
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Antonino Fiorino | + 2650 word(s) | 2650 | 2021-09-23 06:26:38 | | | |
| 2 | Catherine Yang | -1 word(s) | 2649 | 2021-10-11 03:05:53 | | |
Video Upload Options
Amelogenins are enamel matrix proteins currently used to treat bone defects in periodontal surgery. Recent studies have highlighted the relevance of amelogenin-derived peptides, named LRAP, TRAP, SP, and C11, in bone tissue engineering. Interestingly, these peptides seem to maintain or even improve the biological activity of the full-length protein, which has received attention in the field of bone regeneration.
1. Introduction
A wide range of pathological phenomena can be directly or indirectly responsible for skeletal tissue loss. Many of the generated defects, known as critical size defects (CSDs), do not heal spontaneously. The best option to treat CSDs is an autologous bone graft that possesses the three main features needed in bone regenerative medicine: osteogenic, osteoinductive, and osteoconductive properties. Unfortunately, autologous bone graft is rarely used due to a number of drawbacks, such as the requirement of a second surgical procedure with serious risks of infection at the donor site and the generation of significant pain.
Regenerative medicine and tissue engineering offer alternative strategies for the treatment of CSDs. In most of the approaches proposed, molecules capable of stimulating cell migration, recruitment, proliferation, and differentiation, as well as biomineralization, play a pivotal role in the formation of de novo bone tissue. Platelet-derived growth factors, insulin-like growth factors, transforming growth factors, and bone morphogenic proteins are examples of biomolecules investigated so far [1]. However, several efforts are still ongoing to individuate effective and safe bone morphogenic biomolecules. Among the biological macromolecules under investigation, amelogenins (AMG) represent an extremely interesting family of proteins with the above-mentioned characteristics for which the bone morphogenic properties are still matter of debate.
AMG-based preparations were first proposed in the dermatological field for the treatment of burns and, only later, in dentistry. The first AMG formulation marketed for periodontal tissue regeneration procedures was Emdogain ® . The product contains a mixture of animal enamel matrix derivatives embedded in an alginate propylene glycol hydrogel. After 20 years, the use of Emdogain ® in periodontal regeneration procedures has shown a statistically significant improvement in the recovery of the periodontal ligament, cement, and alveolar bone [2].
Although for over four decades, AMG was considered a specific enamel protein expressed in periodontal tissues, such as cementoblasts, periodontal ligament (PDL) cells, or Hertwig’s epithelial root sheath (HERS) [3][4][5][6], its expression has also been reported, at a lower level, in non-dental cell types, such as stem cells, bone cells, brain, and other soft tissue [7][8][9]. Of interest are some observations suggesting that specific AMG splicing products may function as epithelial-mesenchymal or mesenchymal-mesenchymal signaling molecules [10][11][12][13]. In the late 1960s and early 1970s, two articles showed the osteoinductive potential of decalcified enamel and dentin extracts in ectopic sites [14][15]. This phenomenon was attributed to the presence of peptides with chondro-/osteoinduction properties derived from AMG gene splicing [16]. AMG-derived peptides are formed by alternative splicing or proteolytic cleavage of the ~20 kDa full-length protein [17][18].
2. LRAP
3. TRAP
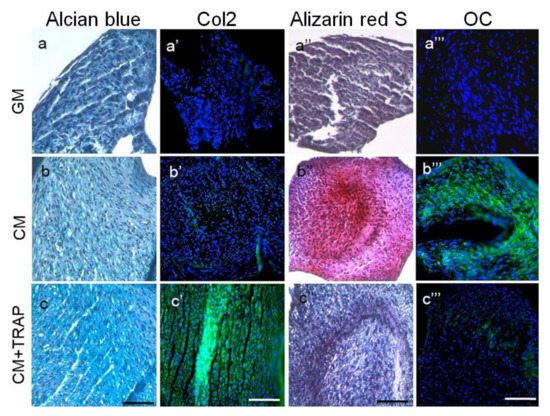
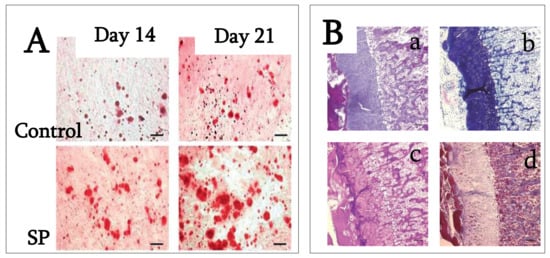
4. SP (Synthetic Peptide)
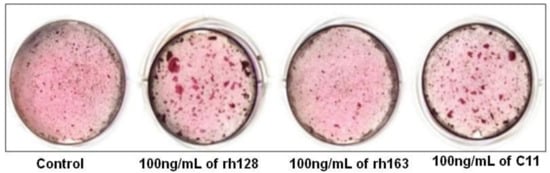
5. C11 (Amelogenin C Peptide, AMG-CP)
References
- Schliephake, H. Bone growth factors in maxillofacial skeletal reconstruction. Int. J. Oral Maxillofac. Surg. 2002, 31, 469–484.
- Miron, R.J.; Sculean, A.; Cochran, D.L.; Froum, S.; Zucchelli, G.; Nemcovsky, C.; Donos, N.; Lyngstadaas, S.P.; Deschner, J.; Dard, M.; et al. Twenty years of enamel matrix derivative: The past, the present and the future. J. Clin. Periodontol. 2016, 43, 668–683.
- Slavkin, H.C.; Bessem, C.; Fincham, A.G.; Bringas, P.; Santos, V.; Snead, M.L.; Zeichner-David, M. Human and mouse cementum proteins immunologically related to enamel proteins. Biochim. Biophys. Acta-Gen. Subj. 1989, 991, 12–18.
- Hammarström, L. The role of enamel matrix proteins in the development of cementum and periodontal tissues. Ciba Found. Symp. 1997, 205, 246–255.
- Fong, C.D.; Hammarström, L. Expression of amelin and amelogenin in epithelial root sheath remnants of fully formed rat molars. Oral Surgery Oral Med. Oral Pathol. Oral Radiol. Endodontology 2000, 90, 218–223.
- Sonoyama, W.; Seo, B.-M.; Yamaza, T.; Shi, S. Human Hertwig’s Epithelial Root Sheath Cells Play Crucial Roles in Cementum Formation. J. Dent. Res. 2007, 86, 594–599.
- Warotayanont, R.; Frenkel, B.; Snead, M.L.; Zhou, Y. Leucine-rich amelogenin peptide induces osteogenesis by activation of the Wnt pathway. Biochem. Biophys. Res. Commun. 2009, 387, 558–563.
- Haze, A.; Taylor, A.L.; Blumenfeld, A.; Rosenfeld, E.; Leiser, Y.; Dafni, L.; Shay, B.; Gruenbaum-Cohen, Y.; Fermon, E.; Haegewald, S.; et al. Amelogenin expression in long bone and cartilage cells and in bone marrow progenitor cells. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2007, 290, 455–460.
- Deutsch, D.; Haze-Filderman, A.; Blumenfeld, A.; Dafni, L.; Leiser, Y.; Shay, B.; Gruenbaum-Cohen, Y.; Rosenfeld, E.; Fermon, E.; Zimmermann, B.; et al. Amelogenin, a major structural protein in mineralizing enamel, is also expressed in soft tissues: Brain and cells of the hematopoietic system. Eur. J. Oral Sci. 2006, 114, 183–189.
- Veis, A.; Tompkins, K.; Alvares, K.; Wei, K.; Wang, L.; Wang, X.S.; Brownell, A.G.; Jengh, S.-M.; Healy, K.E. Specific Amelogenin Gene Splice Products Have Signaling Effects on Cells in Culture and in Implants in Vivo. J. Biol. Chem. 2000, 275, 41263–41272.
- Tompkins, K.; Veis, A. Polypeptides translated from alternatively spliced transcripts of the amelogenin gene, devoid of the exon 6a, b, c region, have specific effects on tooth germ development in culture. Connect. Tissue Res. 2002, 43, 224–231.
- Veis, A. Amelogenin gene splice products: Potential signaling molecules. Cell. Mol. Life Sci. 2003, 60, 38–55.
- Tompkins, K.; Alvares, K.; George, A.; Veis, A. Two related low molecular mass polypeptide isoforms of amelogenin have dis-tinct activities in mouse tooth germ differentiation in vitro. J. Bone Miner. Res. 2005, 20, 341–349.
- Urist, M.R. Bone histogenesis and morphogenesis in implants of demineralized enamel and dentin. J. Oral. Surg. 1971, 29, 88–102.
- Yeomans, D.J.; Urist, M.R. Bone induction by decalcified dentin implanted into oral osseous and muscle tissues. Arch. Oral Biol. 1967, 12, 999–1008.
- Nebgen, D.; Inoue, H.; Sabsay, B.; Wei, K.; Ho, C.-S.; Veis, A. Identification of the chondrogenic-inducing activity from bovine dentin (bCIA) as a low-molecular-mass amelogenin polypeptide. J. Dent. Res. 1999, 78, 1484–1494.
- Fincham, A.; Moradianoldak, J. Amelogenin Post-translational Modifications: Carboxy-Terminal Processing and the Phosphorylation of Bovine and Porcine “TRAP” and “LRAP” Amelogenins. Biochem. Biophys. Res. Commun. 1993, 197, 248–255.
- Nagano, T.; Kakegawa, A.; Yamakoshi, Y.; Tsuchiya, S.; Hu, J.-C.; Gomi, K.; Arai, T.; Bartlett, J.; Simmer, J. Mmp-20 and Klk4 Cleavage Site Preferences for Amelogenin Sequences. J. Dent. Res. 2009, 88, 823–828.
- Yuan, Z.; Collier, P.; Rosenbloom, J.; Gibson, C. Analysis of amelogenin mRNA during bovine tooth development. Arch. Oral Biol. 1996, 41, 205–213.
- Fincham, A.G.; Belcourt, A.B.; Termine, J.D.; Butler, W.T.; Cothran, W.C. Dental enamel matrix: Sequences of two amelogenin polypeptides. Biosci. Rep. 1981, 1, 771–778.
- Masica, D.L.; Gray, J.J.; Shaw, W.J. Partial High-Resolution Structure of Phosphorylated and Non-phosphorylated Leucine-Rich Amelogenin Protein Adsorbed to Hydroxyapatite. J. Phys. Chem. C 2011, 115, 13775–13785.
- Lu, J.X.; Xu, Y.S.; Buchko, G.W.; Shaw, W.J. Mineral association changes the secondary structure and dynamics of murine amelog-enin. J. Dent. Res. 2013, 92, 1000–1004.
- Tarasevich, B.J.; Perez-Salas, U.; Masica, D.L.; Philo, J.; Kienzle, P.; Krueger, S.; Majkrzak, C.F.; Gray, J.L.; Shaw, W.J. Neutron reflectometry studies of the adsorbed structure of the amelogen-in, LRAP. J. Phys. Chem. B 2013, 117, 3098–3109.
- Yamazaki, H.; Beniash, E.; Yamakoshi, Y.; Simmer, J.P.; Margolis, H.C. Protein Phosphorylation and Mineral Binding Affect the Secondary Structure of the Leucine-Rich Amelogenin Peptide. Front. Physiol. 2017, 8.
- Ma, C.-W.; Zhang, J.; Dong, X.-Q.; Lu, J.-X. Amyloid structure of high-order assembly of Leucine-rich amelogenin revealed by solid-state NMR. J. Struct. Biol. 2019, 206, 29–35.
- Boabaid, F.; Gibson, C.W.; Kuehl, M.A.; Berry, J.E.; Snead, M.L.; Nociti, F.H.; Katchburian, E.; Somerman, M.J. Leucine-Rich Amelogenin Peptide: A Candidate Signaling Molecule During Cementogenesis. J. Periodontol. 2004, 75, 1126–1136.
- Warotayanont, R.; Zhu, D.; Snead, M.L.; Zhou, Y. Leucine-rich amelogenin peptide induces osteogenesis in mouse embryonic stem cells. Biochem. Biophys. Res. Commun. 2008, 367, 1–6.
- Wen, X.; Cawthorn, W.P.; MacDougald, O.A.; Stupp, S.I.; Snead, M.L.; Zhou, Y. The influence of Leucine-rich amelogenin peptide on MSC fate by induc-ing Wnt10b expression. Biomaterials 2011, 32, 6478–6486.
- Le, T.Q.; Zhang, Y.; Li, W.; Denbesten, P.K. The effect of LRAP on enamel organ epithelial celldifferentiation. J. Dent Res. 2007, 86, 1095–1099.
- Le Norcy, E.; Kwak, S.Y.; Wiedemann-Bidlack, F.B.; Beniash, E.; Yamakoshi, Y.; Simmer, J.P.; Margolis, H.C. Leucine-rich amelogenin peptides regulate mineralization in vitro. J. Dent. Res. 2011, 90, 1091–1097.
- Xia, Y.; Ren, A.; Pugach, M.K. Truncated amelogenin and LRAP transgenes improve Amelx null mouse enamel. Matrix Biol. 2016, 52–54, 198–206.
- Habelitz, S.; DenBesten, P.K.; Marshall, S.J.; Marshall, G.W.; Li, W. Self-assembly and effect on crystal growth of the leucine-rich amelogenin peptide. Eur. J. Oral Sci. 2006, 114, 315–319.
- Tarasevich, B.J.; Lea, S.; Shaw, W.J. The leucine rich amelogenin protein (LRAP) adsorbs as monomers or dimers onto sur-faces. J. Struct. Biol. 2010, 169, 266–276.
- Shaw, W.J.; Campbell, A.A.; Paine, M.L.; Snead, M.L. The COOH terminus of the amelogenin, LRAP, is oriented next to the hydrax-yapatite surface. J. Biol. Chem. 2004, 279, 40263–40266.
- Shaw, W.J.; Ferris, K.; Tarasevich, B.; Larson, J.L. The Structure and Orientation of the C-Terminus of LRAP. Biophys. J. 2008, 94, 3247–3257.
- Le, T.Q.; Gochin, M.; Featherstone, J.D.B.; Li, W.; DenBesten, P.K. Comparative calcium binding of leucine-rich amelogenin pep-tide and full-length amelogenin. Eur. J. Oral Sci. 2006, 114, 320–326.
- Amin, H.D.; Olsen, I.; Knowles, J.C.; Donos, N. Differential Effect of Amelogenin Peptides on Osteogenic Differentiation In Vitro: Identification of Possible New Drugs for Bone Repair and Regeneration. Tissue Eng. Part A 2012, 18, 1193–1202.
- Stout, B.M.; Alent, B.J.; Pedalino, P.; Holbrook, R.; Gluhak-Heinrich, J.; Cui, Y.; Harris, M.A.; Gemperli, A.C.; Cochran, D.L.; Deas, D.E.; et al. Enamel Matrix Derivative: Protein Components and Osteoinductive Properties. J. Periodontol. 2014, 85, e9–e17.
- Amin, H.D.; Olsen, I.; Knowles, J.; Dard, M.; Donos, N. A tyrosine-rich amelogenin peptide promotes neovasculogenesis in vitro and ex vi-vo. Acta Biomater. 2014, 10, 1930–1939.
- Amin, H.D.; Olsen, I.; Knowles, J.; Dard, M.; Donos, N. A procedure for identifying stem cell compartments with multi-lineage differentia-tion potential. Analyst 2011, 136, 1440–1449.
- Amin, H.D.; Olsen, I.; Knowles, J.C.; Dard, M.; Donos, N. Effects of enamel matrix proteins on multi-lineage differentiation of periodontal ligament cells in vitro. Acta Biomater. 2013, 9, 4796–4805.
- Jonke, E.; Gemperli, A.C.; Zhang, T.; Özdemir, B.; Dard, M.; Rausch-Fan, X.; Andrukhov, O. Effect of tyrosine-rich amelogenin peptide on behavior and differentiation of en-dothelial cells. Clin. Oral Investig. 2016, 20, 2275–2284.
- Aspriello, S.D.; Zizzi, A.; Spazzafumo, L.; Rubini, C.; Lorenzi, T.; Marzioni, D.; Bullon, P.; Piemontese, M. Effects of Enamel Matrix Derivative on Vascular Endothelial Growth Factor Expression and Microvessel Density in Gingival Tissues of Periodontal Pocket: A Comparative Study. J. Periodontol. 2011, 82, 606–612.
- Thoma, D.S.; Villar, C.C.; Carnes, D.L.; Dard, M.; Chun, Y.-H.P.; Cochran, D.L. Angiogenic activity of an enamel matrix derivative (EMD) and EMD-derived proteins: An experimental study in mice. J. Clin. Periodontol. 2010, 38, 253–260.
- Schlueter, S.R.; Carnes, D.L.; Cochran, D.L. In Vitro Effects of Enamel Matrix Derivative on Microvascular Cells. J. Periodontol. 2007, 78, 141–151.
- Kasaj, A.; Meister, J.; Lehmann, K.; Stratul, S.-I.; Schlee, M.; Stein, J.M.; Willershausen, B.; Schmidt, M. The influence of enamel matrix derivative on the angiogenic activity of primary endothelial cells. J. Periodontal Res. 2011, 47, 479–487.
- Bertl, K.; An, N.; Bruckmann, C.; Dard, M.; Andrukhov, O.; Matejka, M.; Rausch-Fan, X. Effects of Enamel Matrix Derivative on Proliferation/Viability, Migration, and Ex-pression of Angiogenic Factor and Adhesion Molecules in Endothelial Cells In Vitro. J. Periodontol. 2009, 80, 1622–1630.
- Yuan, K.; Chen, C.-L.; Lin, M.T. Enamel matrix derivative exhibits angiogenic effect in vitro and in a murine model. J. Clin. Periodontol. 2003, 30, 732–738.
- Martin, P. Wound Healing--Aiming for Perfect Skin Regeneration. Science 1997, 276, 75–81.
- Albelda, S.M.; Smith, C.W.; Ward, P.A. Adhesion molecules and inflammatory injury. FASEB J. 1994, 8, 504–512.
- Bao, P.; Kodra, A.; Tomic-Canic, M.; Golinko, M.S.; Ehrlich, H.P.; Brem, H. The Role of Vascular Endothelial Growth Factor in Wound Healing. J. Surg. Res. 2009, 153, 347–358.
- Peters, K.G.; De Vries, C.; Williams, L.T. Vascular endothelial growth factor receptor expression during embryogenesis and tissue repair suggests a role in endothelial differentiation and blood vessel growth. Proc. Natl. Acad. Sci. USA 1993, 90, 8915–8919.
- Jones, N.; Iljin, K.; Dumont, D.J.; Alitalo, K. Tie receptors: New modulators of angiogenic and lymphangiogenic responses. Nat. Rev. Mol. Cell Biol. 2001, 2, 257–267.
- Amin, H.D.; Ethier, C.R. Differential effects of tyrosine-rich amelogenin peptide on chondrogenic and osteogenic differenti-ation of adult chondrocytes. Cell Tissue Res. 2016, 364, 219–224.
- Tanimoto, K.; Kunimatsu, R.; Tanne, Y.; Huang, Y.-C.; Michida, M.; Yoshimi, Y.; Miyauchi, M.; Takata, T.; Tanne, K. Differential Effects of Amelogenin on Mineralization of Cementoblasts and Periodontal Ligament Cells. J. Periodontol. 2012, 83, 672–679.
- Kim, N.H.; Tominaga, K.; Tanaka, A. Analysis of eosinophilic round bodies formed after injection of enamel matrix deriva-tive into the backs of rats. J. Periodontol. 2005, 76, 1934–1941.
- Kawanaka, A.; Tominaga, K.; Tanaka, A. Effect of peptide derived from Emdogain on human periodontal ligament fibro-blast. J. Osaka Dent. Univ. 2009, 43, 111–117.
- Taguchi, Y.; Yasui, N.; Takahashi, S.; Tominaga, K.; Kato, H.; Komasa, S.; Shida, M.; Hayashi, H.; Tanaka, A.; Umeda, M. Hard Tissue Formation by Human Periodontal Ligament Fibroblast Cells Treated with an Emdogain-Derived Oligopeptide in vitro. J. Hard Tissue Biol. 2012, 21, 375–384.
- Yasui, N.; Taguchi, Y.; Tanaka, A.; Ueda, M.; Umeda, M. Biologic effects of Emdogain Derived Oligopeptides on rat bone marrow cells. J. Oral Tissue Eng. 2012, 9, 126–135.
- Kato, H.; Katayama, N.; Taguchi, Y.; Tominaga, K.; Umeda, M.; Tanaka, A. A synthetic oligopeptide derived from enamel matrix derivative promotes the dif-ferentiation of human periodontal ligament stem cells into osteoblast-like cells with increased mineralization. J. Periodontol. 2013, 84, 1476–1483.
- Hida, T.; Tominaga, K.; Tanaka, A. Tissue Reaction to Synthetic Oligopeptide Derived from Enamel Matrix Derivative in Rats. Oral Sci. Int. 2010, 7, 26–33.
- Katayama, N.; Kato, H.; Taguchi, Y.; Tanaka, A.; Umeda, M. The Effects of Synthetic Oligopeptide Derived from Enamel Matrix Derivative on Cell Proliferation and Osteoblastic Differentiation of Human Mesenchymal Stem Cells. Int. J. Mol. Sci. 2014, 15, 14026–14043.
- Moradian-Oldak, J.; Bouropoulos, N.; Wang, L.; Gharakhanian, N. Analysis of self-assembly and apatite binding properties of amelogenin proteins lacking the hydrophilic C-terminal. Matrix Biol. 2002, 21, 197–205.
- Zhu, L.; Tanimoto, K.; Le, T.; DenBesten, P.K.; Li, W. Functional Roles of Prolines at Amelogenin C Terminal during Tooth Enamel Formation. Cells Tissues Organs 2008, 189, 203–206.
- Ryu, O.; Fincham, A.; Hu, C.-C.; Zhang, C.; Qian, Q.; Bartlett, J.; Simmer, J. Characterization of recombinant pig enamelysin activity and cleavage of recombinant pig and mouse amelogenins. J. Dent. Res. 1999, 78, 743–750.
- Yoshimi, Y.; Kunimatsu, R.; Hirose, N.; Awada, T.; Miyauchi, M.; Takata, T.; Li, W.; Zhu, L.; Denbesten, P.; Tanne, K.; et al. Effects of C-Terminal Amelogenin Peptide on Proliferation of Human Cemento-blast Lineage Cells. J. Periodontol. 2016, 87, 820–827.
- Ando, K.; Kunimatsu, R.; Awada, T.; Yoshimi, Y.; Tsuka, Y.; Sumi, K.; Horie, K.; Abe, T.; Nakajima, K.; Tanimoto, K. Effects of Human Full-length Amelogenin and C-terminal Amelogenin Peptide on the Proliferation of Human Mesenchymal Stem Cells Derived from Adipose Tissue. Curr. Pharm. Des. 2018, 24, 2993–3001.
- Kunimatsu, R.; Awada, T.; Yoshimi, Y.; Ando, K.; Hirose, N.; Tanne, Y.; Sumi, K.; Tanimoto, K. The C-terminus of the amelogenin peptide influences the proliferation of hu-man bone marrow mesenchymal stem cells. J. Periodontol. 2018, 89, 496–505.
- Kunimatsu, R.; Yoshimi, Y.; Hirose, N.; Awada, T.; Miyauchi, M.; Takata, T.; Li, W.; Zhu, L.; DenBesten, P.; Tanimoto, K. The C-terminus of amelogenin enhances osteogenic differentiation of human cementoblast lineage cells. J. Periodontal Res. 2016, 52, 218–224.
- Awada, T.; Kunimatsu, R.; Yoshimi, Y.; Hirose, N.; Mitsuyoshi, T.; Sumi, K.; Tanimoto, K. Effects of C-terminal amelogenin pep-tides on the metabolism of osteoblasts. Biochem. Biophys. Res. Commun. 2017, 482, 1154–1159.





