Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Norman Toro | + 1279 word(s) | 1279 | 2021-10-12 12:13:43 | | | |
| 2 | Rita Xu | Meta information modification | 1279 | 2021-10-13 06:02:45 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Toro, N. Copper Heap Leaching. Encyclopedia. Available online: https://encyclopedia.pub/entry/14992 (accessed on 09 March 2026).
Toro N. Copper Heap Leaching. Encyclopedia. Available at: https://encyclopedia.pub/entry/14992. Accessed March 09, 2026.
Toro, Norman. "Copper Heap Leaching" Encyclopedia, https://encyclopedia.pub/entry/14992 (accessed March 09, 2026).
Toro, N. (2021, October 13). Copper Heap Leaching. In Encyclopedia. https://encyclopedia.pub/entry/14992
Toro, Norman. "Copper Heap Leaching." Encyclopedia. Web. 13 October, 2021.
Copy Citation
Heap leaching is a firm extractive metallurgical technology facilitating the economical processing of different kinds of low-grade ores that are otherwise not exploited.
hydrometallurgy
leaching
gangues
clays minerals
heap leaching
agglomeration
1. Introduction
According to Toro et al. [1], copper mining is an industry that is in constant growth, and approximately 25 million tons are produced annually worldwide [2]. Among the copper minerals on the planet, the vast majority correspond to sulfide ores [3]. Within these copper minerals, chalcopyrite stands out as the most abundant, representing 70% of all minerals that contain copper in the Earth’s crust [4][5][6][7]. Copper is recovered from these minerals mainly through flotation, followed by pyrometallurgical processing, representing 80–85% of world’s copper production [8][9]. However, pyrometallurgical treatment is difficult and expensive for low-grade copper ores producing high emissions of sulfur dioxide (SO2), NOx, and CO2, which cause problems, such as acid rain and increased local pollution [10][11][12].
In addition, flotation techniques generate a large amount of waste, which results in tailings dams with a high possibility of generating acid mine drainage (AMD) due to the oxidation of minerals with a high presence of pyrite [13]. The latter is essential to consider since the drainage of mining waste rocks is one of the most important environmental challenges facing the global mining industry due to its dynamics and persistence [14][15][16][17]. AMD creates a severe environmental problem allied with mining and mineral processing due to its very low pH (<3.0) and high concentrations of possibly toxic dissolved metals, metalloids, and sulfate. Without appropriate management, AMD can result in considerable environmental degradation, water, and soil contamination, severe health deterioration among neighboring communities, and damaged biodiversity in aquatic ecosystems [18][19][20][21].
All of the above has led to the need to investigate the development of a profitable hydrometallurgical process to treat these minerals since hydrometallurgy is a good alternative to process both oxidized minerals and sulfide minerals environmentally friendly [22][23][24]. Heap leaching is a hydrometallurgical approach and continuously developing mineral processing and extraction technology that is gaining attractiveness and recognition in the mineral industry. Heap leaching has solid benefits over traditional metallurgical methods where economically viable options have become limited [25].
2. Heap Leaching as an Alternative Route in Hydrometallurgy
For Watling et al. [26], certain issues motivate the use of hydrometallurgical methods, even for sulfide ores, for example, the high copper demand; the continuous decay of the ore grades; and the extensive exploitation of oxide and secondary sulfide minerals. The low-grade may eventually leave large amounts of low-grade chalcopyrite ores as an important, but so far, uneconomical source of copper. This has prompted the use of processes such as heap leaching. Heap leaching began to be used in the middle of the 20th century.
Nevada’s gold and silver heap leaching as the “birthplace” of modern gold heap leaching [25][27][28]. The first modern copper heap leach operation may have been the Bluebird copper oxide mine in 1968, followed in the early 1970s by other small operations in the United States. Uranium producers have already utilized the heap leaching of uranium through either acid or alkaline solutions since the late 1950s. Large-scale heap leaching can be said to have started in 1980 when three major copper projects were commissioned in Chile, and, at approximately the same time, a large number of gold projects were commissioned in the United States [25].
Heap leaching has been developed for many different types of minerals, climates, and operations of any size [25]. Further than copper oxide, uranium, and gold, today there are an extensive variety of applications, including copper sulfide ores, gold-bearing pyritic ores, and non-metallic minerals (such as saltpeter [29]) as well as soil remediation [25][30][31]. Heap leaching is typically applied for low-grade deposits; however, it might also be applied to small higher-grade deposits in remote or politically high-risk locations to reduce capital cost. Heap leaching from low-grade ores has contributed to the total global production of copper, gold, silver, and uranium [25][32][33]. Heap leaching has also been considered for zinc [25][34][35] and nickel [36] and more lately for platinum group metal (PGM)-bearing ores and electronic scrap [37][38].
In heap leaching, the crushed ore is stacked on an impermeable pad, and leaching reagents (a strong acid, commonly sulfuric acid for copper or nickel ores or a dilute cyanide solution for gold and silver-bearing ores) are added by irrigation from the top. The wanted mineral is extracted, and the solution is gradually loaded as it penetrates through the pile. Leaching may be aided by microorganisms resident within the ore bed, particularly in the existence of sulfide minerals. A drainage system collects the pregnant leach solution (PLS) at the base of the heap. The PLS is then pumped to the processing units to extract the value metal.
The barren leach solution (BLS) is sent to the barren solution pond, from where, after solution makeup, it is reapplied to the heap’s surface [25]. A typical heap leaching circuit is shown in Figure 1. This process is conducted in leaching piles, where their typical height is between 4 and 10 m, although in some cases, they can reach 18 m [28]. In addition, the largest sizes generally range between 10 and 40 mm in heap leaching, and sizes less than 6 mm are unacceptable. This is because small-sized particles affect the heap’s permeability, mainly clay minerals result in increased clogging of heaps over time due to swelling and gradual decrepitation [39].
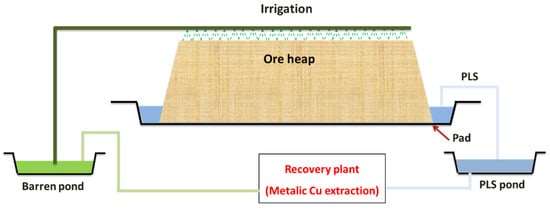
Figure 1. Typical heap leach flow diagram for copper (modified from [25]).
For the leaching process to be efficient, the fine particles tend to agglomerate around the larger particles with water and concentrated sulfuric acid, a process known as “curing.” This process improves the strength of the material while having good mineral permeability in heap leaching. In addition, it helps to achieve adequate heap heights, improve copper recovery rates, and control processing times [40][41]. It is worth mentioning that another emerging method is bio-hydrometallurgy, which plays an important role in the recovery of copper with economic, environmental, and social benefits. To date, it has been reported that many investigations on the acid bioleaching of secondary [42][43][44][45][46] and primary sulfides have presented good results.
3. Effect of Ore Mineralogy on Copper Heap Leaching Performance
Copper heap leach projects are sometimes evaluated without adequate mineralogy, despite the lack of a clear and comprehensive mineralogical sturdy, which could significantly affect the heap efficiency and expected recovery and operating costs [47][48][49]. Heap leaching processes operate over approximately three months for sulfide minerals in chlorinated media and lower ore grades in typical operations. This is why several studies have emphasized the essential need to characterize the mineral’s physical, chemical, and mineralogical properties to be leached [49][50].
Problems with copper heap leaching may arise from the ore mineralogy, more specifically, the presence of reagent consuming gangues and clays minerals. Ghomi et al. [51] analyzed the effect of polar organic reagents on chalcopyrite leaching, Scanning Electron Microscopy-Energy Dispersive X-ray Spectroscopy (SEM-EDS) analysis was performed (Figure 2), where the presence of aluminosilicate or clay-type gangues was detected in area 4, with a high percentage of aluminum, silicon, and oxygen, which can be corroborated by Figure 3.
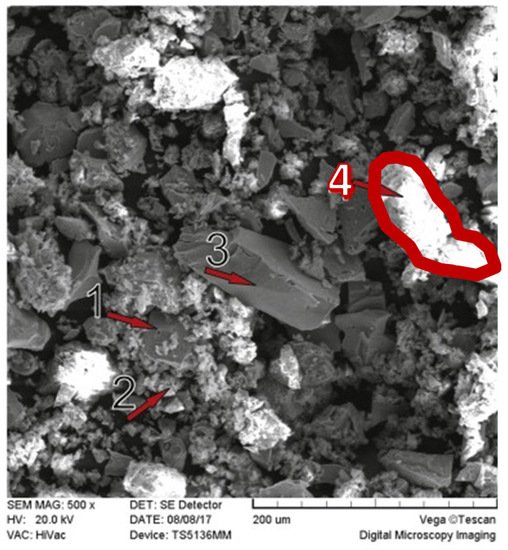
Figure 2. SEM image of the chalcopyrite leaching residue after 120 min of leaching in 1.5 mol/L of [H2SO4], 2 mol/L of [H2O2], and 2 mol/L of isopropanol solution at 65 °C (Modified from [51]).
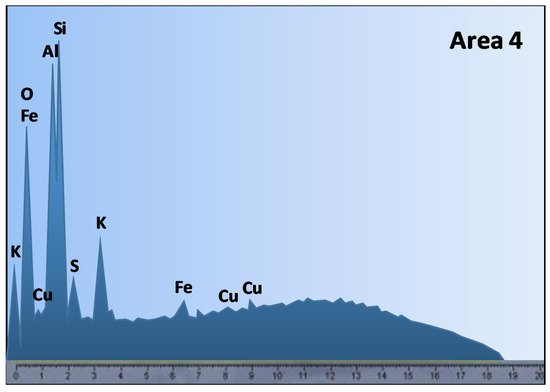
Figure 3. EDS microanalysis of areas indicated in the SEM (Modified from [51]).
For their part, Helle and Kelm [52] studied leaching with sulfuric acid, focusing on the retention of copper by the reactive gangue. Gangue minerals can considerably affect acid consumption and copper recovery and change the acid requirements in different unit operations [53][54]. Critical factors for acid consumption in oxidized copper ores include the presence of carbonate; the presence of other short-term and long-term acid consumers; and the degree of acid adsorption by different non-carbonate minerals (e.g., clays, oxides of hydrated iron, highly porous copper minerals, and/or mineral-forming silts) [48].
References
- Toro, N.; Pérez, K.; Saldaña, M.; Salinas-Rodríguez, E.; Hernández, P. Treatment of black copper with the use of iron scrap—Part I. Hem. Ind. Ind. 2020, 74, 237–245.
- Flanagan, D.M. Copper Commodity Summaries 2021; USGS: Reston, VA, USA, 2021.
- Pérez, K.; Toro, N.; Saldaña, M.; Salinas-Rodríguez, E.; Robles, P.; Torres, D.; Jeldres, R.I. Statistical Study for Leaching of Covellite in a Chloride Media. Metals 2020, 10, 477.
- Taboada, M.E.; Hernández, P.C.; Padilla, A.P.; Jamett, N.E.; Graber, T.A. Effects of Fe+2 and Fe+3 in Pretreatment and Leaching on a Mixed Copper Ore in Chloride Media. Metals 2021, 11, 866.
- Rodríguez, M.; Ayala, L.; Robles, P.; Sepúlveda, R.; Torres, D.; Carrillo-Pedroza, F.R.; Jeldres, R.I.; Toro, N. Leaching chalcopyrite with an imidazolium-based ionic liquid and bromide. Metals 2020, 10, 183.
- Bogdanović, G.D.; Petrović, S.; Sokić, M.; Antonijević, M.M. Chalcopyrite leaching in acid media: A review. Metall. Mater. Eng. 2020, 26, 177–198.
- Torres, D.; Ayala, L.; Jeldres, R.I.; Cerecedo-Sáenz, E.; Salinas-Rodríguez, E.; Robles, P.; Toro, N. Leaching Chalcopyrite with High MnO2 and Chloride Concentrations. Metals 2020, 10, 107.
- Saldaña, M.; Rodríguez, F.; Rojas, A.; Pérez, K.; Angulo, P. Development of an empirical model for copper extraction from chalcocite in chloride media. Hem. Ind. 2020, 74, 285–292.
- Saldaña, M.; Neira, P.; Flores, V.; Robles, P.; Moraga, C. A Decision Support System for Changes in Operation Modes of the Copper Heap Leaching Process. Metals 2021, 11, 1025.
- Nikoloski, A.N.; O’Malley, G.P. The acidic ferric sulfate leaching of primary copper sulfides under recycle solution conditions observed in heap leaching. Part 1. Effect of standard conditions. Hydrometallurgy 2018, 178, 231–239.
- Dijkstra, R. Economical abatement of high-strength SO2 off-gas from a smelter. J. S. Afr. Inst. Min. Metall. 2017, 117, 1003–1007.
- Toro, N.; Briceño, W.; Pérez, K.; Cánovas, M.; Trigueros, E.; Sepúlveda, R.; Hernández, P. Leaching of pure chalcocite in a chloride media using sea water and waste water. Metals 2019, 9, 780.
- Rodríguez, F.; Moraga, C.; Castillo, J.; Gálvez, E.; Robles, P.; Toro, N. Submarine Tailings in Chile—A Review. Metals 2021, 11, 780.
- Dinelli, E.; Tateo, F. Different types of fine-grained sediments associated with acid mine drainage in the Libiola Fe–Cu mine area (Ligurian Apennines, Italy). Appl. Geochem. 2002, 17, 1081–1092.
- Doula, M.; Ioannou, A.; Dimirkou, A. Copper Adsorption and Si, Al, Ca, Mg, and Na Release from Clinoptilolite. J. Colloid Interface Sci. 2002, 245, 237–250.
- Johnson, D.B.; Hallberg, K.B. Acid mine drainage remediation options: A review. Sci. Total Environ. 2005, 338, 3–14.
- White, A.F.; Brantley, S.L. The effect of time on the weathering of silicate minerals: Why do weathering rates differ in the laboratory and field? Chem. Geol. 2003, 202, 479–506.
- Galhardi, J.A.; Bonotto, D.M. Hydrogeochemical features of surface water and groundwater contaminated with acid mine drainage (AMD) in coal mining areas: A case study in southern Brazil. Environ. Sci. Pollut. Res. 2016, 23, 18911–18927.
- Shim, M.J.; Choi, B.Y.; Lee, G.; Hwang, Y.H.; Yang, J.-S.; O’Loughlin, E.J.; Kwon, M.J. Water quality changes in acid mine drainage streams in Gangneung, Korea, 10 years after treatment with limestone. J. Geochem. Explor. 2015, 159, 234–242.
- Anawar, H.M. Sustainable rehabilitation of mining waste and acid mine drainage using geochemistry, mine type, mineralogy, texture, ore extraction and climate knowledge. J. Environ. Manag. 2015, 158, 111–121.
- Albanese, S.; De Vivo, B.; Lima, A.; Frattasio, G.; Kříbek, B.; Nyambe, I.; Majer, V. Prioritising environmental risk at the regional scale by a GIS aided technique: The Zambian Copperbelt Province case study. J. Geochem. Explor. 2014, 144, 433–442.
- Velásquez-Yévenes, L.; Torres, D.; Toro, N. Leaching of chalcopyrite ore agglomerated with high chloride concentration and high curing periods. Hydrometallurgy 2018, 181, 215–220.
- Turan, M.D.; Orhan, R.; Turan, M.; Nizamoğlu, H. Use of Ammonia Salts in Selective Copper Extraction from Tailings. Mining, Metall. Explor. 2020, 37, 1349–1356.
- Turan, M.D.; Sarı, Z.A.; Nizamoğlu, H. Pressure leaching of chalcopyrite with oxalic acid and hydrogen peroxide. J. Taiwan Inst. Chem. Eng. 2021, 118, 112–120.
- Ghorbani, Y.; Franzidis, J.-P.; Petersen, J. Heap leaching technology—Current state, innovations and future directions: A review. Miner. Process. Extr. Metall. Rev. 2015, 37, 73–119.
- Watling, H.R.; Shiers, D.W.; Li, J.; Chapman, N.M.; Douglas, G.B. Effect of water quality on the leaching of a low-grade copper sulfide ore. Miner. Eng. 2014, 58, 39–51.
- Scheffel, R. Copper Heap Leach Design and Practice. In Mineral Processing Plant Design, Practice, and Control; Chapter 12; Mular, A.L., Halbe, D.N., Barratt, D.J., Eds.; SME: Englewood, CO, USA, 2002; Volume 2, pp. 1571–1605.
- Kappes, D.W. Precious metal heap leach design and practice. In Mineral Processing Plant Design, Practice, and Control; SME: Englewood, CO, USA, 2002; Volume 2, pp. 1606–1630. ISBN 0-87335-223-8.
- Valencia, J.A.; Méndez, D.A.; Cueto, J.Y.; Cisternas, L.A. Saltpeter extraction and modelling of caliche mineral heap leaching. Hydrometallurgy 2008, 90, 103–114.
- Padilla, G.A.; Cisternas, L.A.; Cueto, J.Y. On the optimization of heap leaching. Miner. Eng. 2008, 21, 673–678.
- Scheffel, R.D. Heap Leaching Design for Success-a Case Study. In Proceedings of the Low-Grade Uranium Dump and Heap Leach Technical Meeting, Vienna, Austria, 29–31 March 2010.
- Petersen, J. Heap leaching as a key technology for recovery of values from low-grade ores—A brief overview. Hydrometallurgy 2016, 165, 206–212.
- Robertson, S.W.; van Staden, P.J.; Seyedbagheri, A. Advances in high-temperature heap leaching of refractory copper sulphide ores. J. S. Afr. Inst. Min. Metall. 2012, 112, 1045–1050.
- Lizama, H.M.; Jensen, S.E.; Stradling, A.W. Dynamic microbial populations in heap leaching of zinc sulphide ore. Miner. Eng. 2012, 25, 54–58.
- Petersen, J.; Dixon, D.G. Modeling and optimization of heap bioleach processes. In Biomining; Springer: Berlin/Heidelberg, Germany, 2007; pp. 153–176.
- Carlsson, E.; Büchel, G. Screening of residual contamination at a former uranium heap leaching site, Thuringia, Germany. Geochemistry 2005, 65, 75–95.
- Mwase, J.M.; Petersen, J.; Eksteen, J.J. A conceptual flowsheet for heap leaching of platinum group metals (PGMs) from a low-grade ore concentrate. Hydrometallurgy 2012, 111–112, 129–135.
- Ilyas, S.; Lee, J.; Chi, R. Bioleaching of metals from electronic scrap and its potential for commercial exploitation. Hydrometallurgy 2013, 131–132, 138–143.
- Saldaña, M.; Toro, N.; Castillo, J.; Hernández, P.; Navarra, A. Optimization of the heap leaching process through changes in modes of operation and discrete event simulation. Minerals 2019, 9, 421.
- Lu, J.; Dreisinger, D.; West-Sells, P. Acid curing and agglomeration for heap leaching. Hydrometallurgy 2017, 167, 30–35.
- Schlesinger, M.; King, M.; Sole, K.; Davenport, W. Extractive Metallurgy of Copper, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2011; ISBN 9780080967899.
- Liu, H.; Xia, J.; Nie, Z.; Ma, C.; Zheng, L.; Hong, C.; Zhao, Y.; Wen, W. Bioleaching of chalcopyrite by Acidianus manzaensis under different constant pH. Miner. Eng. 2016, 98, 80–89.
- Lee, J.; Acar, S.; Doerr, D.L.; Brierley, J.A. Comparative bioleaching and mineralogy of composited sulfide ores containing enargite, covellite and chalcocite by mesophilic and thermophilic microorganisms. Hydrometallurgy 2011, 105, 213–221.
- Ruan, R.; Zou, G.; Zhong, S.; Wu, Z.; Chan, B.; Wang, D. Why Zijinshan copper bioheapleaching plant works efficiently at low microbial activity—Study on leaching kinetics of copper sulfides and its implications. Miner. Eng. 2013, 48, 36–43.
- Ma, L.; Wang, X.; Liu, X.; Wang, S.; Wang, H. Intensified bioleaching of chalcopyrite by communities with enriched ferrous or sulfur oxidizers. Bioresour. Technol. 2018, 268, 415–423.
- Zhang, R.; Sun, C.; Kou, J.; Zhao, H.; Wei, D.; Xing, Y. Enhancing the Leaching of Chalcopyrite Using Acidithiobacillus ferrooxidans under the Induction of Surfactant Triton X-100. Minerals 2018, 9, 11.
- Moraga, C.; Cerecedo-Saenz, E.; González, J.; Robles, P.; Carrillo-Pedroza, F.R.; Toro, N. Comparative Study of MnO2 Dissolution from Black Copper Minerals and Manganese Nodules in an Acid Medium. Metals 2021, 11, 817.
- Jansen, M.; Taylor, A. Overview of gangue mineralogy issues in oxide copper heap leaching. In Proceedings of the ALTA Conference, Perth, WA, Australia, 19–24 May 2003; p. 32.
- Ghorbani, Y.; Becker, M.; Petersen, J.; Mainza, A.N.; Franzidis, J.-P. Investigation of the Effect of mineralogy as rate-limiting factors in large particle leaching. Miner. Eng. 2013, 52, 38–51.
- Bouffard, S.C. Review of Agglomeration Practice and Fundamentals in Heap Leaching. Miner. Process. Extr. Metall. Rev. 2005, 26, 233–294.
- Ghomi, M.A.; Mozammel, M.; Moghanni, H.; Shahkar, L. Atmospheric leaching of chalcopyrite in the presence of some polar organic reagents: A comparative study and optimization. Hydrometallurgy 2019, 189, 105120.
- Helle, S.; Kelm, U. Experimental leaching of atacamite, chrysocolla and malachite: Relationship between copper retention and cation exchange capacity. Hydrometallurgy 2005, 78, 180–186.
- Baum, W. The use of a mineralogical data base for production forecasting and troubleshooting in copper leach operations. In Proceedings of the C. 99, Copper 99 Fourth International Conference, Phoenix, AZ, USA, 10–13 October 1999; pp. 393–408.
- Baum, W.; Ausburn, K. Daily process mineralogy: A metallurgical tool for optimized copper leaching. In Proceedings of the 5th International Seminar on Process Hydrometallurgy, Santiago, Chile, 10–12 July 2013; pp. 49–56.
More
Information
Subjects:
Engineering, Chemical
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
7.2K
Revisions:
2 times
(View History)
Update Date:
13 Oct 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





