 542-550, 10.1002/ehf2.12591.
- Christine Bachmayer; Anne Kemmer; Nadine Ehrmann; Till Hasenberg; Alexander Lammert; Hans-Peter Hammes; Adipokines and endothelial dysfunction in obesity WHO°III. Microvascular Research 2013, 89, 129-133, 10.1016/j.mvr.2013.04.007.
- Pilar Codoñer-Franch; Joaquin Carrasco-Luna; Paula Allepuz; Alan Codoñer-Alejos; Vicent Guillem; Association of RBP4 genetic variants with childhood obesity and cardiovascular risk factors. Pediatric Diabetes 2015, 17, 576-583, 10.1111/pedi.12339.
- Mansour Shajarian; Laleh Rafiee; Hajar Naji-Esfahani; Shaghayegh Haghjooy Javanmard; Sarrafzadegan Nizal; Association of RBP4 gene variants with adverse lipid profile and obesity. Gene 2015, 561, 1-5, 10.1016/j.gene.2014.12.071.
- RavindraNath Duggirala; John Blangero; Laura Almasy; Thomas D. Dyer; Kenneth L. Williams; Robin J. Leach; Peter O'connell; Michael P. Stern; Linkage of Type 2 Diabetes Mellitus and of Age at Onset to a Genetic Location on Chromosome 10q in Mexican Americans. The American Journal of Human Genetics 1999, 64, 1127-1140, 10.1086/302316.
- James B. Meigs; Carolien I. M. Panhuysen; Richard H. Myers; Peter W.F. Wilson; L. Adrienne Cupples; A Genome-Wide Scan for Loci Linked to Plasma Levels of Glucose and HbA1c in a Community-Based Sample of Caucasian Pedigrees: The Framingham Offspring Study. Diabetes 2002, 51, 833-840, 10.2337/diabetes.51.3.833.
- Jennifer L. Shea; Guang Sun; J. Concepción Loredo-Osti; Association of RBP4 Gene Variants and Serum HDL Cholesterol Levels in the Newfoundland Population. Obesity 2009, 18, 1393-1397, 10.1038/oby.2009.398.
- Peter Kovacs; Michaela Geyer; Janin Berndt; Nora Klöting; Timothy E. Graham; Yvonne Böttcher; Beate Enigk; Anke Tönjes; Rit Schleinitz; Michael P Schön; et al.Barbara B. KahnMatthias BlüherMichael Stumvoll Effects of Genetic Variation in the Human Retinol Binding Protein-4 Gene (RBP4) on Insulin Resistance and Fat Depot Specific mRNA Expression. Diabetes 2007, 56, 3095-3100, 10.2337/db06-1647.
- Ying Wu; Huaixing Li; Ruth J F Loos; Qibin Qi; Frank B. Hu; Yong Liu; Xu Lin; RBP4 variants are significantly associated with plasma RBP4 levels and hypertriglyceridemia risk in Chinese Hanss⃞. Journal of Lipid Research 2009, 50, 1479-1486, 10.1194/jlr.P900014-JLR200.
- Katie Goodwin; Michal Abrahamowicz; Gabriel Leonard; Michel Perron; Louis Richer; Suzanne Veillette; Daniel Gaudet; Tomas Paus; Zdenka Pausova; Dietary Vitamin A and Visceral Adiposity: A Modulating Role of the Retinol-Binding Protein 4 Gene. Journal of Nutrigenetics and Nutrigenomics 2015, 8, 164-173, 10.1159/000442090.
- Ke Wan; Jianxun Zhao; Ying Deng; Xi Chen; Qing Zhang; Zhi Zeng; Li Zhang; Yucheng Chen; A Genetic Polymorphism in RBP4 Is Associated with Coronary Artery Disease. International Journal of Molecular Sciences 2014, 15, 22309-22319, 10.3390/ijms151222309.
- Mathias Fasshauer; Matthias Blüher; Adipokines in health and disease. Trends in Pharmacological Sciences 2015, 36, 461-470, 10.1016/j.tips.2015.04.014.
- Emilia Korek; Magdalena Gibas-Dorna; Zuzanna Chęcińska-Maciejewska; Hanna Krauss; Małgorzata Łagiedo-Żelazowska; Barbara Kołodziejczak; Paweł Bogdański; Serum RBP4 positively correlates with triglyceride level but not with BMI, fat mass and insulin resistance in healthy obese and non-obese individuals. Biomarkers 2018, 23, 683-688, 10.1080/1354750x.2018.1479770.
- Hanna Wessel; Ali Saeed; Janette Heegsma; Margery A. Connelly; Klaas Nico Faber; Robin P F Dullaart; Plasma Levels of Retinol Binding Protein 4 Relate to Large VLDL and Small LDL Particles in Subjects with and without Type 2 Diabetes.. Journal of Clinical Medicine 2019, 8, 1792, 10.3390/jcm8111792.
- Marcin Majerczyk; Piotr Kocełak; P. Choręza; H. Arabzada; Aleksander Jerzy Owczarek; M. Bozentowicz-Wikarek; Anna Brzozowska; A. Szybalska; Monika Puzianowska-Kuznicka; Tomasz Grodzicki; et al.A. WięcekMagdalena Olszanecka-GlinianowiczJerzy Chudek Components of metabolic syndrome in relation to plasma levels of retinol binding protein 4 (RBP4) in a cohort of people aged 65 years and older. Journal of Endocrinological Investigation 2018, 41, 1211-1219, 10.1007/s40618-018-0856-6.
- Gary Huang; Dan Wang; Unab I. Khan; Irfan Zeb; JoAnn E. Manson; Virginia M. Miller; Howard N. Hodis; Matthew J. Budoff; George R. Merriam; S. Mitchell Harman; et al.Eliot A. BrintonMarcelle I. CedarsYali SuRogerio A LoboFrederick NaftolinNanette SantoroHugh S. TaylorRachel P. Wildman Associations between retinol-binding protein 4 and cardiometabolic risk factors and subclinical atherosclerosis in recently postmenopausal women: cross-sectional analyses from the KEEPS study. Cardiovascular Diabetology 2012, 11, 52-52, 10.1186/1475-2840-11-52.
- C. Bachmayer; Till Hasenberg; H.-P. Hammes; A. Lammert; Healthy Obese and Post Bariatric Patients – Metabolic and Vascular Patterns. Experimental and Clinical Endocrinology & Diabetes 2013, 121, 483-487, 10.1055/s-0033-1347248.
- Dominik Georg Haider; Karin Schindler; Gerhard Prager; A. Bohdjalian; Anton Luger; Michael Wolzt; Bernhard Ludvik; Serum Retinol-Binding Protein 4 Is Reduced after Weight Loss in Morbidly Obese Subjects. The Journal of Clinical Endocrinology & Metabolism 2007, 92, 1168-1171, 10.1210/jc.2006-1839.
- Alexander Tschoner; Wolfgang Sturm; Julia Engl; Susanne Kaser; Markus Laimer; Elisabeth Laimer; Helmut Weiss; Josef R. Patsch; Christoph F. Ebenbichler; Retinol-binding Protein 4, Visceral Fat, and the Metabolic Syndrome: Effects of Weight Loss. Obesity 2008, 16, 2439-2444, 10.1038/oby.2008.391.
- Nora Klöting; Timothy E. Graham; Janin Berndt; Susan Kralisch; Peter Kovacs; Christopher J. Wason; Mathias Fasshauer; Michael P. Schön; Michael Stumvoll; Matthias Blüher; et al.Barbara B. Kahn Serum Retinol-Binding Protein Is More Highly Expressed in Visceral than in Subcutaneous Adipose Tissue and Is a Marker of Intra-abdominal Fat Mass. Cell Metabolism 2007, 6, 79-87, 10.1016/j.cmet.2007.06.002.
- Ji-Won Lee; Jee-Aee Im; Hye-Ree Lee; Jae-Yong Shim; Byung-S. Youn; Duk Chul Lee; Visceral Adiposity Is Associated with Serum Retinol Binding Protein-4 Levels in Healthy Women*. Obesity 2007, 15, 2225-2232, 10.1038/oby.2007.264.
- Justin P Zachariah; Rene Quiroz; Kerrie P. Nelson; Zhaoyang Teng; John F. Keaney; Lisa Sullivan; Ramachandran S. Vasan; Prospective Relation of Circulating Adipokines to Incident Metabolic Syndrome: The Framingham Heart Study. Journal of the American Heart Association 2017, 6, e004974, 10.1161/jaha.116.004974.
- Vera Karamfilova; Antoaneta Gateva; Asen Alexiev; Nadejda Zheleva; Tsvetelina Velikova; Radina Ivanova-Boyanova; Raya Ivanova; Nikolay Cherkezov; Zdravko Kamenov; Ludmila Mateva; et al. The association between retinol-binding protein 4 and prediabetes in obese patients with nonalcoholic fatty liver disease.. Archives of Physiology and Biochemistry 2019, 63, 1-6, 10.1080/13813455.2019.1673429.
- R G Vink; Nadia J. Roumans; Edwin C. Mariman; Marleen A. Van Baak; Dietary weight loss‐induced changes in RBP4, FFA, and ACE predict weight regain in people with overweight and obesity. Physiological Reports 2017, 5, e13450, 10.14814/phy2.13450.