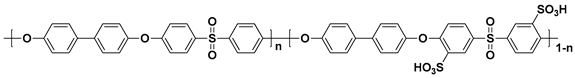
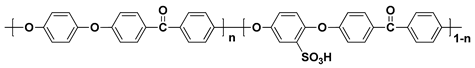
2.1. Block Copolymer-Based PEMs
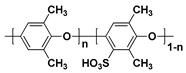
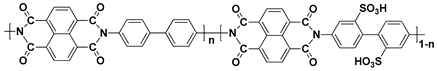
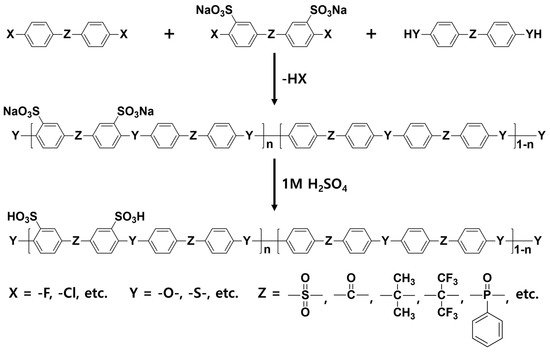
In general, sulfonated hydrocarbon polymers synthesized via the nucleophilic aromatic substitution reaction between dihalo monomers with or without sulfonic acid groups and difunctional monomers with nucleophiles (e.g., dihydroxy and dithiol) are composed of randomly distributed hydrophilic and hydrophobic moieties due to the random distribution of hydrophilic sulfonic acid groups (
Figure 3). Therefore, the PEMs prepared by random copolymers usually exhibit lower proton conductivity compared to the PFSA-based PEMs, especially at low relative humidity (% RH) conditions, due to the low hydrophilic/hydrophobic phase separation behavior which forms the small ion-conducting channels
[57][58][57,58]. Therefore, structural engineering of SHP-based polymer beginning with the synthetic process is highly required to control the nano-phase structures of the resulting PEMs.
Figure 3. Synthetic procedures of sulfonated hydrocarbon-based random copolymers.
It is well known that block copolymers synthesized by assembling the hydrophilic and hydrophobic oligomers forming di-block, tri-block and multi-blocks can effectively control the nano-phase structure and facilitate the distinct phase separation characteristics of the hydrophilic/hydrophobic moieties
[59][60][61][62][59,60,61,62]. Therefore, the block copolymer-based PEMs are able to exhibit outstanding proton conductivity even under low RH conditions and greatly reduce the conductivity dependence on temperature and humidity changes. In addition, due to the well-defined phase-separated structure, the dimensional and chemical stabilities of the block copolymer-based PEMs can be improved compared to those of the random copolymer-based PEMs having similar ion exchange capacities (IECs).
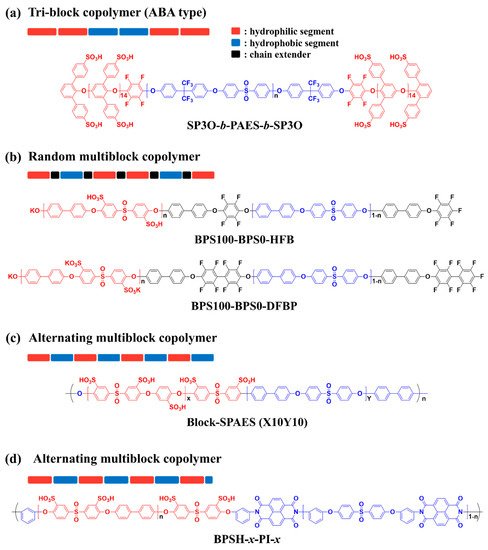
Representatively, Michael D. Guiver et al. reported development of a PEM with ‘ABA’ type tri-block copolymer (SP3O-
b-PAES-
b-SP3O) composed of sulfonated poly(2,6-diphenyl-1,4-phenylene oxide)s (SP3O) as a hydrophilic ‘A’ block and poly(arylene ether sulfone) (PAES) as a hydrophobic ‘B’ block (
Figure 4a)
[63]. Although the IEC value (0.97 meq g
−1) of the SP3O-
b-PAES-
b-SP3O membrane was found to be smaller than those of other reported hydrocarbon-based PEMs, showing the values from 1.50 to 2.34 meq g
−1, the size of the ionic clusters observed by atomic force microscopy (AFM) and transmission electron microscopy (TEM) were 15 nm and 5–10 nm, respectively, and these are comparable to or larger than those of Nafion
® 112. Therefore, the proton conductivities of the SP3O-
b-PAES-
b-SP3O membrane are comparable to those of Nafion
® 112 under low humidity conditions from 30 to 50% RH.
James E. McGrath et al. developed multi-block copolymers using phenoxide-terminated sulfonated poly(arylene ether sulfone) (BPS100) with different chain lengths as the hydrophilic oligomers and poly(arylene ether sulfone) (BPS0) with different chain lengths as the hydrophobic. Two different types of block copolymers were prepared by incorporating different types of perfluoroaryl chain extenders such as hexafluorobenzene (HFB) and decafluorobiphenyl (DFBP), as shown in
Figure 4b
[64]. The resulting multi-block copolymer-based membranes revealed well-defined ion-conducting channels with distinct phase separation between hydrophilic and hydrophobic domains. In addition, the membrane properties including ion conductivity could be adjusted by changing the hydrophilic/hydrophobic block length (e.g., 5k–5k, 10k–10k, 15k–15k, etc.)
[65][66][65,66]. Furthermore, it was noted that membranes formed using DFBP as the chain extender, BPS100-BPS0-DFBP, showed a more distinct phase-separated structure than with HFB as the chain extender (BPS100-BPS0-HFB). This occurs due to the higher content of fluorine moieties in DFBP, which increases the hydrophobicity of the BPS0 oligomers and the acidity of sulfonic acid groups in the hydrophilic BPS100. This observation indicates that the molecular structure of the chain extender affects the properties of the resulting multi-block copolymer-based PEMs.
The incorporation of highly reactive reagents such as HFB and DFBP as chain extenders can efficiently increase the molecular weight of block copolymer by increasing the reaction rate of hydrophilic and hydrophobic segments. However, the structure of the resulting block copolymer is usually random multiblock copolymers due to the same telechelic functionality of the hydrophilic and hydrophobic oligomers. Recently, Byungchan Bae et al. reported detailed synthetic strategies for the development of alternating multi-block copolymer with high-molecular weight by using hydrophilic and hydrophobic segments with different telechelic functional groups. A transparent and flexible PEM with IEC value of 2.9 meq g
−1 could be obtained using this alternating multi-block copolymer (X10-Y10), as shown in
Figure 4c
[67]. Interestingly, the proton conductivity of this block copolymer PEM is four times higher than that of PFSA ionomer-based PEMs such as Nafion
® and Aquivion
® at 80 °C and RH 90%. As well, the conductivity is also comparable to that of the PFSA ionomer-based PEMs even under low RH conditions (≤50%). The distinct phase-separated structure confirmed by TEM as well as the large IEC value of the alternating multi-block copolymer membrane could support its outstanding proton conductivity.
A multi-block copolymer incorporating polyimide (PI) moieties into the main chain was developed by James E. McGrath et al. (
Figure 4d)
[68]. As PI is vulnerable to acidic conditions, a modified PI was adopted to improve the hydrolysis resistance of the PI-based PEMs under fuel cell operating conditions. Multi-block copolymers, utilizing sulfonated poly(arylene ether sulfone)-
b-polyimide (BPSH-
x-PI-
x, where
x indicated the chain length of the hydrophilic and hydrophobic oligomers, respectively), were synthesized via imidization using hydrophilic BPSH oligomers with amine functional end groups and hydrophobic PI oligomers with anhydride end groups. The experimental IEC of the BPSH-
x-PI-
x membrane was found to diminish as the chain lengths of the hydrophilic and hydrophobic moieties increased, although all IEC values showed a similar range from 1.22 to 1.65 (meq g
−1). AFM analysis of the BPSH-
x-PI-
x membranes indicated that the connectivity and size of the ion-conducting channels were better formed as the chain length of each block increased. Accordingly, water uptake of the membranes increased as the length of each block increased, but it decreased sharply when hydrophilic/hydrophobic chain lengths were each over 20 k (i.e., BPSH-
20-PI-
20) due to the enhanced phase separation as well as entanglement between intermolecular chains. In terms of proton conductivity, the BPSH-
15-PI-
15 membrane possessed moderate ion-conducting channels, and the largest water absorption behavior exhibited the largest conductivity values among the samples and these values were larger than those of Nafion
® 112 at 80 °C. Based on these results, it can be concluded that optimizing chain lengths of the hydrophilic/hydrophobic blocks can effectively engineer the ion-conducting channels and thereby control membrane properties
[65][69][65,69]. Due to the outstanding proton conductivity of the block copolymer-based PEMs by constructing the well-defined phase-separated structures, the MEAs prepared with these PEMs have been tried to apply in fuel cell vehicles operating at low RH conditions. The PEM properties of the above-described block copolymer-based PEMs including IEC, water uptake and proton conductivity are summarized in
Table 3.
Figure 4. Representative chemical structures of sulfonated hydrocarbon-based block copolymers
[63][64][67][68][63,64,67,68].
Table 3. Properties of block copolymer-based polymer electrolyte membranes.
| Sample |
IEC
(meq g | −1 | ) |
Water Uptake |
Proton Conductivity |
References |
Value
(%) |
Conditions
(°C, % RH) |
Value
(mS cm | −1 | ) |
Conditions
(°C, % RH) |
| SP3O- | b | -PAES- | b | -SP3O |
X100 |
0.97 |
47.4 |
20, 100 |
9 |
90, 30 |
[63] |
| SPAE100-BPS0-HFB |
5k–5k |
1.30 |
35 |
rt, 100 |
50 |
30, 100 |
[64] |
| 10k–10k |
1.38 |
68 |
|
100 |
|
|
| 15k–15k |
1.40 |
79 |
|
110 |
|
|
| SPAE100-BPS0-DFBP |
10k–5k |
1.83 |
100 |
|
160 |
|
|
| 15k–10k |
1.71 |
90 |
|
140 |
|
|
| Block-SPAES |
X10Y10 |
2.90 |
390 |
rt, 100 |
480 |
80, 90 |
[67] |
| BPSH- | x | -PI- | x |
5–5 |
1.65 |
59 |
rt, 100 |
80 |
30, 100 |
[68] |
| 15–15 |
1.55 |
85 |
|
100 |
|
|
| 20–20 |
1.22 |
57 |
|
100 |
|
|