
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maria Stefania Sinicropi | + 2351 word(s) | 2351 | 2021-09-27 10:55:46 | | | |
| 2 | Catherine Yang | Meta information modification | 2351 | 2021-10-14 03:52:08 | | |
Video Upload Options
Thallium, as a pharmaceutical cosmetic product, is applied for facial hair removal and fungal infections of the scalp. Thallium acetate is currently used as a catalyst in organic synthesis in the oxidation of olefins and hydrocarbons, and in epoxidation and polymerization reactions. Detection of Tl is a challenging task because its concentration in environmental samples may be at a nanogram per gram level or lower.
1. Introduction
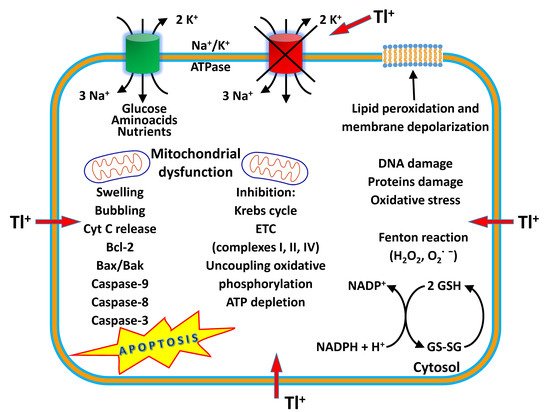
Thallium (Tl) is a rare earth bluish-white heavy metal (81 atomic number, 204.38 atomic mass, 11.85 g/cm 3), and is soft, malleable, and exists in two oxidation states (I and III). The name thallium derives from Greek thallos, a young olive-green shoot. Although thallium is present in the natural environment in low concentration, it is widely distributed in water environments [1]. The European COST Action TD1407 included Tl in the list of technology-critical elements, with associated environmental impacts and potential human health threats [2]. This element is a non-essential metal present in low concentration in human tissues but is endowed with high potential toxicity. Indeed, it has been considered one of the most toxic among the heavy metals, more toxic to humans than mercury, cadmium, lead, copper, or zinc [3][4]. Acute Tl poisoning in humans induces pathological changes in organs such as the stomach, liver, kidneys, brain, intestine, cardiovascular and nervous systems, along with chronic effects such as mental disorders or polyneuritis, and may even result in death [5]. The lethal dose of Tl for an adult human is only 8–10 mg/kg. Monovalent thallium is similar to potassium in ionic radius and electrical charge, and these factors contribute to its toxic nature. Thallium acts on several organs, interfering with cellular metabolism, affecting vital potassium-dependent processes and mitochondrial metabolism, affecting uncoupling mitochondrial oxidative phosphorylation. In addition, thallium increases reactive oxygen species (ROS) formation and phospholipid peroxidation, alters the mitochondrial membrane potential (MMP), causing mitochondrial depolarization and swelling with a release of cytochrome C from the inner mitochondrial membrane. These processes are likely to account for the neurotoxic effects of the metal [6]. Long-term, low-dose prenatal Tl exposure may cause dysfunction in the mother [7], whereas absorbed Tl can affect the developing fetus because it can cross the placental barrier [8]. Several treatment modalities have been used for thallium toxicity, but no single antidote has been shown to be effective in severe toxicity. However, combinations of different treatments have been proven to be beneficial in several cases. Prussian blue (PB) has been the most prescribed antidote to treat thallium poisoning. This chelator agent is administered by the oral route, decreasing the absorption of Tl to the enterohepatic circulation, and therefore increasing the elimination of Tl into feces [9]. Despite its effectiveness as an antidote, in severe cases of human thallotoxicosis, its administration is still ineffective. Other chelating agents have been administered alone or in combination with PB, such as sodium diethyldithiocarbamate and D-penicillamine [10][11]. The combined treatment of PB and metallothionein has proven to be a good antidotal option against thallotoxicosis [12]. Blood purification treatments are also a beneficial treatment option, especially for patients with severe thallium poisoning [13]. Despite its toxicity, known since the 1970s [14][15], this metal is applied in cardiovascular scintigraphy and as a tool for imaging malignant tumors such as lung cancer, breast cancer and osteosarcoma bone cancer [16]. Thallium-201 chloride ( 201Tl-thallous chloride) was the first radiopharmaceutical clinically used for cardiac imaging technique in the evaluation of ischemic heath disease. Short-lived radioactive thallium (emitting X-rays and gamma-rays) is administered by the intravenous route in the human body. It is obtained from metal mining, ore processing or smelting operations and is discarded as a by-product in the environment [17]. In recent years, Tl contamination incidences have been reported in many countries, mostly due to industrial activities such as the mining and smelting of Tl-rich sulfide ores, metallurgical production, coal combustion, and cement production [18]. Recent studies have shown that elevated Tl levels often occur in soils, waters, sediments, and agricultural products in the vicinity of industrial sites using Tl-bearing mineral resources [19]. Thallium can be released into the environment as waste from the production of cadmium, lead and zinc, and cement factories, and by the combustion of coal in coal-fired power plants. There is an increasing contemporary demand for this metal in the advanced technology field, in infrared spectrometers and other optical systems, electronic devices, alloys, semiconductors, and the laser industry [20][21]. High levels of thallium have been found in drinking water, vegetables, fruits and food due to anthropogenic activities [19][22]. Human beings are subjected to thallium exposure through the intake of contaminated fruits, vegetables, and other food, water, through the inhalation of polluted air, and living near industrial facilities such as cement and coal-fired power plants, mines and ore smelting. Aprea et al. reported a study which represents the most extensive human biomonitoring campaign for the evaluation of thallium exposure available at international level [22]. Staff et al. showed that urinary thallium concentrations were higher in thallium workers than in non-occupationally exposed people and general workers [23]. Therefore, the removal of this element from soil and water is vital to eradicate its health impacts. Phytoremediation is a green technology that uses plants to remove toxic and radioactive metals, and organic compounds such as pesticides and detergents from soil and water, and it is used for its cost-effectiveness and environmental friendliness [24]. Some plants are ideal for the phytoremediation process, especially when they grow fast and have a high biomass [25]. Solanum nigrum L., Brassica oleracea acephala L., Brassica napus , Brassica juncea , Iberis intermedia and Callitriche cophocarpa have been identified as good species for the phytoremediation of soil and waters contaminated by thallium [26][27][28].
2. Thallium Toxicity
| References | |
|---|---|
| Cardiac symptoms | [31][32] |
| Coma | |
| Hypotension | |
| Lethargy | |
| Tachycardia | |
| Dermatological symptoms | [32] |
| Alopecia (after about 3 weeks) | |
| Anhidrosis | |
| Hypohidrosis | |
| Mees lines on the nails (after about 1 month) | |
| Gastrointestinal symptoms | [32] |
| Diarrhea or constipation | |
| Nausea and vomiting | |
| Stool containing blood | |
| Hematologic symptoms | [32] |
| Anemia | |
| Eosinophilia | |
| Leukocytosis | |
| Neutrophilia | |
| Thrombocytopenia | |
| Neurologic symptoms (after 3–5 days) | [31][32] |
| Ataxia | |
| Convulsion | |
| Death | |
| Distal muscle weakness of the hands or feet | |
| Hallucination | |
| Headache | |
| Insomnia | |
| Paresthesia | |
| Tremor | |
| Ocular symptoms | [32] |
| Atrophy of the optic nerve | |
| Cranial nerve 7th palsy | |
| Nystagmus | |
| Lens opacity | |
| Optic neuropathy | |
| Ptosis |
3. Uses of Thallium
References
- Karbowska, B. Presence of thallium in the environment: Sources of contaminations, distribution and monitoring methods. Environ. Monit. Assess. 2016, 188, 640.
- Cobelo-García, A.; Filella, M.; Croot, P.; Frazzoli, C.; Du Laing, G.; Ospina-Alvarez, N.; Rauch, S.; Salaun, P.; Schäfer, J.; Zimmermann, S. COST action TD1407: Network on technology-critical elements (NOTICE)—from environmental processes to human health threats. Environ. Sci. Pollut. Res. 2015, 22, 15188–15194.
- Sinicropi, M.S.; Caruso, A.; Capasso, A.; Palladino, C.; Panno, A.; Saturnino, C. Heavy metals: Toxicity and carcinogenicity. Pharmacologyonline 2010, 2, 329–333.
- Carocci, A.; Rovito, N.; Sinicropi, M.S.; Genchi, G. Mercury toxicity and neurodegenerative effects. Rev. Environ. Contam. Toxicol. 2014, 229, 1–18.
- Osorio-Rico, L.; Santamaria, A.; Galván-Arzate, S. Thallium Toxicity: General Issues, Neurological Symptoms, and Neurotoxic Mechanisms. Adv. Neurobiol. 2017, 18, 345–353.
- Osorio-Rico, L.; Villeda-Hernández, J.; Santamaría, A.; Königsberg, M.; Galván-Arzate, S. The N-methyl-d-aspartate receptor antagonist MK-801 prevents thallium-induced behavioral and biochemical alterations in the rat brain. Int. J. Toxicol. 2015, 34, 505–513.
- Zhu, B.; Liang, C.; Yan, S.; Li, Z.; Huang, K.; Xia, X.; Hao, J.; Zhu, P.; Tao, F. Association between serum thallium in early pregnancy and risk of gestational diabetes mellitus: The Ma’anshan birth cohort study. J. Trace Elem. Med. Biol. 2019, 52, 151–156.
- Wu, M.; Shu, Y.; Song, L.; Liu, B.; Zhang, L.; Wang, L.; Liu, Y.; Bi, J.; Xiong, C.; Cao, Z.; et al. Prenatal exposure to thallium is associated with decreased mitochondrial DNA copy number in newborns: Evidence from a birth cohort study. Environ. Int. 2019, 129, 470–477.
- Altagracia-Martínez, M.; Kravzov-Jinich, J.; Martínez-Núñez, J.M.; Ríos-Castañeda, C.; López-Naranjo, F. Prussian blue as an antidote for radioactive thallium and cesium poisoning. Orphan Drugs Res. Rev. 2012, 2, 13–21.
- Kamerbeek, H.H.; Rauws, A.G.; Ten Ham, M.; Van Heijst, A.N.P. Dangerous redistribution of thallium by treatment with sodium diethyldithiocarbamate. Acta Med. Scandinav. 1971, 189, 149–154.
- Montes, S.; Pérez-Barrón, G.; Rubio-Osornio, M.; Ríos, C.; Diaz-Ruíz, A.; Altagracia-Martínez, M.; Monroy-Noyola, A. Additive effect of DL-penicillamine plus Prussian blue for the antidotal treatment of thallotoxicosis in rats. Environ. Toxicol. Pharmacol. 2011, 32, 349–355.
- Anaya-Ramos, L.; Díaz-Ruíz, A.; Ríos, C.; Montes, S.; Aguirre-Vidal, Y.; García-Jiménez, S.; Baron-Flores, V.; Monroy-Noyola, A. Metallothionein alone or in combination with Prussian blue attenuates acute thallium systemic toxicity in rats. Res. Sq. 2020.
- Ghannoum, M.; Nolin, T.D.; Goldfarb, D.S.; Darren, M.R.; Mactier, R.; Mowry, J.B.; Dargan, P.I.; MacLaren, R.; Hoegberg, L.C.; Laliberté, M.; et al. Extracorporeal treatment for thallium poisoning: Recommendations from the EXTRIP Workgroup. Clin. J. Am. Soc. Nephrol. 2012, 7, 1682–1690.
- Lebowitz, E.; Greene, M.V.; Fairchild, R.; Bradley-Moore, P.R.; Atkins, H.L.; Ansari, A.N.; Richards, P.; Belgrave, E. Thallium-201 for medical use. I. J. Nucl. Med. 1975, 16, 151–155.
- Ritchie, J.L.; Trobaugh, G.B.; Hamilton, G.W.; Gould, K.L.; Naraharn, K.A.; Williams, D.L. Myocardial imaging with thallium-201 at rest and during exercise: Comparison with coronary arteriography and resting and stress electrocardiography. Circulation 1977, 56, 66–71.
- Poudyal, B.; Shrestha, P.; Chowdhury, Y.S. Thallium-201. StatPearls . 21 July 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK560586/ (accessed on 11 August 2021).
- U.S. Geological Survey (U.S. GS). Thallium Statistics and Information. Available online: http://minerals.usgs.gov/minerals/pubs/commodity/thallium/ (accessed on 29 June 2021).
- Wang, J.; Zhou, Y.; Dong, X.; Yin, M.; Tsang, D.C.; Sun, J.; Liu, J.; Song, G.; Liu, Y. Temporal sedimentary record of thallium pollution in an urban lake: An emerging thallium pollution source from copper metallurgy. Chemosphere 2020, 242, 125172.
- Liu, J.; Wei, X.; Zhou, Y.; Tsang, D.C.W.; Bao, Z.; Yin, M.; Lippold, H.; Yuan, W.; Wang, J.; Feng, Y.; et al. Thallium contamination, health risk assessment and source apportionment in common vegetables. Sci. Total Environ. 2020, 703, 135547.
- Sander, S.; Kappenstein, O.; Ebner, I.; Fritsch, K.A.; Schmidt, R.; Ptaff, K.; Luch, A. Release of aluminium and thallium ions from uncoated food contact materials made of aluminium alloys into food and food simulant. PLoS ONE 2018, 13, e0200778.
- Datta, A.; Fiala, J.; Becla, P.; Motakef, S. Stable room-temperature thallium bromide semiconductor radiation detectors. APL Mater. 2017, 5, 106109.
- Aprea, M.C.; Nuvolone, D.; Petri, D.; Voller, F.; Bertelloni, S.; Aragona, I. Human biomonitoring to assess exposure to thallium following the contamination of drinking water. PLoS ONE 2020, 15, e0241223.
- Staff, J.F.; Cotton, R.J.; Warren, N.D.; Morton, J. Comparison of urinary thallium levels in non-occupationally ex-posed people and workers. Int. Arch. Occup. Environ. Health 2014, 87, 275–284.
- Farooqi, Z.U.R. Phytoremediation of inorganic pollutants: An eco-friendly approach, its types and mechanisms. Plant Environ. 2012, 1, 110–129.
- Rai, G.K.; Bhat, B.A.; Mushtaq, M.; Tariq, L.; Rai, P.K.; Basu, U.; Dar, A.A.; Islam, S.T.; Dar, T.U.H.; Bhat, J.A. Insights into decontamination of soils by phytoremediation: A detailed account on heavy metal toxicity and mitigation strategies. Physiol. Plant. 2021, 173, 287–304.
- Al-Najar, H.; Schulz, R.; Römheld, V. Phytoremediation of thallium contaminated soils by brassicaceae. In Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2005; pp. 187–196.
- Rader, S.T.; Maier, R.M.; Barton, M.D.; Mazdab, F.K. Uptake and fractionation of thallium by Brassica juncea in a geogenic thallium-amended substrate. Environ. Sci. Technol. 2019, 53, 2441–2449.
- Wu, Q.; Leung, J.Y.S.; Huang, X.; Yao, B.; Yuan, X.; Ma, J.; Guo, S. Evaluation of the ability of black nightshade Solanum nigrum L. for phytoremediation of thallium-contaminated soil. Environ. Sci. Pollut. Res. 2015, 22, 11478–11487.
- Carocci, A.; Catalano, A.; Lauria, G.; Sinicropi, M.S.; Genchi, G. Lead toxicity, antioxidant defense andenvironment. Rev. Environ. Contam. Toxicol. 2016, 238, 45–67.
- Genchi, G.; Sinicropi, M.S.; Carocci, A.; Lauria, G.; Catalano, A. Mercury exposure and heart diseases. Int. J. Environ. Res. Public Health 2017, 14, 74.
- Zavaliy, L.B.; Petrikov, S.S.; Simonova, A.Y.; Potskhveriya, M.M.; Zaker, F.; Ostapenko, Y.N.; Ilyashenko, K.K.; Dikaya, T.I.; Shakhova, O.B.; Evseev, A.K.; et al. Diagnosis and treatment of persons with acute thallium poisoning. Toxicol. Rep. 2021, 8, 277–281.
- Kemnic, T.R.; Coleman, M. Thallium Toxicity; StatPearls Publishing: Treasure Island, NV, USA, 2021.
- Yumoto, T.; Tsukahara, K.; Naito, H.; Iida, A.; Nakao, A. A successfully treated case of criminal thallium poisoning. J. Clin. Diagn. Res. 2017, 11, OD01–OD02.
- Ghaderi, A.; Vahdati-Mashhadian, N.; Oghabian, Z.; Moradi, V.; Afshari, R.; Mehrpour, O. Thallium exists in opioid poisoned patients. DARU J. Pharm. Sci. 2015, 23, 1–4.
- Molavi, N.; Ghaderi, A.; Banafshe, H.R. Determination of thallium in urine, blood, and hair in illicit opioid users in Iran. Hum. Exp. Toxicol. 2020, 39, 808–815.
- Tyagi, R.; Rana, P.; Khan, A.R.; Bhatnagar, D.; Devi, M.M.; Chaturvedi, S.; Tripathi, R.P.; Khushu, S. Study of acute biochemical effects of thallium toxicity in mouse urine by NMR spectroscopy. J. Appl. Toxicol. 2011, 31, 663–670.
- Nagaraja, P.; Ghllab Saeed Al-Tayar, N.; Shivakumar, A.; Shresta, A.K.; Gowda, A.K. Spectrophotometric determination of the trace amount of thallium in water and urine samples by novel oxidative coupling reaction. E-J. Chem. 2009, 6, 1153–1163.
- Eskandari, M.R.; Mashayekhi, V.; Aslani, M.; Hosseini, M.J. Toxicity of thallium on isolated rat liver mitochondria: The role of oxidative stress and MPT pore opening. Environ. Toxicol. 2005, 30, 232–241.
- International Programme on Chemical Safety (IPCS). Thallium. Environmental Health Criteria; World Health Organization: Geneva, Switzerland, 1996; Volume 182.
- Cvjetko, P.; Cvjetko, I.; Pavlica, M. Thallium Toxicity in Humans. Arh. Hig. Rada Toksikol. 2010, 61, 111–119.
- Hoffman, R.S. Thallium toxicity and the role of Prussian blue in therapy. Toxicol. Rev. 2003, 22, 29–40.
- Rodríguez-Mercado, J.J.; Altamirano-Lozano, M.A. Genetic toxicology of thallium: A review. Drug Chem. Toxicol. 2013, 36, 369–383.
- Douglas, K.T.; Bunni, M.A.; Baindur, S.R. Thallium in biochemistry. Int. J. Biochem. 1990, 22, 429–438.
- Riyaz, R.; Pandalai, S.L.; Schwartz, M.; Kazzi, Z.N. A fatal case of thallium toxicity: Challenges in management. J. Med. Toxicol. 2013, 9, 75–78.
- Stockinger, H.E. Thallium. In Patty’s Industrial Hygiene and Toxicology: Vol. 2A: Toxicology, 3rd ed.; John Wiley and Sons Publishing: New York, NY, USA; Chichester: Brisbane, Australia; Toronto, ON, Canada, 1987; pp. 1914–1931.
- Blain, F.; Kazantzis, G. Thallium. In Handbook on the Toxicology of Metals, 4th ed.; Nordberg, G.F., Fowler, B.A., Nordberg, M., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 1129–1240. ISBN 9780123973399.
- Lei, C.; Xu, X. Determination of Thallium in Drinking Water and Source Water by Using Inductively Coupled Plasma-mass Spectrometry. Fujian Anal. Test. 2012, 3, 27–29.
- Asadoulahi, T.; Dadfarnia, S.; Shabani, A.M.H. Determination of thallium traces by ETAAS after on-line matrix separation and preconcentration in a flow injection system. J. Braz. Chem. Soc. 2007, 18, 1353–1359.
- Lukaszewski, Z.; Jakubowska, M.; Zembrzuski, W.; Karbowska, B.; Pasieczna, A. Flow-injection differential-pulse anodic stripping voltammetry as a tool for thallium monitoring in the environment. Electroanalysis 2010, 22, 1963–1966.




