
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kichul Cho | + 2084 word(s) | 2084 | 2021-10-27 08:33:33 | | | |
| 2 | Vicky Zhou | Meta information modification | 2084 | 2021-10-27 09:37:33 | | |
Video Upload Options
Retinoic acid (RA) is an important biological metabolite synthesized from the retinol content (known as “vitamin A”) via a sequential cellular process in the retinoid signaling pathway (RSP). RSP-mediated RA biosynthesis is a vital physiological process in chordates, since RA interacts with the nuclear receptor superfamily, namely nuclear RA receptors (RARs) and retinoid X receptors (RXRs), bound to the RA response elements (RAREs) in the promoter region of RA target genes. Retinaldehyde dehydrogenases (RALDH) belongs to the oxidoreductase family and plays a critical role in RA synthesis from the retinaldehyde content; therefore, this enzyme is considered to be one of the key regulators of RA-related retinol metabolism and embryonic development.
1. Pleiotropic Roles of Retinoic acid (RA) and RALDH Inhibitors
| Role of Retinoic Acid (RA) | References | |
|---|---|---|
| Embryogenesis | Neural tube development | [19] |
| Posterior foregut derivatives development and liver growth | [20] | |
| craniofacial morphogenesis | [21] | |
| Skin morphogenesis | [22] | |
| Eye development | [23][24] | |
| posterior hindbrain structure expansion | [26] | |
| Early somite formation | [6][31] | |
| Heart anteroposterior patterning | [6][31] | |
| Kidney formation | [6][32] | |
| Others (immunity, cell differentiation) |
Neuron differentiation | [6][14][27] |
| Anti-inflammatory naïve T cells differentiation | [33] | |
| HL-60 cells differentiation | [34] |
2. RA Signaling-Associated Eye Development and RALDH-Relevant AOP (AOP297)
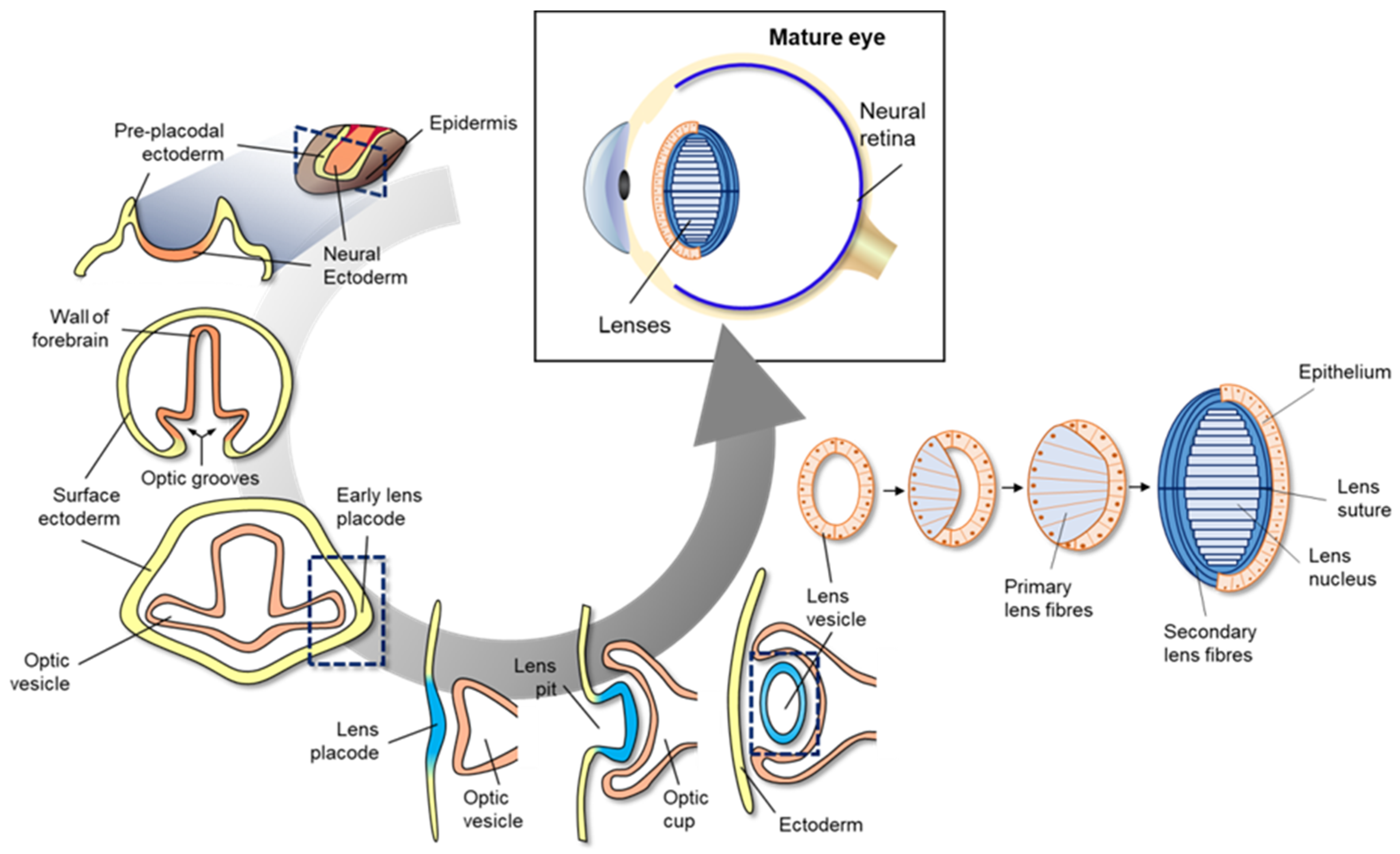 Figure 1. Schematic diagram of mammalian eye development. Eye development in vertebrates occurs in the neural ectoderm origin, surface ectoderm, and neural crest-derived periocular mesenchyme of embryonic cell. Mammalian eye development starts with formation of the wall of the forebrain on either side of the optic grooves. The optic grooves gradually deepen as the neural folds become elevated, and the neural tube is closed by surface ectoderm. The optic grooves extend to the near ectoderm to form early lens placode by thickening of the adjacent area. During the interaction of optic vesicles with the ectoderm, the generated lens placode invaginates to form the lens pit, and optic vesicles invaginate to form the double-layered optic cup. Lens vesicles are generated from the lens pit and are surrounded by the double-layered optic cup; finally, the generated lens vesicle becomes the fetal lens and mature lens after the development of primary lens fibers, secondary lens fibers, lens suture, and lens nucleus.
Figure 1. Schematic diagram of mammalian eye development. Eye development in vertebrates occurs in the neural ectoderm origin, surface ectoderm, and neural crest-derived periocular mesenchyme of embryonic cell. Mammalian eye development starts with formation of the wall of the forebrain on either side of the optic grooves. The optic grooves gradually deepen as the neural folds become elevated, and the neural tube is closed by surface ectoderm. The optic grooves extend to the near ectoderm to form early lens placode by thickening of the adjacent area. During the interaction of optic vesicles with the ectoderm, the generated lens placode invaginates to form the lens pit, and optic vesicles invaginate to form the double-layered optic cup. Lens vesicles are generated from the lens pit and are surrounded by the double-layered optic cup; finally, the generated lens vesicle becomes the fetal lens and mature lens after the development of primary lens fibers, secondary lens fibers, lens suture, and lens nucleus.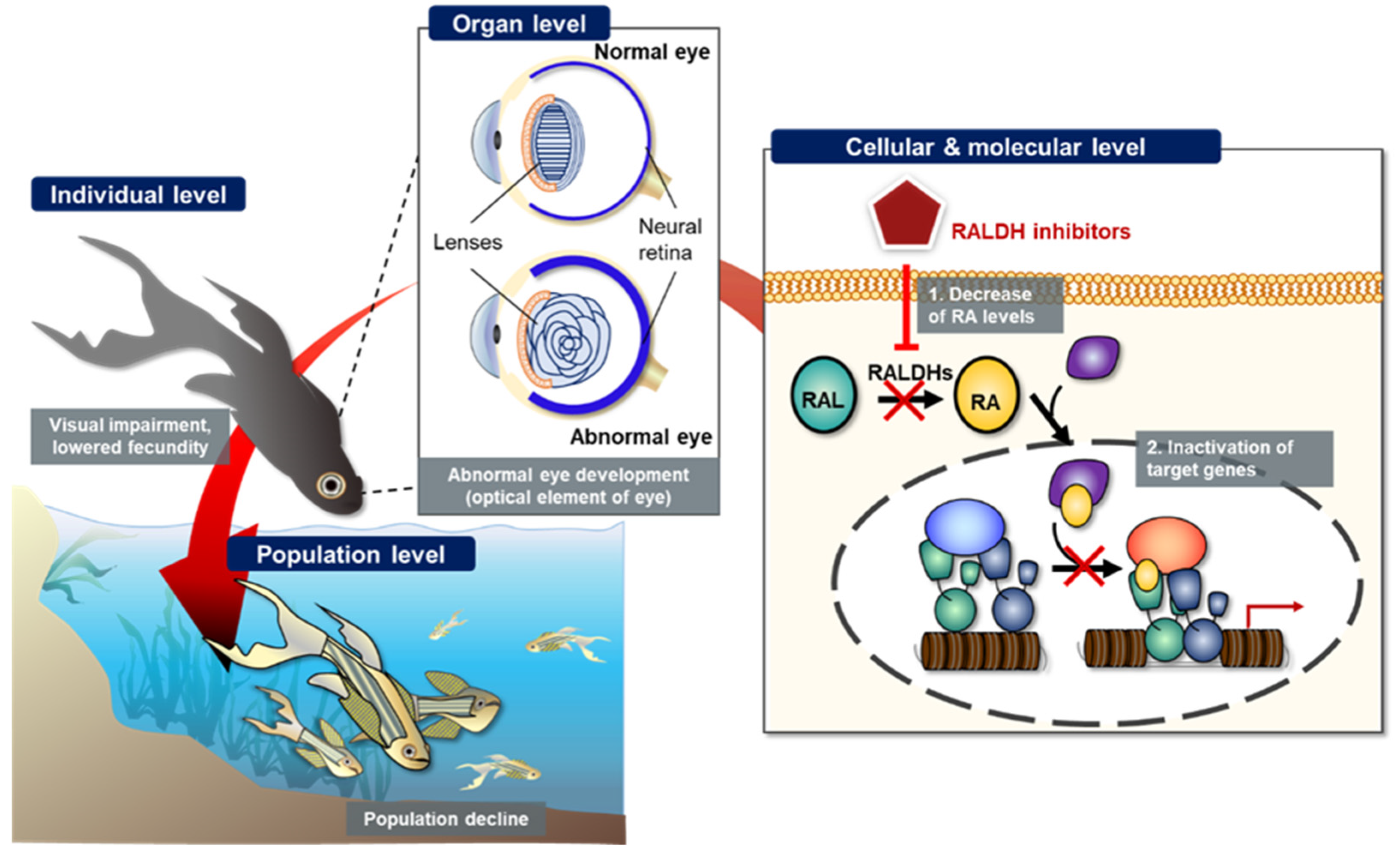
3. Conclusions
References
- Farres, J.; Wang, T.T.Y.; Cunningham, S.J.; Weiner, H. Investigation of the active site cysteine residue of rat liver mitochondrial aldehyde dehydrogenase by site-directed mutagenesis. Biochemistry 1995, 34, 2592–2598.
- Hempel, J.; Lindahl, R. Class III aldehyde dehydrogenase from rat liver: Super-family relationship to classes I and II and functional interpretations. Prog. Clin. Biol. Res. 1989, 290, 3–17.
- Lamb, A.L.; Newcomer, M.E. The structure of retinal dehydrogenase type II at 2.7 Å resolution: Implications for retinal specificity. Biochemistry 1999, 38, 6003–6011.
- Graham, C.E.; Brocklehurst, K.; Pickersgill, R.W.; Warren, M.J. Characterization of retinaldehyde dehydrogenase 3. Biochem. J. 2006, 394, 67–75.
- Moore, S.A.; Baker, H.M.; Blythe, T.J.; Kitson, K.E.; Kitson, T.M.; Baker, E.N. Sheep liver cytosolic aldehyde dehydrogenase: The structure reveals the basis for the retinal specificity of class 1 aldehyde dehydrogenases. Structure 1998, 6, 1541–1551.
- Duester, G. Retinoic acid synthesis and signaling during early organogenesis. Cell 2008, 134, 921–931.
- Pontén, F.; Jirström, K.; Uhlen, M. The Human Protein Atlas—A tool for pathology. J. Pathol. 2008, 216, 387–393.
- Niederreither, K.; Fraulob, V.; Garnier, J.M.; Chambon, P.; Dollé, P. Differential expression of retinoic acid-synthesizing (RALDH) enzymes during fetal development and organ differentiation in the mouse. Mech. Dev. 2002, 110, 165–171.
- Mey, J.; Babiuk, R.P.; Clugston, R.; Zhang, W.; Greer, J.J. Retinal dehydrogenase-2 is inhibited by compounds that induce congenital diaphragmatic hernias in rodents. Am. J. Pathol. 2003, 162, 673–679.
- Kot-Leibovich, H.; Fainsod, A. Ethanol induces embryonic malformations by competing for retinaldehyde dehydrogenase activity during vertebrate gastrulation. Dis. Models Mech. 2009, 2, 295–305.
- Lee, J.Y.; Moon, Y.J.; Lee, H.O.; Park, A.K.; Choi, S.A.; Wang, K.C.; Han, J.W.; Joung, J.G.; Kang, H.S.; Kim, J.E.; et al. Deregulation of retinaldehyde dehydrogenase 2 leads to defective angiogenic function of endothelial colony–Forming cells in pediatric moyamoya disease. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1670–1677.
- Cunningham, T.J.; Duester, G. Mechanisms of retinoic acid signalling and its roles in organ and limb development. Nat. Rev. Mol. 2015, 16, 110–123.
- Niederreither, K.; Dollé, P. Retinoic acid in development: Towards an integrated view. Nat. Rev. Genet. 2008, 9, 541–553.
- Påhlman, S.; Ruusala, A.I.; Abrahamsson, L.; Mattsson, M.E.K.; Esscher, T. Retinoic acid-induced differentiation of cultured human neuroblastoma cells: A comparison with phorbolester-induced differentiation. Cell Differ. 1984, 14, 135–144.
- Rhinn, M.; Dollé, P. Retinoic acid signalling during development. Development 2012, 139, 843–858.
- Rohwedel, J.; Guan, K.; Wobus, A.M. Induction of cellular differentiation by retinoic acid in vitro. Cells Tissues Organs 1999, 165, 190–202.
- Bayha, E.; Jørgensen, M.C.; Serup, P.; Grapin-Botton, A. Retinoic acid signaling organizes endodermal organ specification along the entire antero-posterior axis. PLoS ONE 2009, 4, e5845.
- Sandell, L.L.; Sanderson, B.W.; Moiseyev, G.; Johnson, T.; Mushegian, A.; Young, K.; Rey, J.P.; Ma, J.X.; Staehling-Hampton, K.; Trainor, P.A. RDH10 is essential for synthesis of embryonic retinoic acid and is required for limb, craniofacial, and organ development. Genes Dev. 2007, 21, 1113–1124.
- Wilson, L.; Gale, E.; Maden, M. The role of retinoic acid in the morphogenesis of the neural tube. J. Anat. 2003, 203, 357–368.
- Wang, Z.; Dollé, P.; Cardoso, W.V.; Niederreither, K. Retinoic acid regulates morphogenesis and patterning of posterior foregut derivatives. Dev. Biol. 2006, 297, 433–445.
- Osumi-Yamashita, N. Retinoic acid and mammalian craniofacial morphogenesis. J. Biosci. 1996, 21, 313–327.
- Asselineau, D.; Bernard, B.A.; Bailly, C.; Darmon, M. Retinoic acid improves epidermal morphogenesis. Dev. Biol. 1989, 133, 322–335.
- Cvekl, A.; Wang, W.L. Retinoic acid signaling in mammalian eye development. Exp. Eye Res. 2009, 89, 280–291.
- Hyatt, G.A.; Dowling, J.E. Retinoic acid. A key molecule for eye and photoreceptor development. Investig. Ophthalmol. Vis. Sci. 1997, 38, 1471–1475.
- Marsh-Armstrong, N.; McCaffery, P.; Gilbert, W.; Dowling, J.E.; Dräger, U.C. Retinoic acid is necessary for development of the ventral retina in zebrafish. Proc. Natl. Acad. Sci. USA 1994, 91, 7286–7290.
- Dupé, V.; Lumsden, A. Hindbrain patterning involves graded responses to retinoic acid signalling. Development 2001, 128, 2199–2208.
- Hack, M.A.; Sugimori, M.; Lundberg, C.; Nakafuku, M.; Götz, M. Regionalization and fate specification in neurospheres: The role of Olig2 and Pax6. Mol. Cell. Neurosci. 2004, 25, 664–678.
- Holland, L.Z. A chordate with a difference. Nature 2007, 447, 153–154.
- Niederreither, K.; Vermot, J.; Schuhbaur, B.; Chambon, P.; Dollé, P. Retinoic acid synthesis and hindbrain patterning in the mouse embryo. Development 2000, 127, 75–85.
- Novitch, B.G.; Wichterle, H.; Jessell, T.M.; Sockanathan, S. A requirement for retinoic acid-mediated transcriptional activation in ventral neural patterning and motor neuron specification. Neuron 2003, 40, 81–95.
- Zhao, X.; Duester, G. Effect of retinoic acid signaling on Wnt/β-catenin and FGF signaling during body axis extension. Gene Expr. Patterns 2009, 9, 430–435.
- Griffin, S.V.; Shankland, S.J. Renal hyperplasia and hypertrophy: Role of cell cycle regulatory proteins. In Seldin and Giebisch’s the Kidney; Academic Press: Cambridge, MA, USA, 2008; pp. 723–742.
- Mucida, D.; Park, Y.; Kim, G.; Turovskaya, O.; Scott, I.; Kronenberg, M.; Cheroutre, H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science 2007, 317, 256–260.
- Breitman, T.R.; Selonick, S.E.; Collins, S.J. Induction of differentiation of the human promyelocytic leukemia cell line (HL-60) by retinoic acid. Proc. Natl. Acad. Sci. USA 1980, 77, 2936–2940.
- Duester, G. Keeping an eye on retinoic acid signaling during eye development. Chem. Biol. Interact. 2009, 178, 178–181.
- Kumar, S.; Dollé, P.; Ghyselinck, N.B.; Duester, G. Endogenous retinoic acid signaling is required for maintenance and regeneration of cornea. Exp. Eye Res. 2017, 154, 190–195.
- Matt, N.; Ghyselinck, N.B.; Pellerin, I.; Dupé, V. Impairing retinoic acid signalling in the neural crest cells is sufficient to alter entire eye morphogenesis. Dev. Biol. 2008, 320, 140–148.
- Nedelec, B.; Rozet, J.M.; Taie, L.F. Genetic architecture of retinoic-acid signaling-associated ocular developmental defects. Hum. Genet. 2019, 138, 937–955.
- Smith, J.N.; Walker, H.M.; Thompson, H.; Collinson, J.M.; Vargesson, N.; Erskine, L. Lens-regulated retinoic acid signalling controls expansion of the developing eye. Development 2018, 145, dev167171.
- Dash, S.; Siddam, A.D.; Barnum, C.E.; Janga, S.C.; Lachke, S.A. RNA-binding proteins in eye development and disease: Implication of conserved RNA granule components. Wiley Interdiscip. Rev. RNA 2016, 7, 527–557.
- Heavner, W.; Pevny, L. Eye development and retinogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008391.
- Fan, X.; Molotkov, A.; Manabe, S.I.; Donmoyer, C.M.; Deltour, L.; Foglio, M.H.; Cuenca, A.E.; Blaner, W.S.; Lipton, S.A.; Duester, G. Targeted disruption of Aldh1a1 (Raldh1) provides evidence for a complex mechanism of retinoic acid synthesis in the developing retina. Mol. Cell. Biol. 2003, 23, 4637–4648.
- Matt, N.; Dupé, V.; Garnier, J.M.; Dennefeld, C.; Chambon, P.; Mark, M.; Ghyselinck, N.B. Retinoic acid-dependent eye morphogenesis is orchestrated by neural crest cells. Development 2005, 132, 4789–4800.
- Molotkov, A.; Molotkova, N.; Duester, G. Retinoic acid guides eye morphogenetic movements via paracrine signaling but is unnecessary for retinal dorsoventral patterning. Development 2006, 133, 1901–1910.
- Dupé, V.; Matt, N.; Garnier, J.M.; Chambon, P.; Mark, M.; Ghyselinck, N.B. A newborn lethal defect due to inactivation of retinaldehyde dehydrogenase type 3 is prevented by maternal retinoic acid treatment. Proc. Natl. Acad. Sci. USA 2003, 100, 14036–14041.
- Mic, F.A.; Molotkov, A.; Molotkova, N.; Duester, G. Raldh2 expression in optic vesicle generates a retinoic acid signal needed for invagination of retina during optic cup formation. Dev. Dyn. 2004, 231, 270–277.
- Hyatt, G.A.; Schmitt, E.A.; Marsh-Armstrong, N.R.; Dowling, J.E. Retinoic acid-induced duplication of the zebrafish retina. Proc. Natl. Acad. Sci. USA 1992, 89, 8293–8297.
- Zhou, C.J.; Molotkov, A.; Song, L.; Li, Y.; Pleasure, D.E.; Pleasure, S.J.; Wang, Y.Z. Ocular coloboma and dorsoventral neuroretinal patterning defects in Lrp6 mutant eyes. Dev. Dyn. 2008, 237, 3681–3689.




