
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kai Hilpert | + 3407 word(s) | 3407 | 2021-10-25 10:12:43 | | | |
| 2 | Amina Yu | -2 word(s) | 3405 | 2021-10-26 03:40:02 | | |
Video Upload Options
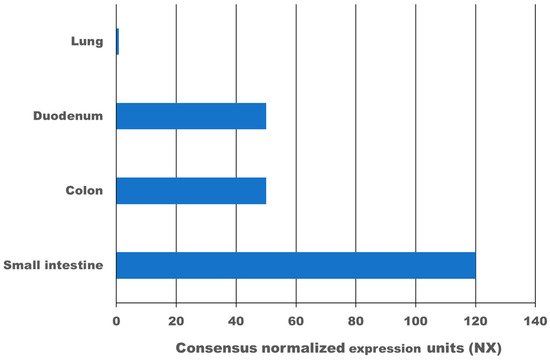
High expression of the transmembrane protein angiotensin I converting enzyme 2 (ACE2), more than 100-times higher as in the lung, and transmembrane serine protease 2 (TMPRSS2) in the gastrointestinal tract leads to infection with SARS-CoV-2. According to meta-analysis data, 9.8–20% of COVID-19 patients experience gastrointestinal symptoms, where diarrhoea is the most frequent, and about 50% shed viruses with high titre through their faeces, where a first faecal transmission was reported. Furthermore, gut inflammation, intestinal damage, and weakening of the gut mucosal integrity that leads to increased permeability has been shown in different studies for COVID-19 patients. This can lead to increased inflammation and bacteraemia. Low mucosal integrity combined with low intestinal damage is a good predictor for disease progression and submission to the intensive care unit (ICU). Several pilot studies have shown that the gut microbiome of COVID-19 patients is changed, microbial richness and diversity were lower, and opportunistic pathogens that can cause bacteraemia were enriched compared to a healthy control group. In a large proportion of these patients, dysbiosis was not resolved at discharge from the hospital and one study showed dysbiosis is still present after 3 months post COVID-19. Consequently, there might be a link between dysbiosis of the gut microbiome in COVID-19 patients and chronic COVID-19 syndrome (CCS). Various clinical trials are investigating the benefit of probiotics for acute COVID-19 patients, the majority of which have not reported results yet. However, two clinical trials have shown that a certain combination of probiotics is beneficial and safe for acute COVID-19 patients. Mortality was 11% for the probiotic treatment group, and 22% for the control group. Furthermore, for the probiotic group, symptoms cleared faster, and an 8-fold decreased risk of developing a respiratory failure was calculated. In conclusion, evidence is arising that inflammation, increased permeability, and microbiome dysbiosis in the gut occur in COVID-19 patients and thus provide new targets for adjuvant treatments of acute and chronic COVID-19. More research in this area is needed.
1. Gastrointestinal Symptoms Caused by SARS-CoV-2
| Number of COVID-19 Patients | Gastro-Intestinal Symptoms | Diarrhoea | Nausea/Vomiting | Abdominal Pain | Number of Studies Used in Meta-Analysis | Reference |
|---|---|---|---|---|---|---|
| 2477 | 13% | 7.8% | 5.5% | 2.7% | 17 | [10] |
| 4243 | 17.6% | 12.5% | 10.2% | 9.2% | 60 | [11] |
| 4805 | Not reported | 7.4% | 4.6% | Not reported | 29 | [12] |
| 5601 | 9.8% | 10.4% | 7.7% | 6.9% | 37 | [13] |
| 17,776 | 20% | 13% | 8% | 4% | 108 | [14] |
| 18,246 | Not reported | 11.5% | 6.3% | 2.3% | 43 | [15] |
2. Expression of ACE2 and TMPRSS2 in the Gastrointestinal Tract
3. Gut Inflammation in COVID-19 Patients
4. SARS-CoV-2 and the Gut Microbiome
| Number of COVID-19 Patients | Healthy Control | Age (Median) | Microbiome Investigated | Enrichment | Loss | Reference | |
|---|---|---|---|---|---|---|---|
| COVID-19 | Control | ||||||
| 15 | 15 (6 with community-acquired pneumonia) | 55 | 48 (50 for Pneumonia) | Gut (faecal sample) | opportunistic pathogens that can cause bacteraemia, including Clostridium hathewayi, Actinomyces viscosus, and Bacteroides nordii | Commensals decreased, for example, Eubacterium, Faecalibacterium prausnitzii, Roseburia, and Lachnospiraceae taxa 1* | [39] |
| 30 | 30 (24 with H1N1) | 55 | 53.5 (48.5 for H1N1) | Gut (faecal sample) | Streptococcus, Rothia, Veillonella, Erysipelatoclostridium, and Actinomyces | mean community richness and microbial diversity were significantly lower in COVID-19 and H1N1 patients 2* | [40] |
| 30 | 30 | 46 | 34 | Gut (faecal sample) | Diversity 2.5-fold higher, for example, Candida albicans, Candida auris, and Aspergillus flavus | [41] | |
| 24 | 48 | 49 | 48 | Oral cavitiy and gut (saliva and facecal sample) | Lipopolysaccharide producing bacteria increased | Microbial diversity decreased, butyric acid-producing bacteria decreased | [42] |
| 14 | 16 | 63.3 | 40.5 | Plasma (from blood) | 65% of COVID-19 patients showed atypical plasma microbiome 3* | [28] | |
5. Targeting the Gut Microbiome as Adjunctive Therapy for COVID-19
6. Is There a Link between Changes in the Gut Microbiome in COVID-19 Patients and Chronic COVID-19 Symptoms?
7. Conclusions
References
- Fehr, A.R.; Perlman, S. Coronaviruses: An overview of their replication and pathogenesis. In Coronaviruses Methods and Protocols; Springer: New York, NY, USA, 2015; pp. 1–23.
- Wood, E.N. An apparently new syndrome of porcine epidemic diarrhoea. Veter. Rec. 1977, 100, 243–244.
- Jung, K.; Annamalai, T.; Lu, Z.; Saif, L.J. Comparative pathogenesis of US porcine epidemic diarrhea virus (PEDV) strain PC21A in conventional 9-day-old nursing piglets vs. 26-day-old weaned pigs. Veter. Microbiol. 2015, 178, 31–40.
- Saif, L.J.; Wang, Q.; Vlasova, A.N.; Jung, K.; Xiao, S. Coronaviruses. In Diseases of Swine; Wiley: Hoboken, NJ, USA, 2019; pp. 488–523.
- Okur, G.S.; Yazici, Z.; Albayrak, H.; Meral, Y. Rotavirus and Coronavirus Prevalances in Healthy Calves and Calves with Diarrhoea. Available online: https://www.researchgate.net/publication/287890228 (accessed on 29 April 2020).
- Rehman, S.U.; Shafique, L.; Ihsan, A.; Liu, Q. Evolutionary trajectory for the emergence of novel coronavirus SARS-CoV-2. Pathogens 2020, 9, 240. Available online: https://www.mdpi.com/2076-0817/9/3/240 (accessed on 28 April 2020).
- Su, S.; Wong, G.; Shi, W.; Liu, J.; Lai, A.C.K.; Zhou, J.; Liu, W.; Bi, Y.; Gao, G.F. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016, 24, 490–502.
- Chen, Z.-R.; Liu, J.; Liao, Z.-G.; Zhou, J.; Peng, H.-W.; Gong, F.; Hu, J.-F.; Zhou, Y. COVID-19 and gastroenteric manifestations. World J. Clin. Cases 2021, 9, 4990–4997.
- Perisetti, A.; Goyal, H.; Gajendran, M.; Boregowda, U.; Mann, R.; Sharma, N. Prevalence, mechanisms, and implications of gastrointestinal symptoms in COVID-19. Front. Med. 2020, 7, 588711.
- Kumar, V.C.S.; Mukherjee, S.; Harne, P.S.; Subedi, A.; Ganapathy, M.K.; Patthipati, V.S.; Sapkota, B. Novelty in the gut: A systematic review and meta-analysis of the gastrointestinal manifestations of COVID-19. BMJ Open Gastroenterol. 2020, 7, 417. Available online: http://bmjopengastro.bmj.com/ (accessed on 5 October 2020).
- Cheung, K.S.; Hung, I.F.; Chan, P.P.; Lung, K.C.; Tso, E.; Liu, R.; Ng, Y.Y.; Chu, M.Y.; Chung, T.W.; Tam, A.R.; et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from the Hong Kong cohort and systematic review and meta-analysis. Gastroenterology 2020, 159, 81–95.
- Parasa, S.; Desai, M.; Chandrasekar, V.T.; Patel, H.K.; Kennedy, K.F.; Roesch, T.; Spadaccini, M.; Colombo, M.; Gabbiadini, R.; Artifon, E.L.; et al. Prevalence of gastrointestinal symptoms and fecal viral shedding in patients with coronavirus disease 2019: A systematic review and meta-analysis. JAMA Netw. Open 2020, 3, e2011335. Available online: https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2767009 (accessed on 27 August 2020).
- Rokkas, T. Gastrointestinal involvement in COVID-19: A systematic review and meta-analysis. Ann. Gastroenterol. 2020, 33, 355–365.
- Dorrell, R.D.; Dougherty, M.K.; Barash, E.L.; Lichtig, A.E.; Clayton, S.B.; Jensen, E.T. Gastrointestinal and hepatic manifestations of COVID-19: A systematic review and meta-analysis. JGH Open 2021, 5, 107–115.
- Silva, F.A.F.D.; Brito, B.B.D.; Santos, M.L.C.; Marques, H.S.; Silva, R.T.D.; Carvalho, L.S.D.; Vieira, E.S.; Oliveira, M.V.; Melo, F.F.D. COVID-19 gastrointestinal manifestations: A systematic review. Rev. Soc. Bras. Med. Trop. 2020, 53, 1–11.
- Fang, D.; Ma, J.; Guang, J.; Wang, M.; Song, Y.; Tian, D. Manifestations of digestive system in hospitalized patients with novel coronavirus pneumonia in Wuhan, China: A single-center, descriptive study. Chin. J. Dig. 2020, 40, E005.
- Guan, W.-J.; Ni, Z.-Y.; Hu, Y.; Liang, W.-H.; Ou, C.-Q.; He, J.-X.; Liu, L.; Shan, H.; Lei, C.-L.; Hui, D.S.; et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720.
- Tian, Y.; Rong, L.; Nian, W.; He, Y. Review article: Gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment. Pharmacol. Ther. 2020, 51, 843–851.
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069.
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280. Available online: https://pubmed.ncbi.nlm.nih.gov/32142651/ (accessed on 14 June 2021).
- Hikmet, F.; Méar, L.; Edvinsson, Å.; Micke, P.; Uhlén, M.; Lindskog, C. The protein expression profile of ACE2 in human tissues. Mol. Syst. Biol. 2020, 16, e9610.
- Xiao, F.; Tang, M.; Zheng, X.; Liu, Y.; Li, X.; Shan, H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology 2020, 6, 1831–1833.
- TMPRSS2 Gene—GeneCards. TMPS2 Protein TMPS2 Antibody. Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=TMPRSS2 (accessed on 9 September 2021).
- Zhang, H.; Kang, Z.; Gong, H.; Xu, D.; Wang, J.; Li, Z.; Li, Z.; Cui, X.; Xiao, J.; Zhan, J.; et al. Digestive system is a potential route of COVID-19: An analysis of single-cell coexpression pattern of key proteins in viral entry process. Gut 2020, 69, 1010–1018.
- Effenberger, M.; Grabherr, F.; Mayr, L.; Schwaerzler, J.; Nairz, M.; Seifert, M.; Hilbe, R.; Seiwald, S.; Scholl-Buergi, S.; Fritsche, G.; et al. Faecal calprotectin indicates intestinal inflammation in COVID-19. Gut 2020, 69, 1543–1544.
- Reuken, P.A.; Wüst, M.; Löffler, B.; Bauer, M.; Stallmach, A. Letter: SARS-CoV-2-induced gastrointestinal inflammation. Aliment. Pharmacol. Ther. 2020, 52, 1748–1749. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7753696/pdf/APT-52-1750.pdf (accessed on 14 June 2021).
- Oliva, A.; Miele, M.C.; Di Timoteo, F.; De Angelis, M.; Mauro, V.; Aronica, R.; Al Ismail, D.; Ceccarelli, G.; Pinacchio, C.; D’Ettorre, G.; et al. Persistent systemic microbial translocation and intestinal damage during coronavirus disease-19. Front. Immunol. 2021, 12, 2810.
- Prasad, R.; Patton, M.J.; Floyd, J.L.; Vieira, C.P.; Fortmann, S.; DuPont, M.; Harbour, A.; See, J.R.C.; Wright, J.; Lamendella, R.; et al. Plasma microbiome in COVID-19 subjects: An indicator of gut barrier defects and dysbiosis. bioRxiv 2021. Available online: https://www.biorxiv.org/content/10.1101/2021.04.06.438634v1 (accessed on 9 September 2021).
- Tsitsiklis, A.; Shoshana, Z.B.; Byrne, A.; Devoe, C.; Levan, S.; Rackaityte, E.; Sunshine, S.; Mick, E.; Ghale, R.; Jauregui, A.; et al. Impaired immune signaling and changes in the lung microbiome precede secondary 1 bacterial pneumonia in COVID-19. Res. Sq. 2021, 1, rs-3.
- Gaibani, P.; Viciani, E.; Bartoletti, M.; Lewis, R.E.; Tonetti, T.; Lombardo, D.; Castagnetti, A.; Bovo, F.; Horna, C.S.; Ranieri, M.; et al. The lower respiratory tract microbiome of critically ill patients with COVID-19. Sci. Rep. 2021, 11, 10103.
- Haiminen, N.; Utro, F.; Seabolt, E.; Parida, L. Functional profiling of COVID-19 respiratory tract microbiomes. Sci. Rep. 2021, 11, 6433.
- Keely, S.; Talley, N.J.; Hansbro, P.M. Pulmonary-intestinal cross-talk in mucosal inflammatory disease. Mucosal Immunol. 2012, 5, 7–18. Available online: www.nature.com/mi (accessed on 3 September 2020).
- Dumas, A.; Bernard-Raichon, L.; Poquet, Y.; Lugo, G.; Neyrolles, O. The role of the lung microbiota and the gut-lung axis in respiratory infectious diseases. Cell. Microbiol. 2018, 20, e12966.
- Dhar, D.; Mohanty, A. Gut microbiota and Covid-19 possible link and implications. Virus Res. 2020, 285, 198018.
- Baud, D.; Dimopoulou Agri, V.; Gibson, G.R.; Reid, G.; Giannoni, E. Using probiotics to flatten the curve of coronavirus disease COVID-2019 pandemic. Front. Public Health 2020, 8, 186.
- Ebrahimi, K.H. SARS-CoV-2 spike glycoprotein-binding proteins expressed by upper respiratory tract bacteria may prevent severe viral infection. FEBS Lett. 2020, 594, 1651–1660.
- Petersen, C.; Round, J.L. Defining dysbiosis and its influence on host immunity and disease. Cell. Microbiol. 2014, 16, 1024–1033.
- Dysbiosis—Wikipedia. Available online: https://en.wikipedia.org/wiki/Dysbiosis (accessed on 9 September 2021).
- Zuo, T.; Zhang, F.; Lui, G.C.; Yeoh, Y.K.; Li, A.Y.; Zhan, H.; Wan, Y.; Chung, A.C.; Cheung, C.P.; Chen, N.; et al. Alterations in gut microbiota of patients with covid-19 during time of hospitalization. Gastroenterology 2020, 159, 944–955.
- Gu, S.; Chen, Y.; Wu, Z.; Chen, Y.; Gao, H.; Lv, L.; Guo, F.; Zhang, X.; Luo, R.; Huang, C.; et al. Alterations of the gut microbiota in patients with coronavirus disease 2019 or H1N1 influenza. Clin. Infect. Dis. 2020, 71, 2669–2678.
- Zuo, T.; Zhan, H.; Zhang, F.; Liu, Q.; Tso, E.Y.; Lui, G.C.; Chen, N.; Li, A.; Lu, W.; Chan, F.K.; et al. Alterations in fecal fungal microbiome of patients with covid-19 during time of hospitalization until discharge. Gastroenterology 2020, 159, 1302–1310.
- Ren, Z.; Wang, H.; Cui, G.; Lu, H.; Wang, L.; Luo, H.; Chen, X.; Ren, H.; Sun, R.; Liu, W.; et al. Alterations in the human oral and gut microbiomes and lipidomics in COVID-19. Gut 2021, 70, 1253–1265.
- Gou, W.; Fu, Y.; Yue, L.; Chen, G.-D.; Cai, X.; Shuai, M.; Xu, F.; Yi, X.; Chen, H.; Zhu, Y.; et al. Gut microbiota, inflammation and molecular signatures of host response to infection. J. Genet. Genom. 2021, in press.
- Vighi, G.; Marcucci, F.; Sensi, L.; DI Cara, G.; Frati, F. Allergy and the gastrointestinal system. Clin. Exp. Immunol. 2008, 153, 3–6.
- Hummel, S.; Veltman, K.; Cichon, C.; Sonnenborn, U.; Schmidt, M.A. Differential targeting of the E-cadherin/β-catenin complex by gram-positive probiotic lactobacilli improves epithelial barrier function. Appl. Environ. Microbiol. 2012, 78, 1140–1147.
- Zelaya, H.; Alvarez, S.; Kitazawa, H.; Villena, J. Respiratory antiviral immunity and immunobiotics: Beneficial effects on inflammation-coagulation interaction during influenza virus infection. Front. Immunol. 2016, 7, 633.
- D’Ettorre, G.; Ceccarelli, G.; Marazzato, M.; Campagna, G.; Pinacchio, C.; Alessandri, F.; Ruberto, F.; Rossi, G.; Celani, L.; Scagnolari, C.; et al. Challenges in the management of SARS-CoV2 infection: The role of oral bacteriotherapy as complementary therapeutic strategy to avoid the progression of COVID-19. Front Med. 2020, 7, 389.
- Ceccarelli, G.; Borrazzo, C.; Pinacchio, C.; Santinelli, L.; Innocenti, G.P.; Cavallari, E.N.; Celani, L.; Marazzato, M.; Alessandri, F.; Ruberto, F.; et al. Oral bacteriotherapy in patients with COVID-19: A retrospective cohort study. Front Nutr. 2021, 7, 341.
- Oxygen-Ozone as Adjuvant Treatment in Early Control of COVID-19 Progression and Modulation of the Gut Microbial Flora—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04366089 (accessed on 13 August 2021).
- Efficacy of L. Plantarum and P. Acidilactici in Adults with SARS-CoV-2 and COVID-19—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/study/NCT04517422 (accessed on 13 August 2021).
- Efficacy of Intranasal Probiotic Treatment to Reduce Severity of Symptoms in COVID19 Infection—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04458519 (accessed on 13 August 2021).
- Efficacy of Probiotics in Reducing Duration and Symptoms of COVID-19—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04621071 (accessed on 13 August 2020).
- Evaluation of the Probiotic Lactobacillus Coryniformis K8 on COVID-19 Prevention in Healthcare Workers—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/study/NCT04366180 (accessed on 12 August 2021).
- Tang, H.; Bohannon, L.; Lew, M.; Jensen, D.; Jung, S.-H.; Zhao, A.; Sung, A.D.; Wischmeyer, P.E. Randomised, double-blind, placebo-controlled trial of probiotics to eliminate COVID-19 transmission in exposed household contacts (PROTECT-EHC): A Clinical trial protocol. BMJ Open 2021, 11, e047069.
- Effect of Lactobacillus on the Microbiome of Household Contacts Exposed to COVID-19—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04399252 (accessed on 12 August 2021).
- Synbiotic Therapy of Gastrointestinal Symptoms During Covid-19 Infection—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04420676 (accessed on 13 August 2021).
- Biliński, J.; Winter, K.; Jasiński, M.; Szczęś, A.; Bilinska, N.; Mullish, B.H.; Małecka-Panas, E.; Basak, G.W. Rapid resolution of COVID-19 after faecal microbiota transplantation. Gut 2021, 1–2.
- The Impact of Fecal Microbiota Transplantation as an Immunomodulation on the Risk Reduction of COVID-19 Disease Progression with Escalating Cytokine Storm and Inflammatory Parameter—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04824222 (accessed on 16 August 2021).
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 long-term effects of COVID-19: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 16144.
- Arzani, M.; Jahromi, S.R.; Ghorbani, Z.; Vahabizad, F.; Martelletti, P.; Ghaemi, A.; Sacco, S.; Togha, M. Gut-brain Axis and migraine headache: A comprehensive review. J. Headache Pain 2020, 21, 1–2.
- Galland, L. The gut microbiome and the brain. J. Med. Food. 2014, 17, 1261–1272. Available online: https://pubmed.ncbi.nlm.nih.gov/25402818/ (accessed on 19 June 2021).
- Matenchuk, B.A.; Mandhane, P.J.; Kozyrskyj, A.L. Sleep, circadian rhythm, and gut microbiota. Sleep Med. Rev. 2020, 53, 101340. Available online: https://pubmed.ncbi.nlm.nih.gov/32668369/ (accessed on 19 June 2021).
- Molina-Torres, G.; Rodriguez-Arrastia, M.; Roman, P.; Labraca, M.N.S.; Cardona, D. Stress and the gut microbiota-brain axis. Behav. Pharmacol. 2019, 30, 187–200.
- Ogawa, Y.; Miyoshi, C.; Obana, N.; Yajima, K.; Hotta-Hirashima, N.; Ikkyu, A.; Kanno, S.; Soga, T.; Fukuda, S.; Yanagisawa, M. Gut microbiota depletion by chronic antibiotic treatment alters the sleep/wake architecture and sleep EEG power spectra in mice. Sci. Rep. 2020, 10, 19554.
- Peirce, J.M.; Alviña, K. The role of inflammation and the gut microbiome in depression and anxiety. J. Neurosci. Res. 2019, 97, 1223–1241.
- Poroyko, V.A.; Carreras, A.; Khalyfa, A.; Khalyfa, A.A.; Leone, V.; Peris, E.; Almendros, I.; Gileles-Hillel, A.; Qiao, Z.; Hubert, N.; et al. Chronic sleep disruption alters gut microbiota, induces systemic and adipose tissue inflammation and insulin resistance in mice. Sci. Rep. 2016, 6, 35405.
- Smith, R.P.; Easson, C.; Lyle, S.M.; Kapoor, R.; Donnelly, C.P.; Davidson, E.J.; Parikh, E.; Lopez, J.V.; Tartar, J.L. Gut microbiome diversity is associated with sleep physiology in humans. PLoS ONE 2019, 14, e0222394.
- Boehme, M.; Guzzetta, K.E.; Bastiaanssen, T.F.S.; van de Wouw, M.; Moloney, G.M.; Gual-Grau, A.; Spichak, S.; Olavarría-Ramírez, L.; Fitzgerald, P.; Morillas, E.; et al. Microbiota from young mice counteracts selective age-associated behavioral deficits. Nat. Aging 2021, 1, 666–676.
- Hilpert, K.; Mikut, R. Is there a connection between gut microbiome dysbiosis occurring in COVID-19 patients and post-COVID-19 symptoms? Front. Microbiol. 2021, 12, 2564.
- Vestad, B.; Ueland, T.; Lerum, T.V.; Dahl, T.B.; Holm, K.; Barratt-Due, A.; Kasine, T.; Dyrhol-Riise, A.M.; Stiksrud, B.; Tonby, K.; et al. Gut microbiota alterations in patients with persistent respiratory dysfunction three months after severe COVID-19. medRxiv 2021. Available online: https://www.medrxiv.org/content/10.1101/2021.07.13.21260412v1 (accessed on 9 September 2021).




