
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kihyun Kim | + 2288 word(s) | 2288 | 2021-10-18 05:54:47 | | | |
| 2 | Rita Xu | Meta information modification | 2288 | 2021-10-22 04:50:47 | | |
Video Upload Options
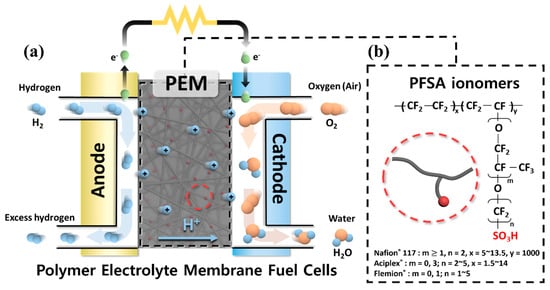
The development of sulfonated hydrocarbon polymer (SHP)-based polymer electrolyte membranes (PEMs) has been pursued in order to overcome drawbacks of the perfluorosulfonic acid ionomer-based PEMs in fuel cell applications. To improve the proton conductivity of SHP-based PEMs without deterioration in physicochemical stability, control of polymeric architecture is necessary to form distinct phase-separated structures between the hydrophilic and hydrophobic domains. By pursuing rational design strategies for the copolymer architectures, it will be possible to develop high-performance SHP-based PEMs in fuel cell applications. This study focused on the synthetic procedures which underlie structure-engineered copolymers and their PEM properties.
1. Introduction
| AFC | MCFC | SOFC | PEMFC | |
|---|---|---|---|---|
| Electrolyte | Aqueous solution of potassium hydroxide soaked in a matrix | Liquid solution of lithium, sodium and/or potassium carbonates, soaked in a matrix | Yttria stabilized zirconie | Solid organic polymer, poly-perfluorosulfonic acid |
| Fuel | Pure H2 | H2, CO, CH4, other | H2, CO, CH4, other | Pure H2 |
| Charge carrier |
OH− | CO32− | O2− | H+ |
| Operating temperature | 90–100 °C | 600–700 °C | 600–1000 °C | 50–100 °C |
| Efficiency | 60% | 45–47% | 35–43% | 53–58% |
| Application | Military, Space | Electric utility, Large distributed generation |
Auxiliary power, Electric utility, Large distributed generation |
Backup power, Portable power, Small distributed generation, Transportation |
| Polymer | Structure |
|---|---|
| SPAES a | 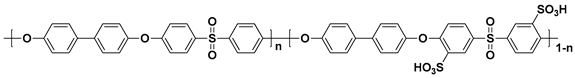 |
| SPEEK b | 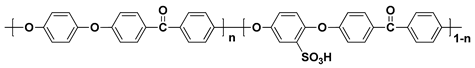 |
| SPPO c | 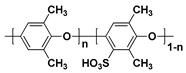 |
| SPI d | 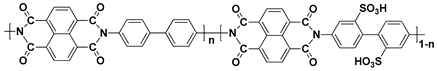 |
a sulfonated poly(arylene ether sulfone); b sulfonated poly(ether ether ketone); c sulfonated poly(phenylene oxide); d sulfonated polyimide.
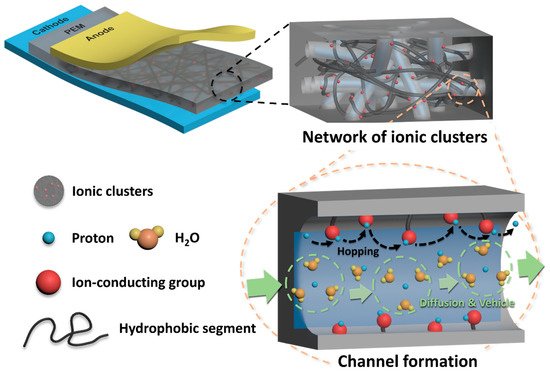
2. Structural Engineering of Sulfonated Hydrocarbon Polymers for PEMFC Applications
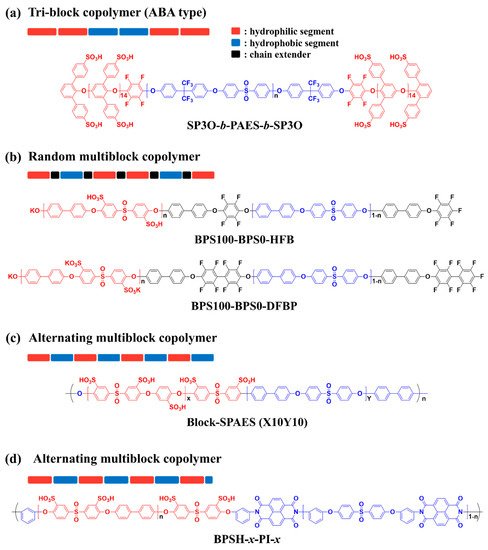
2.1. Block Copolymer-Based PEMs
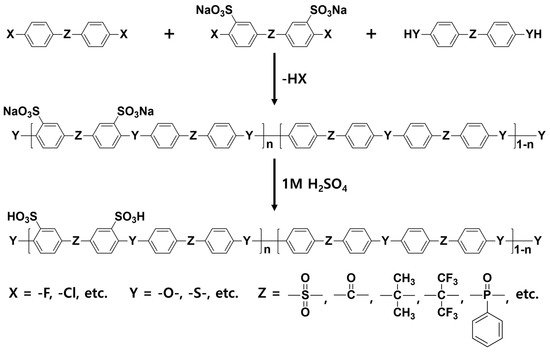
| Sample | IEC (meq g−1) |
Water Uptake | Proton Conductivity | References | |||
|---|---|---|---|---|---|---|---|
| Value (%) |
Conditions (°C, % RH) |
Value (mS cm−1) |
Conditions (°C, % RH) |
||||
| SP3O-b-PAES-b-SP3O | X100 | 0.97 | 47.4 | 20, 100 | 9 | 90, 30 | [63] |
| SPAE100-BPS0-HFB | 5k–5k | 1.30 | 35 | rt, 100 | 50 | 30, 100 | [64] |
| 10k–10k | 1.38 | 68 | 100 | ||||
| 15k–15k | 1.40 | 79 | 110 | ||||
| SPAE100-BPS0-DFBP | 10k–5k | 1.83 | 100 | 160 | |||
| 15k–10k | 1.71 | 90 | 140 | ||||
| Block-SPAES | X10Y10 | 2.90 | 390 | rt, 100 | 480 | 80, 90 | [67] |
| BPSH-x-PI-x | 5–5 | 1.65 | 59 | rt, 100 | 80 | 30, 100 | [68] |
| 15–15 | 1.55 | 85 | 100 | ||||
| 20–20 | 1.22 | 57 | 100 | ||||
References
- Vijayalekshmi, V.; Khastgir, D. Eco-friendly methanesulfonic acid and sodium salt of dodecylbenzene sulfonic acid doped cross-linked chitosan based green polymer electrolyte membranes for fuel cell applications. J. Membr. Sci. 2017, 523, 45–59.
- Mitra, M. A study on advances in hydrogen fuel cells. Elect. Eng. Open A Open J. 2019, 1, 1–4.
- Férriz, A.M.; Bernad, A.; Mori, M.; Fiorot, S. End-of-life of fuel cell and hydrogen products: A state of the art. Int. J. Hydrog. Energy 2019, 44, 12872–12879.
- UNFCCC, V. Adoption of the Paris Agreement. I: Proposal by the President (Draft Decision); United Nations Office: Geneva, Switzerland, 2015.
- Carrette, L.; Friedrich, K.; Stimming, U. Fuel cells-fundamentals and applications. Fuel Cells 2001, 1, 5–39.
- Scofield, M.E.; Liu, H.; Wong, S.S. A concise guide to sustainable PEMFCs: Recent advances in improving both oxygen reduction catalysts and proton exchange membranes. Chem. Soc. Rev. 2015, 44, 5836–5860.
- Mekhilef, S.; Saidur, R.; Safari, A. Comparative study of different fuel cell technologies. Renew. Sustain. Energy Rev. 2012, 16, 981–989.
- Song, C. Fuel processing for low-temperature and high-temperature fuel cells: Challenges, and opportunities for sustainable development in the 21st century. Catal. Today 2002, 77, 17–49.
- Hickner, M.A.; Ghassemi, H.; Kim, Y.S.; Einsla, B.R.; McGrath, J.E. Alternative polymer systems for proton exchange membranes (PEMs). Chem. Rev. 2004, 104, 4587–4612.
- Smitha, B.; Sridhar, S.; Khan, A. Solid polymer electrolyte membranes for fuel cell applications—A review. J. Membr. Sci. 2005, 259, 10–26.
- Kim, K.; Bae, J.; Lim, M.-Y.; Heo, P.; Choi, S.-W.; Kwon, H.-H.; Lee, J.-C. Enhanced physical stability and chemical durability of sulfonated poly(arylene ether sulfone) composite membranes having antioxidant grafted graphene oxide for polymer electrolyte membrane fuel cell applications. J. Membr. Sci. 2017, 525, 125–134.
- Lee, K.H.; Chu, J.Y.; Mohanraj, V.; Kim, A.R.; Song, M.H.; Yoo, D.J. Enhanced ion conductivity of sulfonated poly(arylene ether sulfone) block copolymers linked by aliphatic chains constructing wide-range ion cluster for proton conducting electrolytes. Int. J. Hydrog. Energy 2020, 45, 29297–29307.
- Sharma, P.P.; Tinh, V.D.C.; Kim, D. Enhanced Ion Cluster Size of Sulfonated Poly(Arylene Ether Sulfone) for Proton Exchange Membrane Fuel Cell Application. Polymers 2021, 13, 1111.
- Wu, L.; Zhang, Z.; Ran, J.; Zhou, D.; Li, C.; Xu, T. Advances in proton-exchange membranes for fuel cells: An overview on proton conductive channels (PCCs). Phys. Chem. Chem. Phys. 2013, 15, 4870–4887.
- De Haro, J.C.; Tatsi, E.; Fagiolari, L.; Bonomo, M.; Barolo, C.; Turri, S.; Bella, F.; Griffini, G. Lignin-Based Polymer Electrolyte Membranes for Sustainable Aqueous Dye-Sensitized Solar Cells. ACS Sustain. Chem. Eng. 2021, 9, 8550–8560.
- Rahman, N.A.; Hanifah, S.A.; Mobarak, N.N.; Ahmad, A.; Ludin, N.A.; Bella, F.; Su’ait, M.S. Chitosan as a paradigm for biopolymer electrolytes in solid-state dye-sensitised solar cells. Polymer 2021, 230, 124092.
- Galliano, S.; Bella, F.; Bonomo, M.; Viscardi, G.; Gerbaldi, C.; Boschloo, G.; Barolo, C. Hydrogel electrolytes based on xanthan gum: Green route towards stable dye-sensitized solar cells. Nanomaterials 2020, 10, 1585.
- Nakazawa, S.; Matsuda, Y.; Ochiai, M.; Inafune, Y.; Yamato, M.; Tanaka, M.; Kawakami, H. Enhancing Lithium ion conductivity and all-solid-state secondary battery performance in polymer composite electrolyte membranes with β-Crystalline-rich Poly(vinylidene fluoride) Nanofibers. Electrochim. Acta 2021, 394, 139114.
- Jiang, H.; Wu, Y.; Ma, J.; Liu, Y.; Wang, L.; Yao, X.; Xiang, H. Ultrathin polymer-in-ceramic and ceramic-in-polymer bilayer composite solid electrolyte membrane for high-voltage lithium metal batteries. J. Membr. Sci. 2021, 640, 119840.
- Amici, J.; Torchio, C.; Versaci, D.; Dessantis, D.; Marchisio, A.; Caldera, F.; Bella, F.; Francia, C.; Bodoardo, S. Nanosponge-Based Composite Gel Polymer Electrolyte for Safer Li-O2 Batteries. Polymers 2021, 13, 1625.
- Piana, G.; Ricciardi, M.; Bella, F.; Cucciniello, R.; Proto, A.; Gerbaldi, C. Poly(glycidyl ether)s recycling from industrial waste and feasibility study of reuse as electrolytes in sodium-based batteries. Chem. Eng. J. 2020, 382, 122934.
- Piana, G.; Bella, F.; Geobaldo, F.; Meligrana, G.; Gerbaldi, C.J. PEO/LAGP hybrid solid polymer electrolytes for ambient temperature lithium batteries by solvent-free,“one pot” preparation. J. Energy Storage 2019, 26, 100947.
- Liang, M.; Fu, C.; Xiao, B.; Luo, L.; Wang, Z. A fractal study for the effective electrolyte diffusion through charged porous media. Int. J. Heat Mass Transf. 2019, 137, 365–371.
- Liang, M.; Liu, Y.; Xiao, B.; Yang, S.; Wang, Z.; Han, H. An analytical model for the transverse permeability of gas diffusion layer with electrical double layer effects in proton exchange membrane fuel cells. Int. J. Hydrog. Energy 2018, 43, 17880–17888.
- Redmond, E.L.; Maruyama, M.; Liu, W.K.; Cleghorn, S. Advancement of Thin, Reinforced Membranes for High-Performing, Long-Lasting Proton Exchange Membrane Fuel Cells. In Proceedings of the AiMES 2018 Meeting, Cancun, Mexico, 30 September–4 October 2018; p. 1455.
- Energies, B. Encyclopedia of Inorganic Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; p. 3.
- Zhang, Y.; Li, J.; Ma, L.; Cai, W.; Cheng, H. Recent developments on alternative proton exchange membranes: Strategies for systematic performance improvement. Energy Technol. 2015, 3, 675–691.
- Akbarian-Feizi, L.; Mehdipour-Ataei, S.; Yeganeh, H. Survey of sulfonated polyimide membrane as a good candidate for nafion substitution in fuel cell. Int. J. Hydrog. Energy 2010, 35, 9385–9397.
- Adjemian, K.; Lee, S.; Srinivasan, S.; Benziger, J.; Bocarsly, A.B. Silicon oxide nafion composite membranes for proton-exchange membrane fuel cell operation at 80–140 °C. J. Electrochem. Soc. 2002, 149, A256.
- Kim, K.; Choi, S.-W.; Park, J.O.; Kim, S.-K.; Lim, M.-Y.; Kim, K.-H.; Ko, T.; Lee, J.-C. Proton conductive cross-linked benzoxazine-benzimidazole copolymers as novel porous substrates for reinforced pore-filling membranes in fuel cells operating at high temperatures. J. Membr. Sci. 2017, 536, 76–85.
- Kim, J.; Kim, K.; Han, J.; Lee, H.; Kim, H.; Kim, S.; Sung, Y.E.; Lee, J.C. End-group cross-linked membranes based on highly sulfonated poly(arylene ether sulfone) with vinyl functionalized graphene oxide as a cross-linker and a filler for proton exchange membrane fuel cell application. J. Polym. Sci. 2020, 58, 3456–3466.
- Ko, H.; Kim, M.; Nam, S.Y.; Kim, K. Research of cross-linked hydrocarbon based polymer electrolyte membranes for polymer electrolyte membrane fuel cell applications. Membr. J. 2020, 30, 395–408.
- Gross, M.; Maier, G.; Fuller, T.; MacKinnon, S.; Gittleman, C. Design rules for the improvement of the performance of hydrocarbon-based membranes for proton exchange membrane fuel cells (PEMFC). In Handbook of Fuel Cells; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2010.
- Gubler, L.; Nauser, T.; Coms, F.D.; Lai, Y.-H.; Gittleman, C.S. Perspective—prospects for durable hydrocarbon-based fuel cell membranes. J. Electrochem. Soc. 2018, 165, F3100.
- Byun, G.H.; Kim, J.A.; Kim, N.Y.; Cho, Y.S.; Park, C.R. Molecular engineering of hydrocarbon membrane to substitute perfluorinated sulfonic acid membrane for proton exchange membrane fuel cell operation. Mater. Today Energy 2020, 17, 100483.
- Shin, D.W.; Lee, S.Y.; Lee, C.H.; Lee, K.-S.; Park, C.H.; McGrath, J.E.; Zhang, M.; Moore, R.B.; Lingwood, M.D.; Madsen, L.A. Sulfonated poly(arylene sulfide sulfone nitrile) multiblock copolymers with ordered morphology for proton exchange membranes. Macromolecules 2013, 46, 7797–7804.
- Oh, K.; Ketpang, K.; Kim, H.; Shanmugam, S. Synthesis of sulfonated poly(arylene ether ketone) block copolymers for proton exchange membrane fuel cells. J. Membr. Sci. 2016, 507, 135–142.
- Han, J.; Kim, K.; Kim, J.; Kim, S.; Choi, S.-W.; Lee, H.; Kim, J.-j.; Kim, T.-H.; Sung, Y.-E.; Lee, J.-C. Cross-linked highly sulfonated poly(arylene ether sulfone) membranes prepared by in-situ casting and thiol-ene click reaction for fuel cell application. J. Membr. Sci. 2019, 579, 70–78.
- Kim, K.; Heo, P.; Han, J.; Kim, J.; Lee, J.-C. End-group cross-linked sulfonated poly(arylene ether sulfone) via thiol-ene click reaction for high-performance proton exchange membrane. J. Power Sources 2018, 401, 20–28.
- Kim, K.; Heo, P.; Hwang, W.; Baik, J.-H.; Sung, Y.-E.; Lee, J.-C. Cross-linked sulfonated poly(arylene ether sulfone) containing a flexible and hydrophobic bishydroxy perfluoropolyether cross-linker for high-performance proton exchange membrane. ACS Appl. Mater. Interfaces 2018, 10, 21788–21793.
- Kim, K.; Kim, S.-K.; Park, J.O.; Choi, S.-W.; Kim, K.-H.; Ko, T.; Pak, C.; Lee, J.-C. Highly reinforced pore-filling membranes based on sulfonated poly(arylene ether sulfone)s for high-temperature/low-humidity polymer electrolyte membrane fuel cells. J. Membr. Sci. 2017, 537, 11–21.
- Kim, K.; Heo, P.; Ko, T.; Kim, K.-H.; Kim, S.-K.; Pak, C.; Lee, J.-C. Poly(arlyene ether sulfone) based semi-interpenetrating polymer network membranes containing cross-linked poly(vinyl phosphonic acid) chains for fuel cell applications at high temperature and low humidity conditions. J. Power Sources 2015, 293, 539–547.
- Park, J.E.; Kim, J.; Han, J.; Kim, K.; Park, S.; Kim, S.; Park, H.S.; Cho, Y.-H.; Lee, J.-C.; Sung, Y.-E. High-performance proton-exchange membrane water electrolysis using a sulfonated poly(arylene ether sulfone) membrane and ionomer. J. Membr. Sci. 2021, 620, 118871.
- Han, J.; Kim, K.; Kim, S.; Lee, H.; Kim, J.; Ko, T.; Bae, J.; Choi, W.J.; Sung, Y.-E.; Lee, J.-C. Cross-linked sulfonated poly(ether ether ketone) membranes formed by poly(2, 5-benzimidazole)-grafted graphene oxide as a novel cross-linker for direct methanol fuel cell applications. J. Power Sources 2020, 448, 227427.
- Şengül, E.; Erdener, H.; Akay, R.G.; Yücel, H.; Bac, N.; Eroğlu, İ. Effects of sulfonated polyether-etherketone (SPEEK) and composite membranes on the proton exchange membrane fuel cell (PEMFC) performance. Int. J. Hydrog. Energy 2009, 34, 4645–4652.
- Asano, N.; Aoki, M.; Suzuki, S.; Miyatake, K.; Uchida, H.; Watanabe, M. Aliphatic/aromatic polyimide ionomers as a proton conductive membrane for fuel cell applications. J. Am. Chem. Soc. 2006, 128, 1762–1769.
- Zhang, H.; Shen, P.K. Advances in the high performance polymer electrolyte membranes for fuel cells. Chem. Soc. Rev. 2012, 41, 2382–2394.
- Ko, T.; Kim, K.; Kim, S.-K.; Lee, J.-C. Organic/inorganic composite membranes comprising of sulfonated Poly(arylene ether sulfone) and core–shell silica particles having acidic and basic polymer shells. Polymer 2015, 71, 70–81.
- Kim, K.; Jung, B.-K.; Ko, T.; Kim, T.-H.; Lee, J.-C. Comb-shaped polysulfones containing sulfonated polytriazole side chains for proton exchange membranes. J. Membr. Sci. 2018, 554, 232–243.
- Robertson, G.P.; Mikhailenko, S.D.; Wang, K.; Xing, P.; Guiver, M.D.; Kaliaguine, S. Casting solvent interactions with sulfonated poly(ether ether ketone) during proton exchange membrane fabrication. J. Membr. Sci. 2003, 219, 113–121.
- Lee, H.-S.; Lane, O.; McGrath, J.E. Development of multiblock copolymers with novel hydroquinone-based hydrophilic blocks for proton exchange membrane (PEM) applications. J. Power Sources 2010, 195, 1772–1778.
- Miyatake, K.; Hay, A.S. Synthesis and properties of poly(arylene ether)s bearing sulfonic acid groups on pendant phenyl rings. J. Polym. Sci. A Polym. Chem. 2001, 39, 3211–3217.
- Ko, T.; Kim, K.; Jung, B.-K.; Cha, S.-H.; Kim, S.-K.; Lee, J.-C. Cross-linked sulfonated poly(arylene ether sulfone) membranes formed by in situ casting and click reaction for applications in fuel cells. Macromolecules 2015, 48, 1104–1114.
- Liu, D.; Xie, Y.; Cui, N.; Han, X.; Zhang, J.; Pang, J.; Jiang, Z. Structure and properties of sulfonated poly(arylene ether)s with densely sulfonated segments containing mono-, di-and tri-tetraphenylmethane as proton exchange membrane. J. Membr. Sci. 2021, 620, 118856.
- Lee, K.H.; Chu, J.Y.; Kim, A.R.; Yoo, D.J. Enhanced performance of a sulfonated poly(arylene ether ketone) block copolymer bearing pendant sulfonic acid groups for polymer electrolyte membrane fuel cells operating at 80% relative humidity. ACS Appl. Mater. Interfaces 2018, 10, 20835–20844.
- Kang, K.; Kim, D. Pendant dual-sulfonated poly(arylene ether ketone) multi-block copolymer membranes for enhanced proton conductivity at reduced water swelling. J. Membr. Sci. 2019, 578, 103–110.
- Endoh, E. Highly durable PFSA membranes. In Handbook of Fuel Cells; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 2010.
- Higashihara, T.; Matsumoto, K.; Ueda, M. Sulfonated aromatic hydrocarbon polymers as proton exchange membranes for fuel cells. Polymers 2009, 50, 5341–5357.
- Devanathan, R. Recent developments in proton exchange membranes for fuel cells. Energy Environ. Sci. 2008, 1, 101–119.
- Tsang, E.M.W.; Shi, Z.; Holdcroft, S. Ionic purity and connectivity of proton-conducting channels in fluorous-ionic diblock copolymers. Macromolecules 2011, 44, 8845–8857.
- Tsang, E.M.; Zhang, Z.; Shi, Z.; Soboleva, T.; Holdcroft, S. Considerations of macromolecular structure in the design of proton conducting polymer membranes: Graft versus diblock polyelectrolytes. J. Am. Chem. Soc. 2007, 129, 15106–15107.
- Elabd, Y.A.; Hickner, M.A. Block copolymers for fuel cells. Macromolecules 2011, 44, 1–11.
- Li, N.; Lee, S.Y.; Liu, Y.-L.; Lee, Y.M.; Guiver, M.D. A new class of highly-conducting polymer electrolyte membranes: Aromatic ABA triblock copolymers. Energy Environ. Sci. 2012, 5, 5346–5355.
- Badami, A.S.; Lane, O.; Lee, H.-S.; Roy, A.; McGrath, J.E. Fundamental investigations of the effect of the linkage group on the behavior of hydrophilic–hydrophobic poly(arylene ether sulfone) multiblock copolymers for proton exchange membrane fuel cells. J. Membr. Sci. 2009, 333, 1–11.
- Li, N.; Guiver, M.D. Ion transport by nanochannels in ion-containing aromatic copolymers. Macromolecules 2014, 47, 2175–2198.
- Shin, D.W.; Guiver, M.D.; Lee, Y.M. Hydrocarbon-based polymer electrolyte membranes: Importance of morphology on ion transport and membrane stability. Chem. Rev. 2017, 117, 4759–4805.
- Lee, S.; Ann, J.; Lee, H.; Kim, J.-H.; Kim, C.-S.; Yang, T.-H.; Bae, B. Synthesis and characterization of crosslink-free highly sulfonated multi-block poly(arylene ether sulfone) multi-block membranes for fuel cells. J. Mater. Chem. A 2015, 3, 1833–1836.
- Lee, H.-S.; Roy, A.; Badami, A.S.; McGrath, J.E. Synthesis and characterization of sulfonated poly(arylene ether) polyimide multiblock copolymers for proton exchange membranes. Macromol. Res. 2007, 15, 160–166.
- Einsla, M.L.; Kim, Y.S.; Hawley, M.; Lee, H.-S.; McGrath, J.E.; Liu, B.; Guiver, M.D.; Pivovar, B.S. Toward improved conductivity of sulfonated aromatic proton exchange membranes at low relative humidity. Chem. Mater. 2008, 20, 5636–5642.




